The Presence of a Single Nuchal Cord in the Third Trimester May Not Affect Tei Index in LGA Fetuses
Abstract
1. Background
2. Methods
3. Statistical Analysis
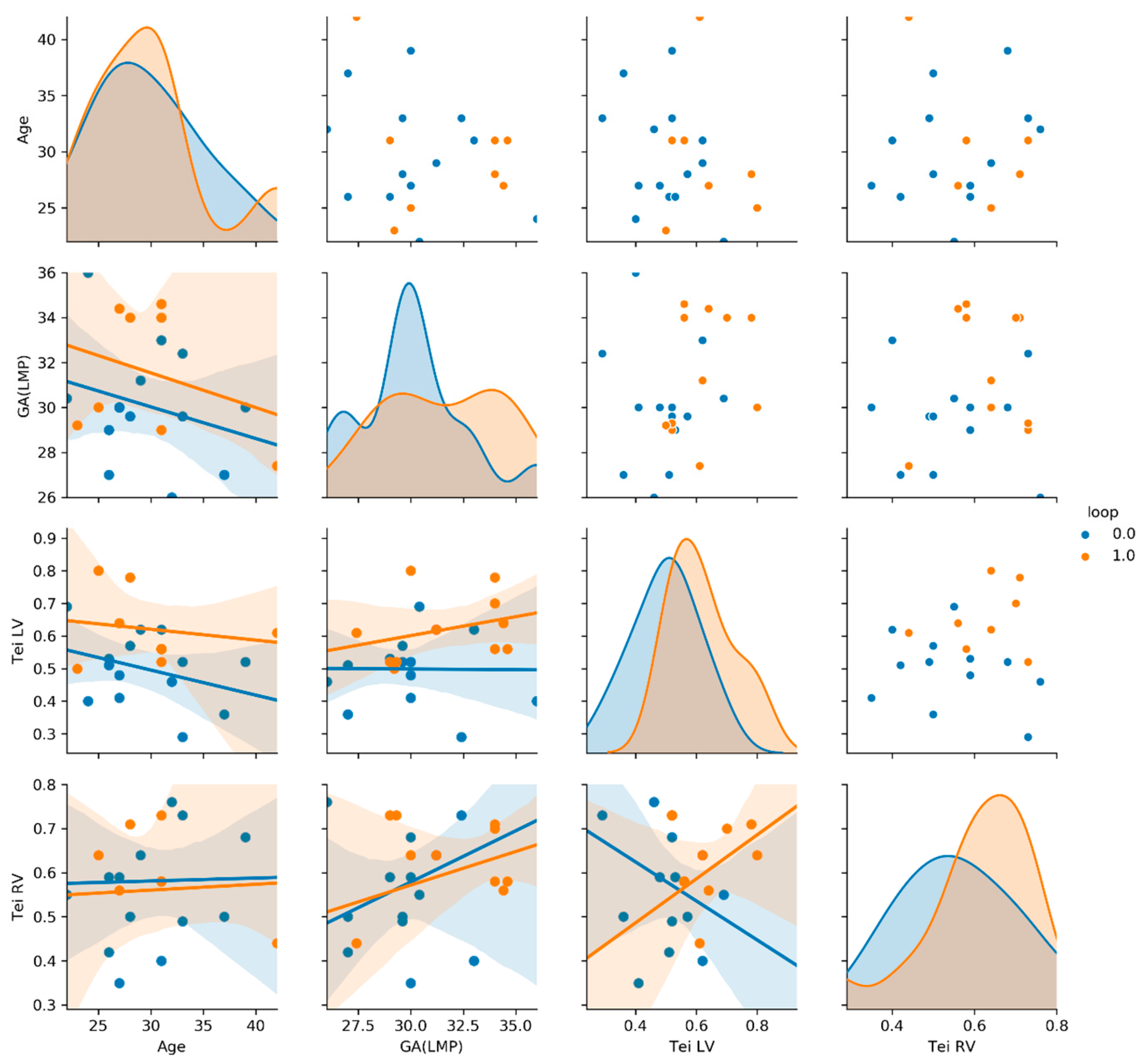
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RV | Right ventricle |
| LV | Left ventricle |
| LGA | Larger for Gestational Age |
| NC | Nuchal cord/fetuses with nuchal cord |
| AGA | Adequated for Gestational Age |
| nNC | Fetuses without nuchal cord |
| GA | Gestational Age |
| LMP | Last Menstrual Period |
| SGA | Smaller for Gestational Age |
| AFI | Amniotic Fluid Index |
| PI UMBA | Pulsatility Index of Umbilical Artery |
| PI DV | Pulsatility Index of Ductus Venosus |
| PI MCA | Pulsatility Index of Middle Cerebral Artery |
| PI LUA | Pulsatility Index of Left Uterine Artery |
| PI RUA | Pulsatility Index of Right Uterine Artery |
| IVCT | Isovolumetric Contraction Time |
| IVRT | Isovolumetric Relaxation Time |
| ET | Ejection Time |
| ALARA | As Low As Reasonably Achievable |
| MV | Mitral Valve |
| TV | Tricuspid Valve |
