Effects of Comprehensive Rehabilitation on Pulmonary Function in Patients Recovering from COVID-19
Abstract
1. Introduction
2. Materials and Methods
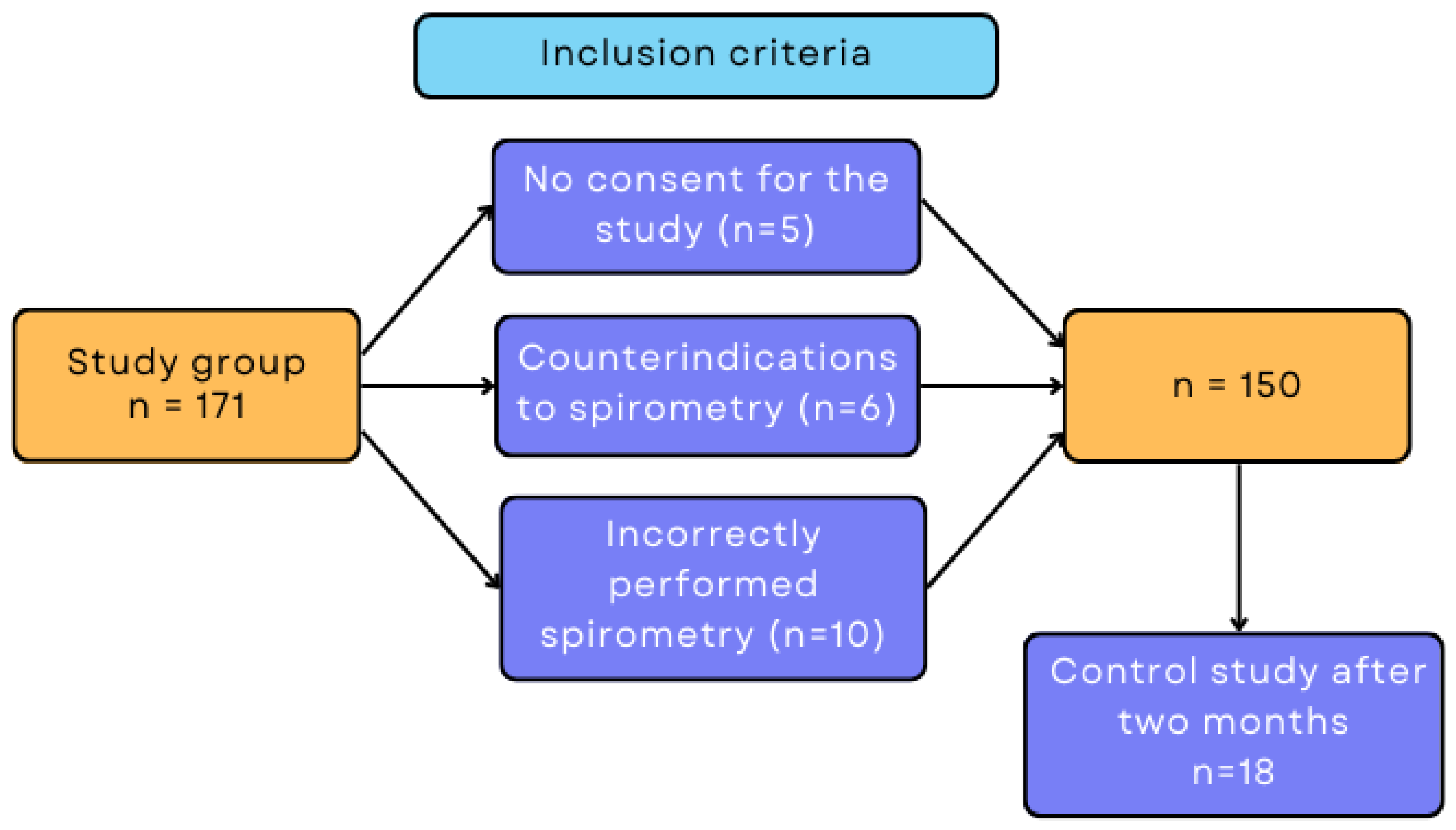
2.1. Characteristics of the Study Group
2.2. Course of the Study
2.3. Statistical Methods
3. Results
3.1. Group Characteristics
3.2. Spirometric Results before and after Rehabilitation
3.3. Spirometric Results Immediately after Rehabilitation and after 2 Months
3.4. Difference in Spirometric Results before and after Rehabilitation vs. Selected Variables
| Variable | Correct Weight (n = 30) | Overweight (n = 53) | 1st Degree Obesity (n = 46) | 2nd Degree Obesity (n = 15) | 3rd Degree Obesity (n = 5) | p | |
|---|---|---|---|---|---|---|---|
| Δ FVC (% predicted) | Me (Q1–Q3) | 10.86 (7.7–20.1) | 4.1 (−1.6–13.06) | 3.1 (−5.5–13.15) | 11.8 (2.7–15.9) | 3.2 (−3.8–7.3) | 0.026 * |
| Δ FEV1 (% predicted) | M ± SD | 12.35 ± 13.76 | 10.87 ± 6.99 | 6.85 ± 16.25 | 12.75 ± 15.24 | −11.99 ± 13.66 | 0.007 * |
| Δ FEV1/FVC (%) | Me (Q1–Q3) | −1.25 (−5.8–6.6) | 3.27 (−4.6–12.6) | 0.7 (−3.7–8.76) | 0.04 (−8.4–10.6) | 0.3 (−5.2–4.9) | 0.712 |
| Δ PEF (% predicted) | M ± SD | 20.15 ± 19.31 | 24.98 ± 24.66 | 18.24 ± 22.32 | 19.73 ± 26.09 | 18.02 ± 15.43 | 0.428 |
| Δ MMEF (% predicted) | M ± SD | 15.38 ± 27.59 | 19.43 ± 16.9 | 14.25 ± 30.94 | 14.47 ± 32.26 | −7.98 ± 13.85 | 0.348 |
| Δ MEF75 (% predicted) | Me (Q1–Q3) | 24.27 (8.02–35.8) | 28.2 (12.1–42.1) | 13.6 (1.28–38.8) | 24.6 (6.3–42.01) | 23.3 (5–29.5) | 0.461 |
| Δ MEF50 (% predicted) | Me (Q1–Q3) | 21.1 (0.24–35.2) | 15.3 (−0.8–35.3) | 6.5 (−4.4–43.8) | 23.1 (−10.4–45.7) | 3.54 (−15–8.3) | 0.456 |
| Δ MEF25 (% predicted) | M ± SD | −5.86 ± 41.98 | 9.98 ± 5.46 | 2.38 ± 48.61 | −5.56 ± 41.47 | −28.61 ± 15.32 | 0.188 |
| Variable | 30–45 Years (n = 12) | 46–60 Years (n = 31) | 61–75 Years (n = 84) | 76–90 Years (n = 23) | p | |
|---|---|---|---|---|---|---|
| Δ FVC (% predicted) | Me (Q1–Q3) | 7.05 (−0.24–19.6) | 8.6 (2.3–16.7) | 6.2 (−3.9–12.6) | 7.47 (−0.26–16.6) | 0.562 |
| Δ FEV1 (% predicted) | M ± SD | 11.63 ± 9.76 | 10.12 ± 18.41 | 8.76 ± 15.41 | 9.91 ± 14.91 | 0.711 |
| Δ FEV1/FVC (%) | Me (Q1–Q3) | 3.2 (−5.04–7.5) | −0.8 (−6.3–6.65) | 2.15 (−4.8–9.7) | 0.3 (−8.9–17.34) | 0.837 |
| Δ PEF (% predicted) | M ± SD | 23.33 ± 17.36 | 14.61 ± 21.03 | 24.35 ± 21.71 | 17.15 ± 16.23 | 0.141 |
| Δ MMEF (% predicted) | Me (Q1–Q3) | 9.6 (−4.25–30.3) | 7.23 (−11.2–24.7) | 17.6 (−5.25–38.9) | 3.7 (−10.8–29.5) | 0.378 |
