Exercise-Induced Elevated BDNF Concentration Seems to Prevent Cognitive Impairment after Acute Exposure to Moderate Normobaric Hypoxia among Young Men
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Size Analysis
2.2. Participants
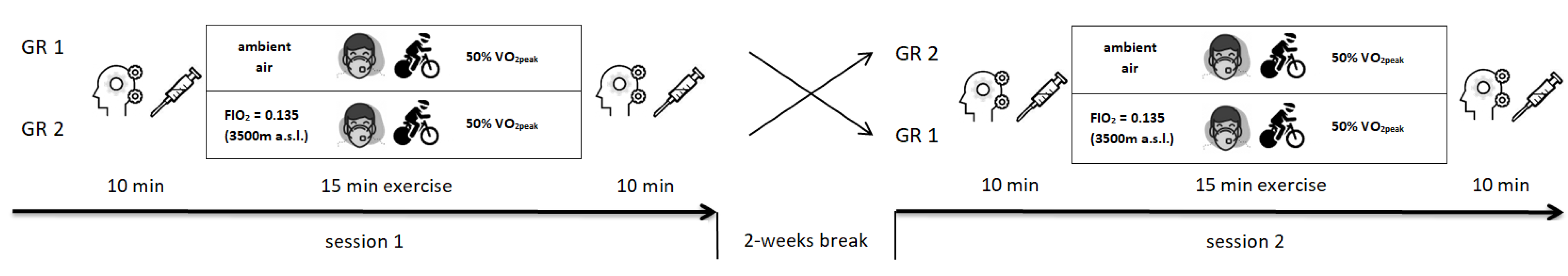
2.3. Study Design
2.4. Anthropometric Measurements
2.5. Hypoxic Conditions
2.6. Assessment of Cognitive Performance
2.7. Collection of Blood Samples
2.8. Statistical Analysis
3. Results
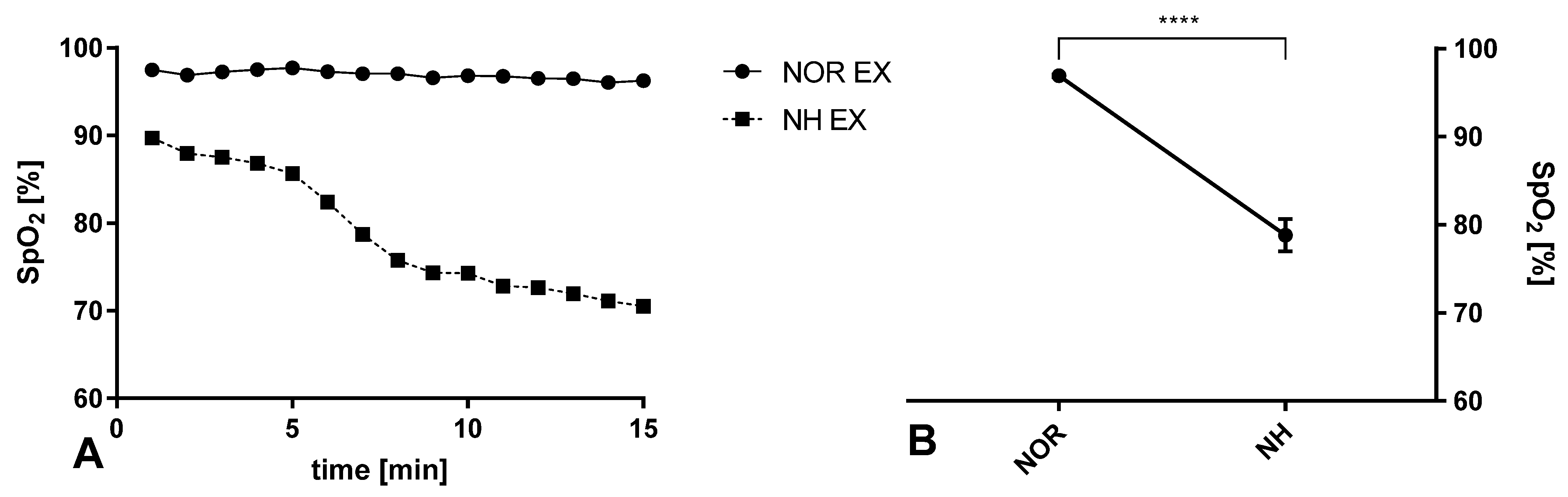
3.1. Blood Saturation during Exercise in Normoxia and Acute Normobaric Hypoxia Conditions
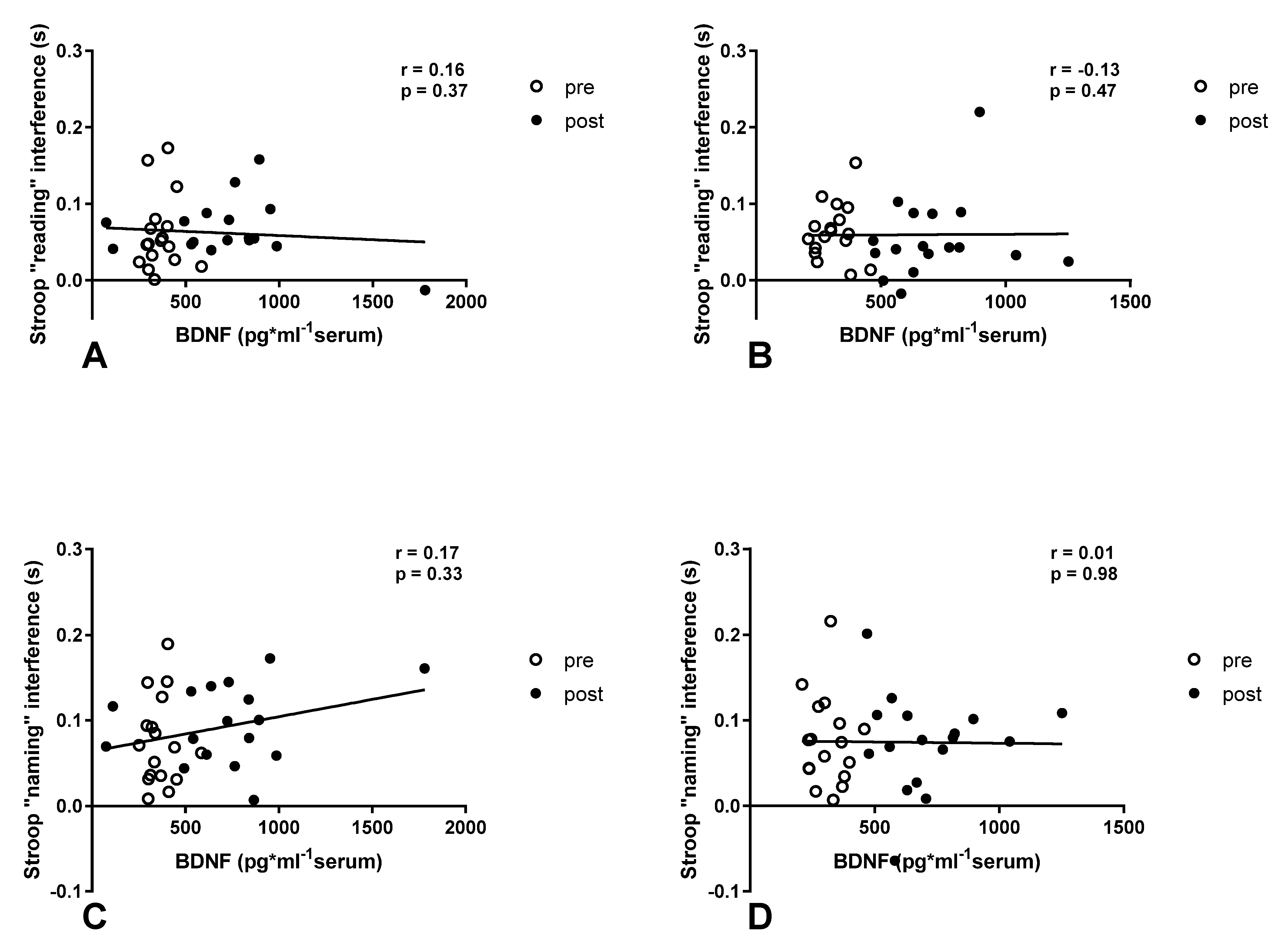
3.2. Stroop Test after Exercise in Normoxia and Acute Normobaric Hypoxia Conditions
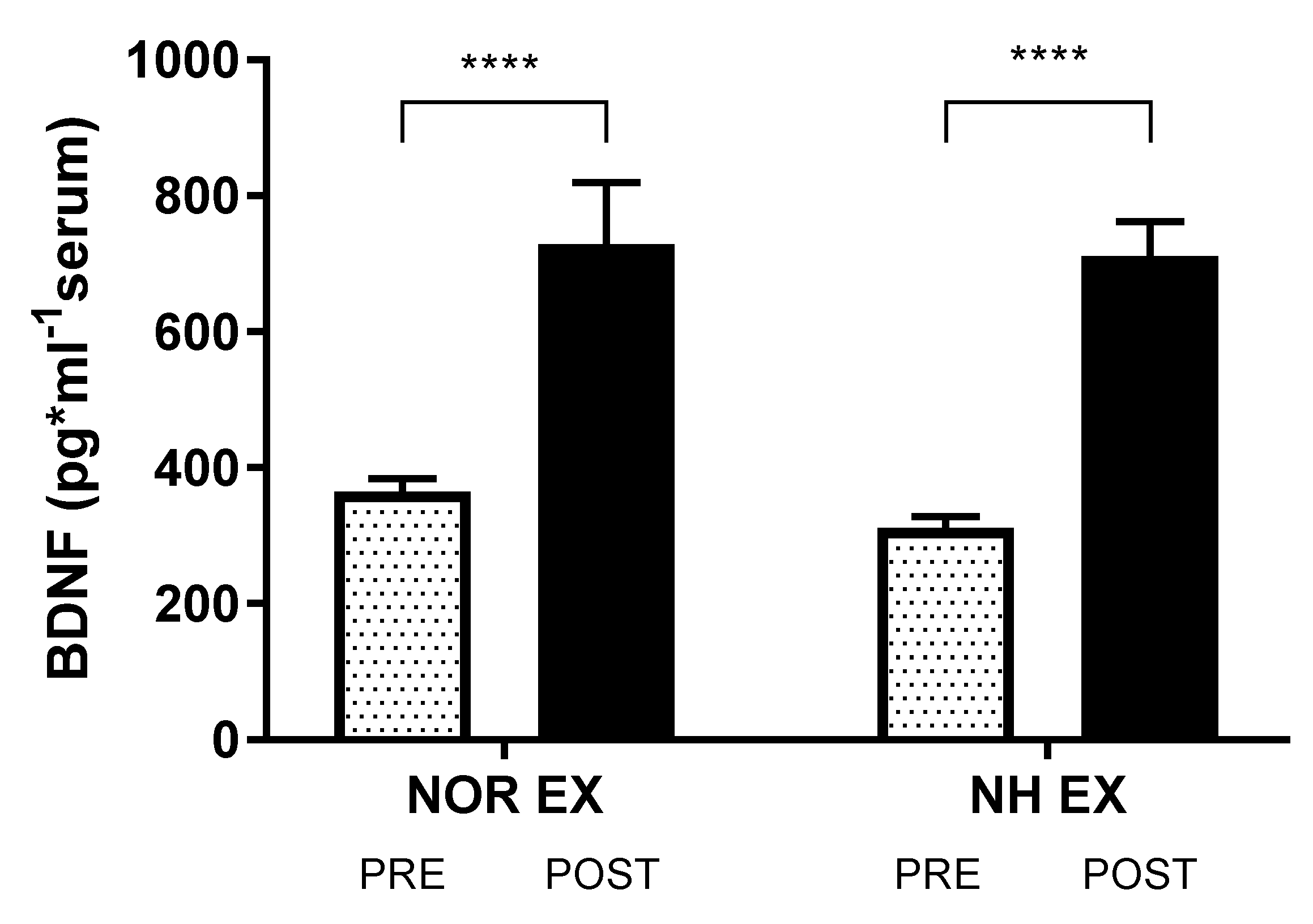
3.3. Blood Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Levitt, S.; Gutin, B. Multiple choice reaction time and movement time during physical exertion. Res. Q. Am. Assoc. Health Phys. Educ. Recreat. 1971, 42, 405–410. [Google Scholar] [CrossRef]
- Travlos, A.K.; Marisi, D.Q. Information processing and concentration as a function of fitness level and exercise-induced activation to exhaustion. Percept. Mot. Ski. 1995, 80, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Chmura, J.; Krysztofiak, H.; Ziemba, A.W.; Nazar, K.; Kaciuba-Uścilko, H. Psychomotor performance during prolonged exercise above and below the blood lactate threshold. Eur. J. Appl. Physiol. Occup. Physiol. 1997, 77, 77–80. [Google Scholar] [CrossRef]
- Chmura, J.; Nazar, K.; Kaciuba-Uscilko, H. Choice reaction time during graded exercise in relation to blood lactate and plasma catecholamine thresholds. Int. J. Sports Med. 1994, 15, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Kruk, B.; Chmura, J.; Krzeminski, K.; Ziemba, A.W.; Nazar, K.; Pekkarinen, H.; Kaciuba-Uścilko, H. Influence of caffeine, cold and exercise on multiple choice reaction time. Psychopharmacology 2001, 157, 197–201. [Google Scholar] [CrossRef]
- Lieberman, P.; Protopapas, A.; Reed, E.; Youngs, J.W.; Kanki, B.G. Cognitive defects at altitude. Nature 1994, 372, 325. [Google Scholar] [CrossRef]