| AoV | Aortic Valve |
| PAV | Pulmonary Artery Valve |
| PP | Python programming |
| SD | Standard deviation |
References
- Peesay, M. Nuchal cord and its implications. Matern. Health Neonatol. Perinatol. 2017, 3, 28. [Google Scholar] [CrossRef] [PubMed]
- Murlewska, J.; Sylwestrzak, O.; Poszwa, P.; Respondek-Liberska, M. The effect of nuchal umbilical cord on fetal cardiac and cerebral circulation-cross-sectional study. J. Perinat. Med. 2021, 49, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Andrade, E.; Lopez-Tenorio, J.; Figueroa-Diesel, H.; Sanin-Blair, J.; Carreras, E.; Cabero, L.; Gratacos, E. A modified myocardial performance (Tei) index based on the use of valve clics improves reproducibility of fetal left cardiac function assessment. Ultrasound Obstet. Gynecol. 2005, 26, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Andrade, E.; Figueroa-Diesel, H.; Kottman, C.; Illanes, S.; Arraztoa, J.; Acosta-Rojas, R.; Gratacos, E. Gestational-age-adjusted reference values for the modified myocardial performance index for evaluation of fetal left cardiac function. Ultrasound Obstet. Gynecol. 2007, 29, 321–325. [Google Scholar] [CrossRef] [PubMed]