| Δ MEF75 (% predicted) | Me (Q1–Q3) | 27.1 (11.7–44.7) | 15.75 (1.9–35.8) | 26.65 (7.2–43.01) | 17.4 (4.9–32.3) | 0.322 |
| Δ MEF50 (% predicted) | Me (Q1–Q3) | 11.4 (3.6–20.5) | 12.8 (−6.03–23.7) | 15.3 (−1.4–44.3) | 7.9 (−6.4–35.7) | 0.556 |
| Δ MEF25 (% predicted) | Me (Q1–Q3) | 10.43 (−7.25–27.3) | −3.3 (−26.7–19.1) | −2.5 (−28–28.6) | −1.7 (−42.8–41.7) | 0.846 |
| Variable | 1–5 Days (n = 5) | 6–10 Days (n = 12) | 11–15 Days (n = 28) | 16–20 Days (n = 15) | Over 20 Days (n = 48) | p | |
|---|---|---|---|---|---|---|---|
| M ± SD | M ± SD | M ± SD | M ± SD | M ± SD | |||
| Δ FVC (% predicted) | Me (Q1–Q3) | 5.46 (2.6–19.5) | 3.65 (−7.6–13.7) | 3.4 (−4.3–16.13) | 6.06 (−1.6–10.6) | 11 (−0.74–14.1) | 0.659 |
| Δ FEV1 (% predicted) | Me (Q1–Q3) | 6.6 (4.5–10.4) | 13.15 (−3.6–22.9) | 3.2 (−2.06–18.02) | 2.5 (0.7–11.5) | 12.02 (4.3–22.4) | 0.231 |
| Δ FEV1/FVC (%) | Me (Q1–Q3) | 1.6 (−27.9–4.9) | 5.7 (−1.4–20.6) | 1.38 (−3.7–12.8) | −1.15 (−8.9–3.7) | 1.94 (−5.3–8.9) | 0.033 * |
| Δ PEF (% predicted) | Me (Q1–Q3) | 19.3 (10.3–27) | 18.9 (8.2–26.3) | 25.3 (8.2–32.5) | 15.3 (2.6–27) | 33.4 (15.7–43.6) | 0.174 |
| Δ MMEF (% predicted) | Me (Q1–Q3) | 11.9 (−1.6–18.4) | 16.4 (2.8–40.8) | 14.7 (−9.2–36.3) | 0.14 (−8.1–17.3) | 23 (−4.9–46.2) | 0.355 |
| Δ MEF75 (% predicted) | M ± SD | 51.21 ± 71.49 | 21.19 ± 18.85 | 20.18 ± 20.94 | 15.75 ± 19.07 | 32.05 ± 25.11 | 0.057 |
| Δ MEF50 (% predicted) | Me (Q1–Q3) | 20.9 (2.7–30.3) | 11.6 (0.9–37.2) | 14.04 (−3–34.6) | 0.71 (−12.8–22.1) | 23.3 (2.17–50.2) | 0.171 |
| Δ MEF25 (% predicted) | Me (Q1–Q3) | −25.11 (−29.5- −2.8) | 22.6 (6.9–43.4) | 0.01 (−33.5–21.6) | −21.8 (−32.4–4.08) | 13.6 (−22.2–34.3) | 0.068 |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fuschillo, S.; Ambrosino, P.; Motta, A.; Maniscalco, M. COVID-19 and diffusing capacity of the lungs for carbon monoxide: A clinical biomarker in postacute care settings. Biom. Med. 2021, 15, 537–539. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.A.; Colley, L.; Agbaedeng, T.A.; Ellison-Hughes, G.M.; Ross, M.D. Vascular manifestations of COVID-19–thromboembolism and microvascular dysfunction. Front. Cardiovasc. Med. 2020, 26, 598400. [Google Scholar] [CrossRef] [PubMed]