- Chroboczek, M.; Kujach, S.; Łuszczyk, M.; Grzywacz, T.; Soya, H. Acute Normobaric Hypoxia Lowers Executive Functions among Young Men despite Increase of BDNF Concentration. Int. J. Environ. Res. Public Health 2022, 19, 10802. [Google Scholar] [CrossRef]
- Taylor, L.; Watkins, S.L.; Marshall, H.; Dascombe, B.J.; Foster, J. The Impact of Different Environmental Conditions on Cognitive Function: A Focused Review. Front. Physiol. 2015, 6, 372. [Google Scholar] [CrossRef] [Green Version]
- McMorris, T.; Hale, B.J.; Barwood, M.; Costello, J.; Corbett, J. Corrigendum to “Effect of acute hypoxia on cognition: A systematic review and meta-regression analysis” Neurosci. Biobehav. Rev. 74 (2017) 225–232. Neurosci. Biobehav. Rev. 2019, 98, 333. [Google Scholar] [CrossRef]
- Jahanshahi, M.; Dirnberger, G.; Fuller, R.; Frith, C.D. The role of the dorsolateral prefrontal cortex in random number generation: A study with positron emission tomography. Neuroimage 2000, 12, 713–725. [Google Scholar] [CrossRef]
- Nielson, K.A.; Powless, M. Positive and negative sources of emotional arousal enhance long-term word-list retention when induced as long as 30 min after learning. Neurobiol. Learn. Mem. 2007, 88, 40–47. [Google Scholar] [CrossRef] [Green Version]
- Hoshino, O. Cognitve enhancement mediated through postsynaptic actions of norepinephrine on ongoing cortical activity. Neural Comput. 2005, 17, 1739–1775. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, K.B.; Sand, T.; Stovner, L.J.; Leistad, R.B.; Westgaard, R.H. Autonomic and muscular responses and recovery to one-hour laboratory mental stress in healthy subjects. BMC Musculoskelet. Disord. 2007, 8, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rothman, S.M.; Griffioen, K.J.; Wan, R.; Mattson, M.P. Brain-derived neurotrophic factor as a regulator of systemic and brain energy metabolism and cardiovascular health. Ann. N. Y. Acad. Sci. 2012, 1264, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Larsen, K.T.; Ried-Larsen, M.; Møller, N.C.; Andersen, L.B. The effects of physical activity and exercise on brain-derived neurotrophic factor in healthy humans: A review. Scand. J. Med. Sci. Sport. 