- McKinney, W. Data Structures for Statistical Computing in Python. In Proceedings of the 9th Python in Science Conference, Austin, TX, USA, 28 June–3 July 2010; pp. 51–56. [Google Scholar]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental Algorithms for Scientific Computing in Python. Nat. Methods 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Liu, H.X.; Xuan, Z.D.; Zhao, L.; Li, J.Z.; Wang, Y.H. Assessments of M-mode color echocardiography on fetal right ventricular diastolic function with umbilical cord around neck. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 2927–2933. [Google Scholar] [PubMed]
- Sherer, D.M.; Sokolovski, M.; Dalloul, M.; Khoury-Collado, F.; Abulafia, O. Is fetal cerebral vascular resistance affected by the presence of nuchal cord(s) in the third trimester of pregnancy? Ultrasound Obstet. Gynecol. 2005, 25, 454–458. [Google Scholar] [CrossRef] [PubMed]
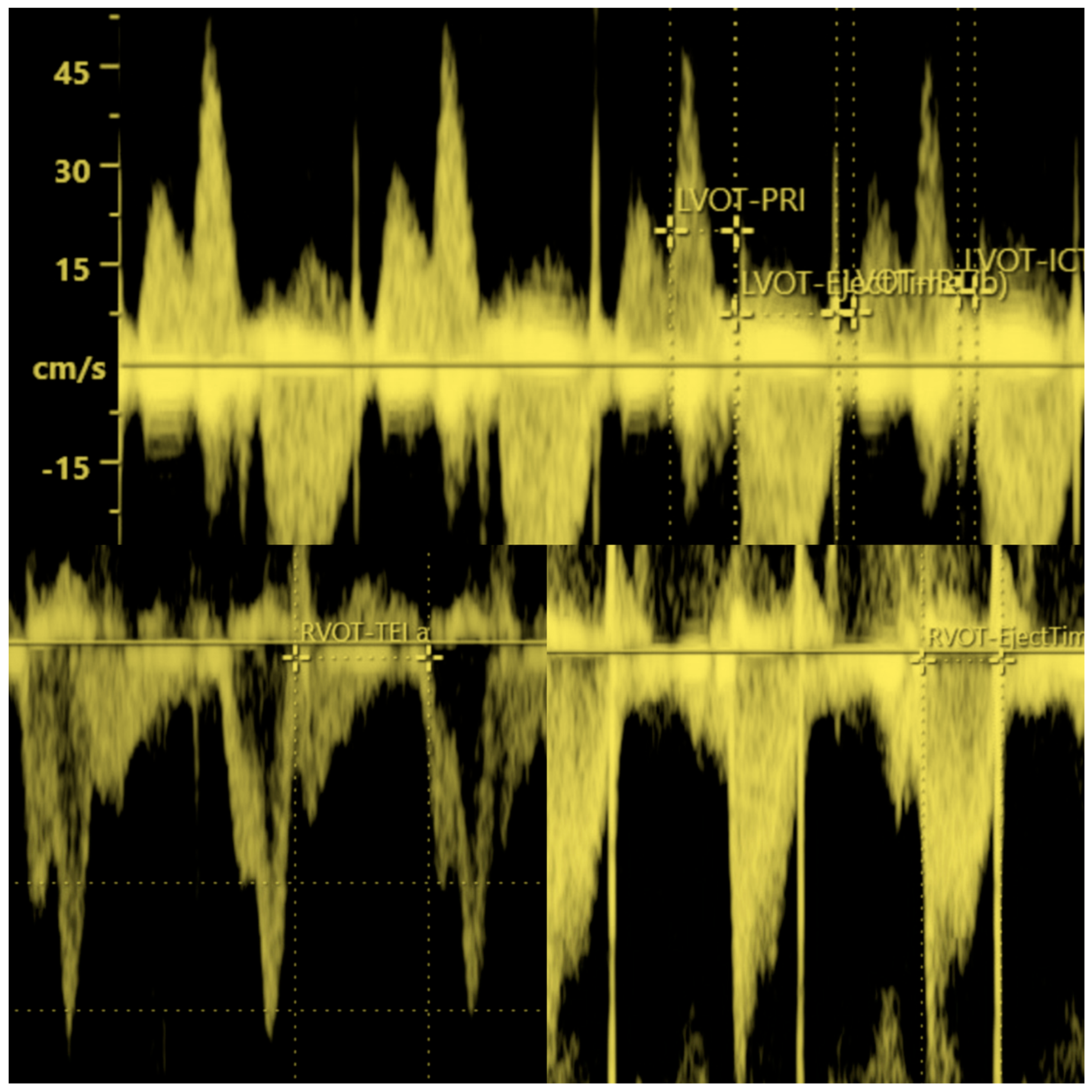
