Stem–Loop Structures in Iron-Regulated mRNAs of Giardia duodenalis
Abstract
1. Introduction
2. Materials and Methods
2.1. Parasite Cultures
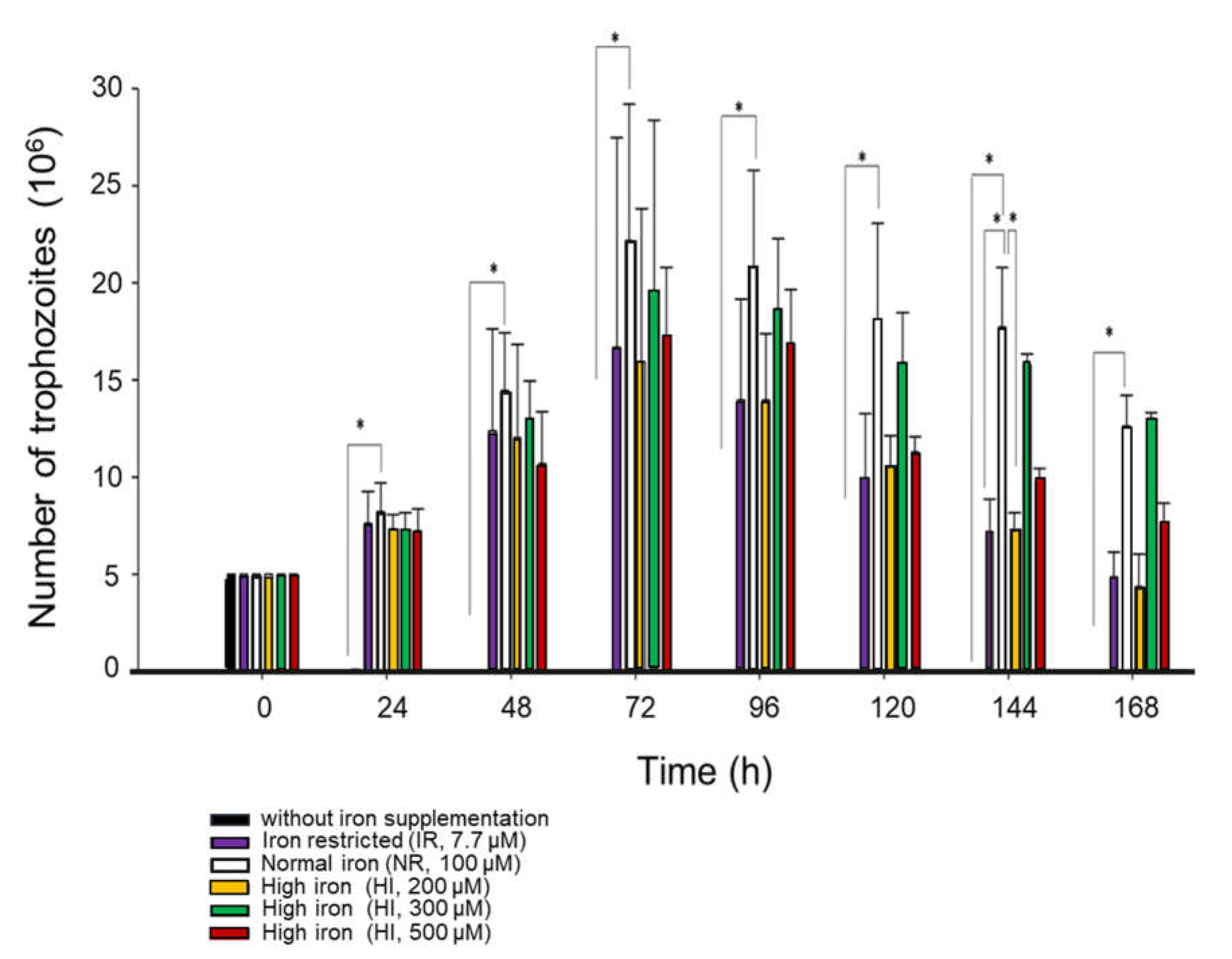
2.2. Iron Effect on the G. duodenalis Growth
2.3. Reverse Transcriptase PCR (RT-PCR) Assays
2.4. Prediction of Stem–Loop Structures in G. duodenalis mRNAs
3. Results
3.1. Iron Affects the Growth and Viability of G. duodenalis
3.2. Actin-Related Protein, Cytochrome b5, and Glucosamine-6-Phosphate Deaminase Are Differentially Modulated by Iron
3.3. Presence of Stem–Loop Structures in G. duodenalis mRNAs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leung, A.K.C.; Leung, A.A.M.; Wong, A.H.C.; Sergi, C.M.; Kam, J.K.M. Giardiasis: An Overview. Recent Pat. Inflamm. Allergy Drug Discov. 2019, 13, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Hotez, P.J.; Molyneux, D.H.; Fenwick, A.; Ottesen, E.; Ehrlich Sachs, S.; Sachs, J.D. Incorporating a rapid-impact package for neglected tropical diseases with programs for HIV/AIDS, tuberculosis, and malaria. PLoS Med. 2006, 3, e102. [Google Scholar] [CrossRef] [PubMed]
- Mark-Carew, M.P.; Adesiyun, A.A.; Basu, A.; Georges, K.A.; Pierre, T.; Tilitz, S.; Wade, S.E.; Mohammed, H.O. Characterization of Giardia duodenalis infections in dogs in Trinidad and Tobago. Vet. Parasitol. 2013, 196, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Sogin, M.L.; Gunderson, J.H.; Elwood, H.J.; Alonso, R.A.; Peattie, D.A. Phylogenetic meaning of the kingdom concept: An unusual ribosomal RNA from Giardia lamblia. Science 1989, 243, 75–77. [Google Scholar] [CrossRef]
- Adam, R.D. Biology of Giardia lamblia. Clin. Microbiol. Rev. 2001, 14, 447–475. [Google Scholar] [CrossRef]
- Svärd, S.G.; Hagblom, P.; Palm, J.E.D. Giardia lamblia—A model organism for eukaryotic cell differentiation. FEMS Microbiol. Lett. 2003, 218, 3–7. [Google Scholar] [CrossRef]