2014, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.K.S.; Ho, C.S.H.; Tam, W.W.S.; Kua, E.H.; Ho, R.C.M. Decreased serum brain-derived neurotrophic factor (BDNF) levels in patients with Alzheimer’s disease (AD): A systematic review and meta-analysis. Int. J. Mol. Sci. 2019, 20, 257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.Z.; Nusslock, R. Exercise-Mediated Neurogenesis in the Hippocampus via BDNF. Front. Neurosci. 2018, 12, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferris, L.T.; Williams, J.S.; Shen, C.L. The effect of acute exercise on serum brain-derived neurotrophic factor levels and cognitive function. Med. Sci. Sports Exerc. 2007, 39, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Kastin, A.J. Polypeptide delivery across the blood-brain barrier. Curr. Drug Targets CNS Neurol. Disord. 2004, 3, 131–136. [Google Scholar] [CrossRef]
- Chang, Y.K.; Labban, J.D.; Gapin, J.I.; Etnier, J.L. The effects of acute exercise on cognitive performance: A meta-analysis. Brain Res. 2012, 1453, 87–101. [Google Scholar] [CrossRef] [Green Version]
- Basso, J.C.; Suzuki, W.A. The Effects of Acute Exercise on Mood, Cognition, Neurophysiology, and Neurochemical Pathways: A Review. Brain Plast. 2017, 2, 127–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bekinschtein, P.; Kent, B.A.; Oomen, C.A.; Clemenson, G.D.; Gage, F.H.; Saksida, L.M.; Bussey, T.J. BDNF in the dentate gyrus is required for consolidation of “pattern-separated” memories. Cell Rep. 2013, 5, 759–768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, J.M.; Bailey, S.P. Possible mechanisms of central nervous system fatigue during exercise. Med. Sci. Sport. Exerc. 1997, 29, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Blogg, S.L.; Gennser, M. Cerebral blood flow velocity and psychomotor performance during acute hypoxia. Aviat. Space Environ. Med. 2006, 77, 107–113. [Google Scholar] [PubMed]