- George, P.M.; Barratt, S.L.; Condliffe, R.; Desai, S.R.; Devaraj, A.; Forrest, I.; Gibbons, M.A.; Hart, N.; Jengins, R.G.; McAuley, D.F.; et al. Respiratory follow-up of patients with COVID-19 pneumonia. Thorax 2020, 75, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Telles, A.; López-Romero, S.; Figueroa-Hurtado, E.; Pou-Aguilar, Y.N.; Wong, A.W.; Milne, K.M.; Ryerson, C.J.; Guenette, J.A. Pulmonary function and functional capacity in COVID-19 survivors with persistent dyspnoea. Respir. Physiol. Neurobiol. 2021, 288, 103644. [Google Scholar] [CrossRef]
- Lerum, T.V.; Aaløkken, T.M.; Brønstad, E.; Aarli, B.; Ikdahl, E.; Lund, K.M.A.; Durheim, M.T.; Rodriguez, J.R.; Meltzer, C.; Tonby, K.; et al. Dyspnoea, lung function and CT findings 3 months after hospital admission for COVID-19. Eur Respir. J. 2021, 57, 2003448. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; Northwell COVID-19 Research Consortium. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Zhang, J.J.; Lee, K.S.; Ang, L.W.; Leo, Y.S.; Young, B.E. Risk factors of severe disease and efficacy of treatment in patients infected with COVID-19: A systematic review, meta-analysis and meta-regression analysis. Clin. Infect. Dis. 2020, 71, 2199–2206. [Google Scholar] [CrossRef]
- Klain, A.; Indolfi, C.; Dinardo, G.; Decimo, F.; Miraglia Del Giudice, M. Covid-19 and spirometry in this age. Ital. J. Pediatr. 2022, 48, 11. [Google Scholar] [CrossRef]
- Komici, K.; Bianco, A.; Perrotta, F.; Dello Iacono, A.; Bencivenga, L.; D’Agnano, V.; Rocca, A.; Bianco, A.; Rengo, G.; Guerra, G. Clinical Characteristics, Exercise Capacity and Pulmonary Function in Post-COVID-19 Competitive Athletes. J. Clin. Med. 2021, 10, 3053. [Google Scholar] [CrossRef]
- Salem, A.M.; Al Khathlan, N.; Alharbi, A.F.; Alghamdi, T.; AlDuilej, S.; Alghamdi, M.; Alfudhaili, M.; Alsunni, A.; Yar, T.; Latif, R.; et al. The Long-Term Impact of COVID-19 Pneumonia on the Pulmonary Function of Survivors. Int. J. Gen. Med. 2021, 14, 3271–3280. [Google Scholar] [CrossRef] [PubMed]
- Esendağli, D.; Yilmaz, A.; Akçay, Ş.; Özlü, T. Post-COVID syndrome: Pulmonary complications. Turk. J. Med. Sci. 2021, 51, 3359–3371. [Google Scholar] [CrossRef] [PubMed]
- Rochester, C.L.; Vogiatzis, I.; Holland, A.E.; Lareau, S.C.; Marciniuk, D.D.; Puhan, M.A.; Spruit, M.A.; Masefield, S.; Casaburi, R.; Clini, E.M.; et al. An Official American Thoracic Society/European Respiratory Society Policy Statement: Enhancing Implementation, Use, and Delivery of Pulmonary Rehabilitation. Am. J. Respir. Crit. Care Med. 2015, 192, 1373–1386. [Google Scholar] [CrossRef] [PubMed]