| nNC n = 158 | NC n = 139 | p Value | Mean ± SD AGA n = 263 | Mean ± SD LGA n = 25 | p Value | LGA/nNC n = 14 | LGA/NC n = 11 | p Value | |
|---|---|---|---|---|---|---|---|---|---|
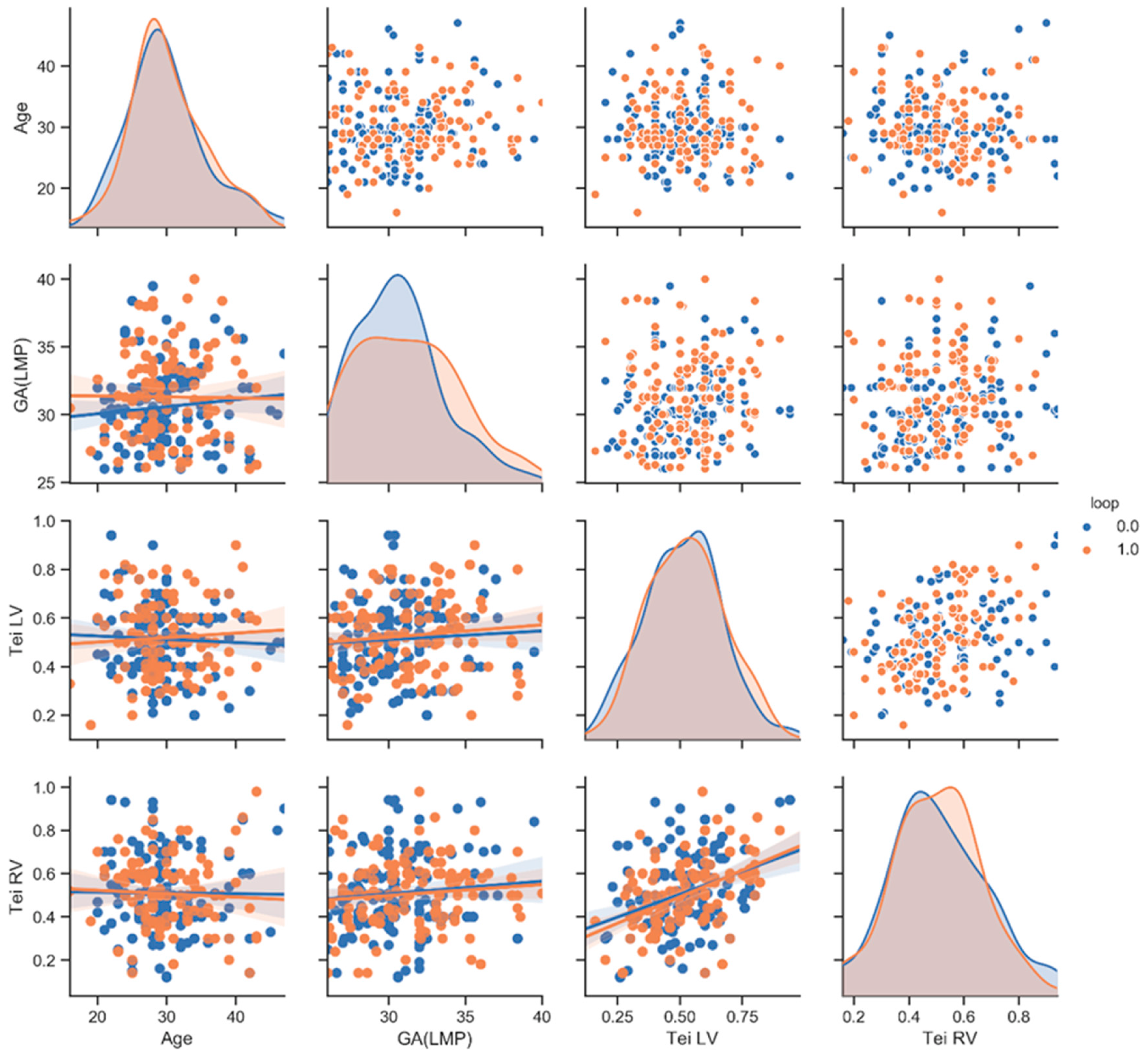
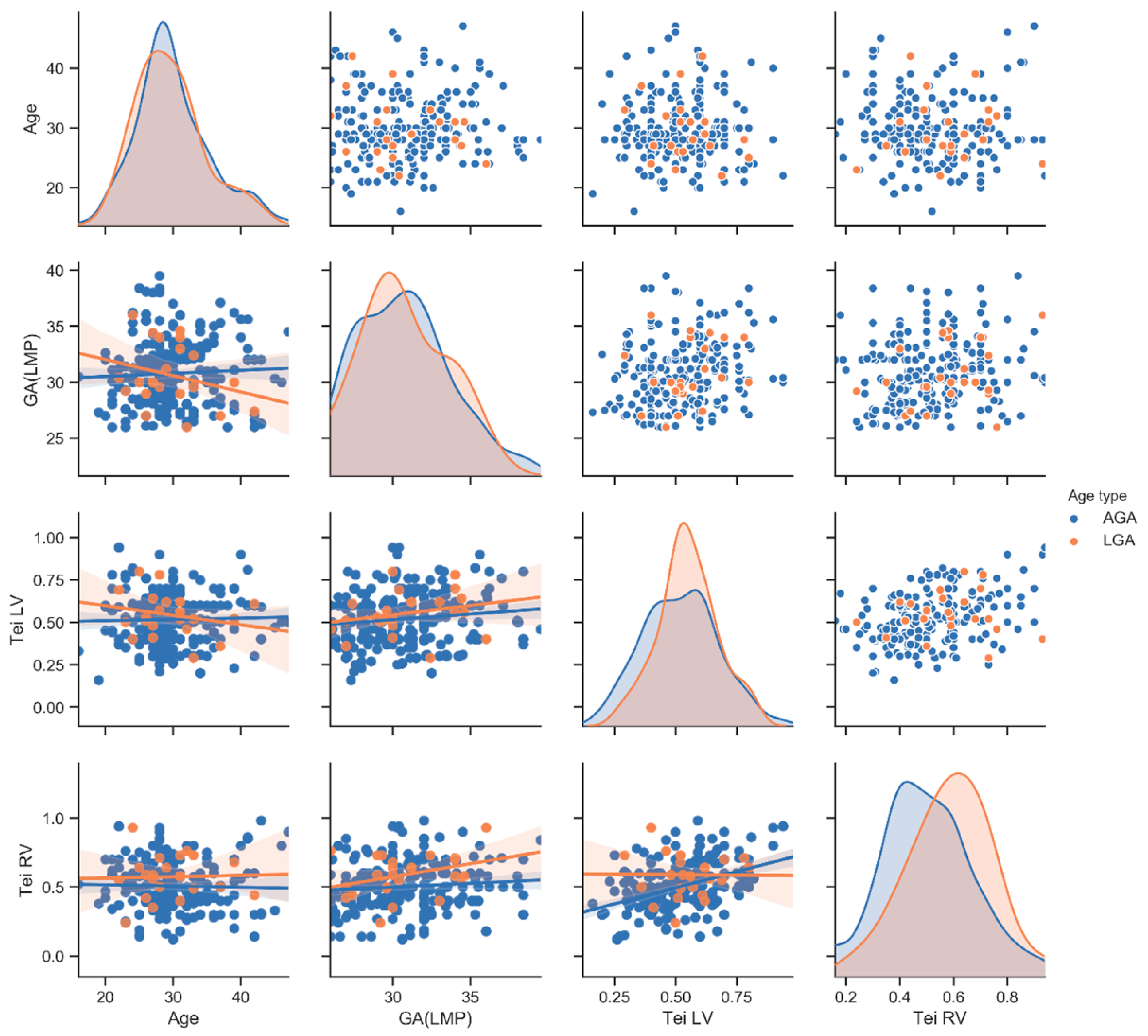
| Tei RV (mean ± SD) | 0.5 ± 0.2 | 0.5 ± 0.2 | 0.8 | 0.5 ± 0.2 | 0.6 ± 0.2 | 0.01 | 0.6 ± 0.2 | 0.6± 0.1 | 0.8 |
| Tei LV (mean ± SD) | 0.5 ± 0.2 | 0.5 ± 0.1 | 0.4 | 0.5 ± 0.2 | 0.6 ± 0.1 | 0.23 | 0.5 ± 0.1 | 0.6 ± 1 | 0.009 |
| Maternal Age (mean ± SD) | 30 ± 5.5 | 30 ± 5.3 | 0.9 | 30 ± 5.5 | 30 ± 5 | 0.73 | 30 ± 4.8 | 30 ± 5.8 | 0.94 |
| GA(LMP) (mean ± SD) | 30 ± 2.8 | 31 ± 3.3 | 10 | 30 ± 3 | 31 ± 2.7 | 0.94 | 30 ± 2.6 | 31 ± 2.7 | 0.2 |
| AFI (mean ± SD) | 16 ± 4.2 | 17 ± 4 | 0.4 | 16 ± 4 | 19 ± 2.8 | 0.0001 | 19 ± 2.8 | 20 ± 2.8 | 0.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murlewska, J.; Poszwa, P.; Sylwestrzak, O.; Respondek-Liberska, M.; Wood, D. The Presence of a Single Nuchal Cord in the Third Trimester May Not Affect Tei Index in LGA Fetuses. Int. J. Environ. Res. Public Health 2023, 20, 3778. https://doi.org/10.3390/ijerph20053778
Murlewska J, Poszwa P, Sylwestrzak O, Respondek-Liberska M, Wood D. The Presence of a Single Nuchal Cord in the Third Trimester May Not Affect Tei Index in LGA Fetuses. International Journal of Environmental Research and Public Health. 2023; 20(5):3778. https://doi.org/10.3390/ijerph20053778
Chicago/Turabian StyleMurlewska, Julia, Przemysław Poszwa, Oskar Sylwestrzak, Maria Respondek-Liberska, and Dennis Wood. 2023. "The Presence of a Single Nuchal Cord in the Third Trimester May Not Affect Tei Index in LGA Fetuses" International Journal of Environmental Research and Public Health 20, no. 5: 3778. https://doi.org/10.3390/ijerph20053778
APA StyleMurlewska, J., Poszwa, P., Sylwestrzak, O., Respondek-Liberska, M., & Wood, D. (2023). The Presence of a Single Nuchal Cord in the Third Trimester May Not Affect Tei Index in LGA Fetuses. International Journal of Environmental Research and Public Health, 20(5), 3778. https://doi.org/10.3390/ijerph20053778