- Temme, L.A.; Still, D.L.; Acromite, M.T. Hypoxia and flight performance of military instructor pilots in a flight simulator. Aviat. Space Environ. Med. 2010, 81, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Kirby, R.L.; Atkinson, S.M.; Donville, J.E.; Urdang, M.F.; Stanley, D.A.; Gupta, S.K.; MacLeod, D.A. Failure of accentuated vertical body movements to induce cardiac-locomotor coupling. J. Appl. Physiol. 1992, 72, 1368–1374. [Google Scholar] [CrossRef] [PubMed]
- De Bartolo, D.; De Giorgi, C.; Compagnucci, L.; Betti, V.; Antonucci, G.; Morone, G.; Paolucci, S.; Iosa, M. Effects of cognitive workload on heart and locomotor rhythms coupling. Neurosci. Lett. 2021, 762, 136140. [Google Scholar] [CrossRef]
- De Bartolo, D.; Belluscio, V.; Vannozzi, G.; Morone, G.; Antonucci, G.; Giordani, G.; Santucci, S.; Resta, F.; Marinozzi, F.; Bini, F.; et al. Sensorized assessment of dynamic locomotor imagery in people with stroke and healthy subjects. Sensors 2020, 20, 4545. [Google Scholar] [CrossRef]
- Zandvoort, C.S.; van Dieën, J.H.; Dominici, N.; Daffertshofer, A. The human sensorimotor cortex fosters muscle synergies through cortico-synergy coherence. Neuroimage 2019, 199, 30–37. [Google Scholar] [CrossRef]
- Patel, P.; Lamar, M.; Bhatt, T. Effect of type of cognitive task and walking speed on cognitive-motor interference during dual-task walking. Neuroscience 2014, 260, 140–148. [Google Scholar] [CrossRef]
- Wrightson, J.G.; Ross, E.Z.; Smeeton, N.J. The effect of cognitive-task type and walking speed on dual-task gait in healthy adults. Mot. Control 2016, 20, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.L.; Kenward, M.G.; Fairclough, D.L.; Horton, N.J. Differential dropout and bias in randomised controlled trials: When it matters and when it may not. BMJ 2013, 346, e8668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shatilo, V.B.; Korkushko, O.V.; Ischuk, V.A.; Downey, H.F.; Serebrovskaya, T.V. Effects of intermittent hypoxia training on exercise performance, hemodynamics, and ventilation in healthy senior men. High Alt. Med. Biol. 2008, 9, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Griffin, E.W.; Mullally, S.; Foley, C.; Warmington, S.A.; O’Mara, S.M.; Kelly, A.M. Aerobic exercise improves hippocampal function and increases BDNF in the serum of young adult males. Physiol. Behav. 2011, 104, 934–941. [Google Scholar] [CrossRef]