- Besnier, F.; Bérubé, B.; Malo, J.; Gagnon, C.; Grégoire, C.-A.; Juneau, M.; Simard, F.; L’Allier, P.; Nigam, A.; Iglésies-Grau, J.; et al. Cardiopulmonary Rehabilitation in Long-COVID-19 Patients with Persistent Breathlessness and Fatigue: The COVID-Rehab Study. Int. J. Environ. Res. Public Health 2022, 19, 4133. [Google Scholar] [CrossRef] [PubMed]
- Rooney, S.; Webster, A.; Paul, L. Systematic Review of Changes and Recovery in Physical Function and Fitness After Severe Acute Respiratory Syndrome–Related Coronavirus Infection: Implications for COVID-19 Rehabilitation. Phys. Ther. 2020, 100, 1717–1729. [Google Scholar] [CrossRef]
- Barker-Davies, R.M.; O’Sullivan, O.; Senaratne, K.P.P.; Baker, P.; Cranley, M.; Dharm-Datta, S.; Ellis, H.; Goodall, D.; Gough, M.; Lewis, S.; et al. The Stanford Hall consensus statement for post-COVID-19 rehabilitation. Br. J. Sports Med. 2020, 54, 949–959. [Google Scholar] [CrossRef]
- Sun, T.; Guo, L.; Tian, F.; Dai, T.; Xing, X.; Zhao, J.; Li, Q. Rehabilitation of patients with COVID-19. Expert Rev. Respir. Med. 2020, 14, 1249–1256. [Google Scholar] [CrossRef]
- Agostini, F.; Mangone, M.; Ruiu, P.; Paolucci, T.; Santilli, V.; Bernetti, A. Rehabilitation setting during and after Covid-19: An overview on recommendations. J. Rehabilitation Med. 2021, 53, jrm00141. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, W.; Yang, Y.; Zhang, J.; Li, Y.; Chen, Y. Respiratory rehabilitation in elderly patients with COVID-19: A randomized controlled study. Complement. Ther. Clin. Pract. 2020, 39, 101166. [Google Scholar] [CrossRef]
- Barbara, C.; Clavario, P.; De Marzo, V.; Lotti, R.; Guglielmi, G.; Porcile, A.; Russo, C.; Griffo, R.; Mäkikallio, T.; Hautala, A.J.; et al. Effects of exercise rehabilitation in patients with long coronavirus disease 2019. Eur. J. Prev. Cardiol. 2022, 29, e258–e260. [Google Scholar] [CrossRef]
- Udina, C.; Ars, J.; Morandi, A.; Vilaró, J.; Cáceres, C.; Inzitari, M. Rehabilitation in adult post-COVID-19 patients in post-acute care with Therapeutic Exercise. J. Frailty Aging 2021, 10, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, R.; Viegi, G.; Brusasco, V.; Crapo, R.O.; Burgos, F.; Casaburi, R.; Coates, A.; Van Der Grinten, C.P.M.; Gustafsson, P.; Hankinson, J.; et al. Interpretative strategies for lung function tests. Eur. Respir. J. 2005, 26, 948–968. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef] [PubMed]