- Maffioletti, E.; Zanardini, R.; Gennarelli, M.; Bocchio-Chiavetto, L. Influence of clotting duration on brain-derived neurotrophic factor (BDNF) dosage in serum. Biotechniques 2014, 57, 111–114. [Google Scholar] [CrossRef] [Green Version]
- Serra-Millas, M. Are the changes in the peripheral brain-derived neurotrophic factor levels due to platelet activation? World J. Psychiatry 2016, 6, 84–101. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Zou, L.; Yu, J.J.; Ryu, S.; Kong, Z.; Yang, L.; Kang, M.; Lin, J.; Li, H.; Smith, L.; et al. Does exercise have a protective effect on cognitive function under hypoxia? A systematic review with meta-analysis. J. Sport Health Sci. 2020, 9, 562–577. [Google Scholar] [CrossRef]
- Das, S.K.; Dhar, P.; Sharma, V.K.; Barhwal, K.; Hota, S.K.; Norboo, T.; Singh, S.B. High altitude with monotonous environment has significant impact on mood and cognitive performance of acclimatized lowlanders: Possible role of altered serum BDNF and plasma homocysteine level. J. Affect. Disord. 2018, 237, 94–103. [Google Scholar] [CrossRef]
- Chroboczek, M.; Kostrzewa, M.; Micielska, K.; Grzywacz, T.; Laskowski, R. Effect of acute normobaric hypoxia exposure on executive functions among young physically active males. J. Clin. Med. 2021, 10, 1560. [Google Scholar] [CrossRef]
- Ochi, G.; Yamada, Y.; Hyodo, K.; Suwabe, K.; Fukuie, T.; Byun, K.; Dan, I.; Soya, H. Neural basis for reduced executive performance with hypoxic exercise. Neuroimage 2018, 171, 75–83. [Google Scholar] [CrossRef]
- Gibson, G.E.; Pulsinelli, W.; Blass, J.P.; Duffy, T.E. Brain dysfunction in mild to moderate hypoxia. Am. J. Med. 1981, 70, 1247–1254. [Google Scholar] [CrossRef]
- Vogiatzis, I.; Louvaris, Z.; Habazettl, H.; Athanasopoulos, D.; Andrianopoulos, V.; Cherouveim, E.; Wagner, H.; Roussos, C.; Wagner, P.D.; Zakynthinos, S. Frontal cerebral cortex blood flow, oxygen delivery and oxygenation during normoxic and hypoxic exercise in athletes. J. Physiol. 2011, 589, 4027–4039. [Google Scholar] [CrossRef]