- Toulgui, E.; Benzarti, W.; Rahmani, C.; Aissa, S.; Ghannouchi, I.; Knaz, A.; Sayhi, A.; Sellami, S.; Mahmoudi, K.; Jemni, S.; et al. Impact of cardiorespiratory rehabilitation program on submaximal exercise capacity of Tunisian male patients with post-COVID19: A pilot study. Front. Physiol. 2022, 13, 1029766. [Google Scholar] [CrossRef] [PubMed]
- Hockele, L.F.; Affonso, J.V.S.; Rossi, D.; Eibel, B. Pulmonary and Functional Rehabilitation Improves Functional Capacity, Pulmonary Function and Respiratory Muscle Strength in Post COVID-19 Patients: Pilot Clinical Trial. Int. J. Environ. Res. Public Health 2022, 19, 14899. [Google Scholar] [CrossRef]
- Douin, C.; Forton, K.; Lamotte, M.; Gillet, A.; Van de Borne, P. Benefits of Cardio-Pulmonary Rehabilitation in Moderate to Severe Forms of COVID-19 Infection. Healthcare 2022, 10, 2044. [Google Scholar] [CrossRef]
- Eksombatchai, D.; Wongsinin, T.; Phongnarudech, T.; Thammavaranucupt, K.; Amornputtisathaporn, N.; Sungkanuparph, S. Pulmonary function and six-minute-walk test in patients after recovery from COVID-19: A prospective cohort study. PLoS ONE 2021, 16, e0257040. [Google Scholar] [CrossRef]
- Guler, S.A.; Ebner, L.; Aubry-Beigelman, C.; Bridevaux, P.-O.; Brutsche, M.; Clarenbach, C.; Garzoni, C.; Geiser, T.K.; Lenoir, A.; Mancinetti, M.; et al. Pulmonary function and radiological features 4 months after COVID-19: First results from the national prospective observational Swiss COVID-19 lung study. Eur. Respir. J. 2021, 57, 2003690. [Google Scholar] [CrossRef]
- Anastasio, F.; Barbuto, S.; Scarnecchia, E.; Cosma, P.; Fugagnoli, A.; Rossi, G.; Parravicini, M.; Parravicini, P. Medium-term impact of COVID-19 on pulmonary function, functional capacity and quality of life. Eur. Respir. J. 2021, 58, 2004015. [Google Scholar] [CrossRef]
- Zhao, Y.-M.; Shang, Y.-M.; Song, W.-B.; Li, Q.-Q.; Xie, H.; Xu, Q.-F.; Jia, J.-L.; Li, L.-M.; Mao, H.-L.; Zhou, X.-M.; et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. Eclinicalmedicine 2020, 25, 100463. [Google Scholar] [CrossRef]
- Liang, L.; Yang, B.; Jiang, N.; Fu, W.; He, X.; Zhou, Y.; Ma, W.-L.; Wang, X. Three-Month Follow-Up Study of Survivors of Coronavirus Disease 2019 after Discharge. J. Korean Med. Sci. 2020, 35, e418. [Google Scholar] [CrossRef] [PubMed]
| Procedure | Guidelines | Time |
|---|---|---|
| General fitness and respiratory improvement exercises | Active breathing exercises, active breathing exercises with resistance, learning to cough and expectorate effectively | 30 min |
| Aerobic training | Stair climbing; climbing 2–3 floors at a leisurely pace; gradually increased intensity by 5–10%; assessment of exercise tolerance by oxygenation checks (pulse oximeter); perceived exercise intensity: 2–3 on the Borg scale | 30 min |
| Continuous/interval endurance training on a bicycle cycloergometer | Initial low or moderate intensity; gradual increase in intensity by 5–10%; assessment of exercise tolerance by oxygenation checks (pulse oximeter); perceived exercise intensity: 2–3 on the Borg scale | 30 min |
| Outdoor march training | Assessment of exercise tolerance by oxygenation checks (pulse oximeter); perceived of exercise intensity: 2–3 on the Borg scale | 2 times a day for 30 min each |
| Strength and endurance training | Training selected individually for the patient on the basis of the RM unit and the patient’s exercise tolerance (assessment of the presence of desaturation); load: 70–85% of 1 RM; volume: 3 series of 8–12 repetitions; rest: 1–2 min; progression: 60–70% of 1 RM | 30 min |
| Variable | n | % | |
|---|---|---|---|
| Gender | female | 94 | 54.97 |
| male | 77 | 45.03 | |
| Age | 30–45 years | 15 | 8.77 |
| 46–60 years | 33 | 19.3 | |
| 61–75 years | 96 | 56.14 | |
| 76–90 years | 27 | 15.79 | |
| Nutritional status (BMI) | 18.5–24.99 (norm) | 37 | 21.76 |
| 25.0–29.9 (overweight) | 61 | 35.88 | |
| 30.0–34.99 (1st degree obesity) | 49 | 28.82 | |
| 35.0–39.99 (2nd degree obesity) | 18 | 10.59 | |
| over 40 (3rd degree obesity) | 5 | 2.94 | |
| Hospitalization | yes | 119 | 69.59 |
| no | 45 | 26.32 | |
| Length of hospitalization | 1–5 days | 5 | 2.92 |
| 6–10 days | 13 | 7.6 | |
| 11–15 days | 32 | 18.71 | |
| 16–20 days | 18 | 10.53 | |
| over 20 days | 51 | 29.82 | |
| Pneumonia in the course of COVID-19 | yes | 127 | 74.27 |
| no | 37 | 21.64 | |
| Duration of rehabilitation | 2–3 weeks | 80 | 46.78 |
| 3–4 weeks | 27 | 15.79 | |
| 4–5 weeks | 23 | 13.45 | |
| 5–6 weeks | 41 | 23.97 | |
| Comorbidities | diabetes | 43 | 25.15 |
| hypertension | 97 | 56.73 | |
| asthma | 19 | 11.11 | |
| COPD | 8 | 4.68 | |
| Variable | Before Rehabilitation (n = 150) | After Rehabilitation (n = 150) | p | |
|---|---|---|---|---|
| FVC (% predicted) | M ± SD | 83.39 ± 22.71 | 89.71 ± 21.47 | 0.016 * |