- Komiyama, T.; Katayama, K.; Sudo, M.; Ishida, K.; Higaki, Y.; Ando, S. Cognitive function during exercise under severe hypoxia. Sci. Rep. 2017, 7, 10000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambourne, K.; Tomporowski, P. The effect of exercise-induced arousal on cognitive task performance: A meta-regression analysis. Brain Res. 2010, 1341, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Campos, C.; Rocha, N.B.; Lattari, E.; Nardi, A.E.; Machado, S. Exercise Induced Neuroplasticity to Enhance Therapeutic Outcomes of Cognitive Remediation in Schizophrenia: Analyzing the Role of Brai nderived Neurotrophic Factor. CNS Neurol. Disord. Drug Targets 2017, 16, 638–651. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, P.; Brassard, P.; Adser, H.; Pedersen, M.V.; Leick, L.; Hart, E.; Secher, N.H.; Pedersen, B.K.; Pilegaard, H. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp. Physiol. 2009, 94, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Piotrowicz, Z.; Chalimoniuk, M.; Płoszczyca, K.; Czuba, M.; Langfort, J. Exercise-induced elevated BDNF level does not prevent cognitive impairment due to acute exposure to moderate hypoxia in well-trained athletes. Int. J. Mol. Sci. 2020, 21, 5569. [Google Scholar] [CrossRef]
- Lei, O.K.; Kong, Z.; Loprinzi, P.D.; Shi, Q.; Sun, S.; Zou, L.; Hu, Y.; Nie, J. Severe Hypoxia Does Not Offset the Benefits of Exercise on Cognitive Function in Sedentary Young Women. Int. J. Environ. Res. Public Health 2019, 16, 1003. [Google Scholar] [CrossRef] [Green Version]
- Seo, Y.; Burns, K.; Fennell, C.; Kim, J.H.; Gunstad, J.; Glickman, E.; McDaniel, J. The Influence of Exercise on Cognitive Performance in Normobaric Hypoxia. High Alt. Med. Biol. 2015, 16, 298–305. [Google Scholar] [CrossRef]
- Curtelin, D.; Morales-Alamo, D.; Torres-Peralta, R.; Rasmussen, P.; Martin-Rincon, M.; Perez-Valera, M.; Siebenmann, C.; Perez-Suarez, I.; Cherouveim, E.; Sheel, A.W.; et al. Cerebral blood flow, frontal lobe oxygenation and intra-arterial blood pressure during sprint exercise in normoxia and severe acute hypoxia in humans. J. Cereb. Blood Flow Metab. 2018, 38, 136–150. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Loprinzi, P.D.; Guan, H.; Zou, L.; Kong, Z.; Hu, Y.; Shi, Q.; Nie, J. The effects of high-intensity interval exercise and hypoxia on cognition in sedentary young adults. Medicina 2019, 55, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dale, E.A.; Ben Mabrouk, F.; Mitchell, G.S. Unexpected benefits of intermittent hypoxia: Enhanced respiratory and nonrespiratory motor function. Physiology 2014, 29, 39–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loprinzi, P.D.; Edwards, M.K.; Frith, E. Potential avenues for exercise to activate episodic memory-related pathways: A narrative review. Eur. J. Neurosci. 2017, 46, 2067–2077. [Google Scholar] [CrossRef] [PubMed]
- Bherer, L.; Kramer, A.F.; Peterson, M.S.; Colcombe, S.; Erickson, K.; Becic, E. Testing the limits of cognitive plasticity in older adults: Application to attentional control. Acta Psychol. 2006, 123, 261–278. [Google Scholar] [CrossRef]
- Hillman, C.H.; Buck, S.M.; Themanson, J.R.; Pontifex, M.B.; Castelli, D.M. Aerobic fitness and cognitive development: Event-related brain potential and task performance indices of executive control in preadolescent children. Dev. Psychol. 2009, 45, 114–129. [Google Scholar] [CrossRef] [Green Version]
- Ludyga, S.; Gerber, M.; Brand, S.; Holsboer-Trachsler, E.; Pühse, U. Acute effects of moderate aerobic exercise on specific aspects of executive function in different age and fitness groups: A meta-analysis. Psychophysiology 2016, 53, 1611–1626. [Google Scholar] [CrossRef]
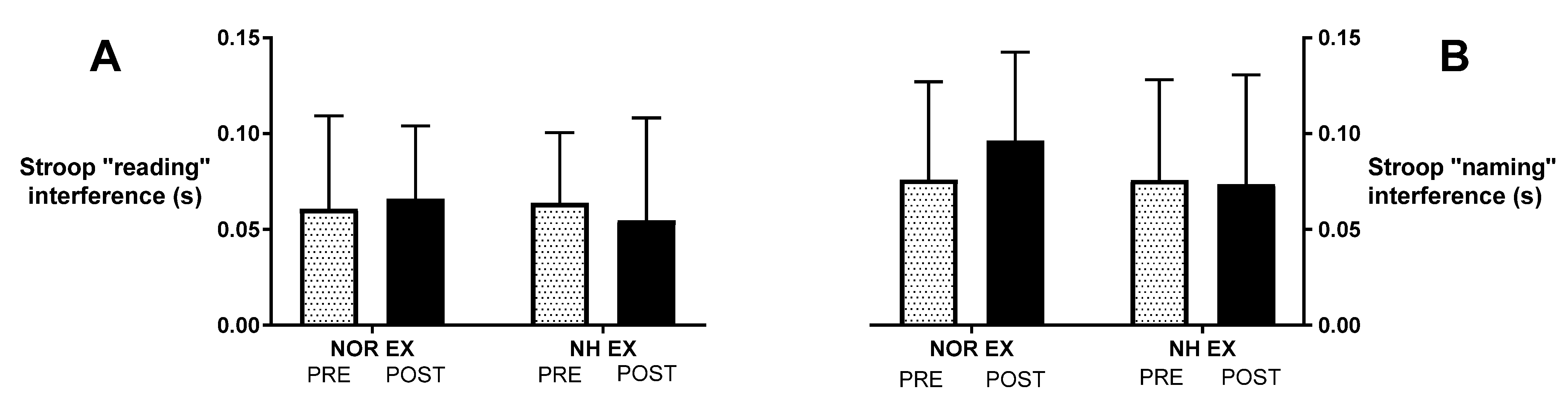
| N = 17 | X | SD |
|---|---|---|
| Age [years] | 20.6 | 0.7 |
| Weight [kg] | 75.6 | 8.3 |
| FAT [%] | 17.9 | 2.9 |
| FAT [kg] | 13.7 | 3.1 |
| FFM [kg] | 62.0 | 6.3 |
| BMI [kg∙m−2] | 23.3 | 2.0 |
| VO2max [mL∙kg−1∙min−1] | 42.1 | 6.5 |
| NOR EX (n = 17) Mean ± SD | NH EX (n = 17) Mean ± SD | Diff | 95% CI | p | ||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| BDNF (pg·mL−1 serum) | ||||||
| Before | 364.19 ± 80.72 | 311.08 ± 70.4 | 53.11 | −121.1 | 227.3 | 0.9731 |
| After | 728.35 ± 375.54 | 711.38 ± 207.96 | 16.97 | −157.2 | 191.1 | >0.9999 |