| FEV1 (% predicted) | Me (Q1–Q3) | 86.36 (68.16–102.75) | 96.81 (80.73–110.43) | <0.001 * |
| FEV1/FVC (%) | Me (Q1–Q3) | 86.32 (78.52–91.51) | 87.39 (81.46–91.93) | 0.071 |
| PEF (% predicted) | Me (Q1–Q3) | 56.5 (36.85–76.05) | 82.13 (60.12–99.36) | <0.001 * |
| MMEF (% predicted) | Me (Q1–Q3) | 82.35 (60.12–108.03) | 99.38 (77.15–125.43) | <0.001 * |
| MEF75 (% predicted) | Me (Q1–Q3) | 53.48 (36.27–81.33) | 85.15 (59.78–106.08) | <0.001 * |
| MEF50 (% predicted) | Me (Q1–Q3) | 73.97 (51.62–102.73) | 95.81 (70.25–119.86) | <0.001 * |
| MEF25 (% predicted) | Me (Q1–Q3) | 106.76 (75.77–141.97) | 108.3 (80.67–146.97) | 0.554 |
| Variable | After Rehabilitation (n = 150) | 2 Months after Rehabilitation (n = 18) | p | |
|---|---|---|---|---|
| M ± SD | M ± SD | |||
| FVC (% predicted) | M ± SD | 89.71 ± 21.47 | 83.63 ± 15.41 | 0.304 |
| FEV1 (% predicted) | M ± SD | 94.71 ± 22.69 | 90.11 ± 15.27 | 0.265 |
| FEV1/FVC (%) | Me (Q1–Q3) | 87.39 (81.46–91.93) | 85.35 (83.44–92.03) | 0.084 |
| PEF (% predicted) | M ± SD | 80.24 ± 27.98 | 92.04 ± 33.26 | 0.235 |
| MMEF (% predicted) | M ± SD | 101.47 ± 39.6 | 103.22 ± 38.43 | 0.814 |
| MEF75 (% predicted) | M ± SD | 84.37 ± 31.65 | 89.94 ± 35.04 | 0.781 |
| MEF50 (% predicted) | M ± SD | 95.69 ± 37.35 | 94.53 ± 35.53 | 0.934 |
| MEF25 (% predicted) | Me (Q1–Q3) | 108.3 (80.67–146.97) | 108.77 (89.04–119.05) | 0.915 |
| Variable | Female (n = 85) | Male (n = 65) | p | |
|---|---|---|---|---|
| Δ FVC (% predicted) | M ± SD | 6.56 ± 14.64 | 6.34 ± 13.45 | 0.601 |
| Δ FEV1 (% predicted) | Me (Q1–Q3) | 5.86 (0.58–18.11) | 10.33 (1.52–16.23) | 0.288 |
| Δ FEV1/FVC (%) | Me (Q1–Q3) | 0.3 (−5.4–8.67) | 2.71 (−3.68–12.57) | 0.562 |
| Δ PEF (% predicted) | M ± SD | 18.95 ± 21.12 | 24.04 ± 20.04 | 0.123 |
| Δ MMEF (% predicted) | Me (Q1–Q3) | 9.07 (−7.91–29.55) | 16.14 (−5.78–36.56) | 0.509 |
| Δ MEF75 (% predicted) | Me (Q1–Q3) | 23.13 (4.56–38.76) | 24.61 (9.16–40.74) | 0.366 |
| Δ MEF50 (% predicted) | Me (Q1–Q3) | 11.4 (−4.48–32.35) | 13.46 (−0.87–37.77) | 0.411 |
| Δ MEF25 (% predicted) | M ± SD | −1.5 ± 46.75 | 6.05 ± 46.56 | 0.192 |
| Variable | Pneumonia (n = 115) | No Pneumonia (n = 32) | p | |
|---|---|---|---|---|
| Δ FVC (% predicted) | M ± SD | 6.66 ± 13.51 | 5.62 ± 16.71 | 0.549 |
| Δ FEV1 (% predicted) | Me (Q1–Q3) | 6.96 (0.5–17.91) | 5.03 (1.41–13.36) | 0.522 |
| Δ FEV1/FVC (%) | Me (Q1–Q3) | 1.76 (−5.17–8.9) | −0.62 (−7.08–13.29) | 0.604 |
| Δ PEF (% predicted) | Me (Q1–Q3) | 22.88 (9.17–35.9) | 11.64 (−1.97–30.35) | 0.079 |
| Δ MMEF (% predicted) | Me (Q1–Q3) | 14.69 (−4.62–34.67) | −2.28 (−11.06–24.97) | 0.069 |
| Δ MEF75 (% predicted) | Me (Q1–Q3) | 23.46 (6.42–40.74) | 20.75 (−2.34–34.6) | 0.221 |
| Δ MEF50 (% predicted) | Me (Q1–Q3) | 15.33 (−1.57–39.04) | 2.92 (−5.74–23.48) | 0.099 |
| Δ MEF25 (% predicted) | Me (Q1–Q3) | 0.44 (−26.69–27.72) | −9.89 (−42.03–12.87) | 0.148 |
| Outcome | OR (95% CI) | p | |
|---|---|---|---|
| Gender | male | 1.190 (0.550–2.574) | 0.659 |
| Age | 30–45 years | 1.000 (-) | - |
| 46–60 years | 1.446 (0.272–7.696) | 0.665 | |
| 61–75 years | 0.644 (0.150–2.776) | 0.555 | |
| 76–90 years | 0.969 (0.184–5.108) | 0.970 | |
| Nutritional status (BMI) | norm | 1.000 (-) | - |
| overweight | 0.176 (0.046–0.678) | 0.012 * | |
| 1st degree obesity | 0.117 (0.030–0.452) | 0.002 * | |
| 2nd degree obesity | 0.619 (0.088–4.340) | 0.629 | |
| 3rd degree obesity | 0.155 (0.018–1.377) | 0.094 | |
| Hospitalization | 1.405 (0.431–4.581) | 0.573 | |
| Pneumonia in the course of COVID-19 | 0.968 (0.271–3.462) | 0.961 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mińko, A.; Turoń-Skrzypińska, A.; Rył, A.; Szylińska, A.; Denisewicz, I.; Rotter, I. Effects of Comprehensive Rehabilitation on Pulmonary Function in Patients Recovering from COVID-19. Int. J. Environ. Res. Public Health 2023, 20, 3985. https://doi.org/10.3390/ijerph20053985
Mińko A, Turoń-Skrzypińska A, Rył A, Szylińska A, Denisewicz I, Rotter I. Effects of Comprehensive Rehabilitation on Pulmonary Function in Patients Recovering from COVID-19. International Journal of Environmental Research and Public Health. 2023; 20(5):3985. https://doi.org/10.3390/ijerph20053985
Chicago/Turabian StyleMińko, Alicja, Agnieszka Turoń-Skrzypińska, Aleksandra Rył, Aleksandra Szylińska, Iwona Denisewicz, and Iwona Rotter. 2023. "Effects of Comprehensive Rehabilitation on Pulmonary Function in Patients Recovering from COVID-19" International Journal of Environmental Research and Public Health 20, no. 5: 3985. https://doi.org/10.3390/ijerph20053985
APA StyleMińko, A., Turoń-Skrzypińska, A., Rył, A., Szylińska, A., Denisewicz, I., & Rotter, I. (2023). Effects of Comprehensive Rehabilitation on Pulmonary Function in Patients Recovering from COVID-19. International Journal of Environmental Research and Public Health, 20(5), 3985. https://doi.org/10.3390/ijerph20053985