| Change | 364.2 ± 359.1 | 400.3 ± 195.4 | ||||
| p | <0.0001 | <0.0001 | ||||
| Stroop “reading” interference (s) | ||||||
| Before | 0.06071 ± 0.04863 | 0.06385 ± 0.03673 | −0.003147 | −0.03842 | 0.03213 | >0.9999 |
| After | 0.066 ± 0.03805 | 0.05465 ± 0.05356 | 0.01135 | −0.2392 | 0.04663 | 0.9256 |
| Change | 0.005294 ± 0.04917 | −0.009206 ± 0.0689 | ||||
| p | >0.9999 | >0.9999 | ||||
| Stroop “naming” interference (s) | ||||||
| Before | 0.07588 ± 0.0512 | 0.07576 ± 0.05245 | 0.0001176 | −0.0407 | 0.04094 | >0.9999 |
| After | 0.09632 ± 0.04619 | 0.07371 ± 0.05698 | 0.02262 | −0.0182 | 0.06344 | 0.4161 |
| Change | 0.02044 ± 0.04423 | −0.002059 ± 0.0553 | ||||
| p | 0.2041 | >0.9999 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chroboczek, M.; Kujach, S.; Łuszczyk, M.; Soya, H.; Laskowski, R. Exercise-Induced Elevated BDNF Concentration Seems to Prevent Cognitive Impairment after Acute Exposure to Moderate Normobaric Hypoxia among Young Men. Int. J. Environ. Res. Public Health 2023, 20, 3629. https://doi.org/10.3390/ijerph20043629
Chroboczek M, Kujach S, Łuszczyk M, Soya H, Laskowski R. Exercise-Induced Elevated BDNF Concentration Seems to Prevent Cognitive Impairment after Acute Exposure to Moderate Normobaric Hypoxia among Young Men. International Journal of Environmental Research and Public Health. 2023; 20(4):3629. https://doi.org/10.3390/ijerph20043629
Chicago/Turabian StyleChroboczek, Maciej, Sylwester Kujach, Marcin Łuszczyk, Hideaki Soya, and Radosław Laskowski. 2023. "Exercise-Induced Elevated BDNF Concentration Seems to Prevent Cognitive Impairment after Acute Exposure to Moderate Normobaric Hypoxia among Young Men" International Journal of Environmental Research and Public Health 20, no. 4: 3629. https://doi.org/10.3390/ijerph20043629
APA StyleChroboczek, M., Kujach, S., Łuszczyk, M., Soya, H., & Laskowski, R. (2023). Exercise-Induced Elevated BDNF Concentration Seems to Prevent Cognitive Impairment after Acute Exposure to Moderate Normobaric Hypoxia among Young Men. International Journal of Environmental Research and Public Health, 20(4), 3629. https://doi.org/10.3390/ijerph20043629