- Bartelt, L.A.; Roche, J.; Kolling, G.; Bolick, D.; Noronha, F.; Naylor, C.; Hoffman, P.; Warren, C.; Singer, S.; Guerrant, R. Persistent Giardia lamblia impairs growth in a murine malnutrition model. J. Clin. Investig. 2013, 123, 2672–2684. [Google Scholar] [CrossRef]
- Olivares, J.L.; Fernández, R.; Fleta, J.; Ruiz, M.Y.; Clavel, A.; Moreno, L.A. Iron deficiency in children with Giardia lamblia and Enterobius vermicularis. Nutr. Res. 2004, 24, 1–5. [Google Scholar] [CrossRef]
- Monajemzadeh, S.M.; Monajemzadeh, M. Comparison of iron and hematological indices in Giardia lamblia before and after treatment in 102 children in Ahwaz, Iran. Med. Sci. Monit. 2008, 14, 19–23. [Google Scholar]
- Al-Mekhlafi, M.S.M.; Azlin, M.; Nor Aini, U.; Shaik, A.; Sa’iah, A.; Fatmah, M.S.; Ismail, M.G.; Ahmad Firdaus, M.S.; Aisah, M.Y.; Rozlida, A.R.; et al. Giardiasis as a predictor of childhood malnutrition in Orang Asli children in Malaysia. Trans. R. Soc. Trop. Med. Hyg. 2005, 9, 686–691. [Google Scholar] [CrossRef]
- Suchan, P.; Vyoral, D.; Petrák, J.; Sut’ák, R.; Rasoloson, D.; Nohýnková, E.; Dolezal, P.; Tachezy, J. Incorporation of iron into Tritrichomonas foetus cell compartments revels ferredoxin as a major iron-binding protein in hydrogenosomes. Microbiology 2003, 149 Pt 7, 1911–1921. [Google Scholar] [CrossRef] [PubMed]
- Peirasmaki, D.; Ma’ayeh, S.Y.; Xu, F.F.; Ferella, M.; Campos, S.; Liu, J.; Svärd, S.G. High Cystine Membrane Proteins (HCMP) are up-regulated during Giardia- host cells interaction. Front. Genet. 2020, 11, 913. [Google Scholar] [CrossRef] [PubMed]
- Pantopoulos, K. Iron metabolism and the IRE/IRP regulatory system: An update. Ann. N. Y. Acad. Sci. 2004, 1012, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Pérez, G.; Vittori, D.; Pregi, N.; Garbossa, G.; Nesse, A. Homeostasis del hierro. Mecanismos de absorción, captación celular y regulación. Acta Bioquímica Clínica Latinoam. 2005, 39, 301–314. [Google Scholar]
- Tandara, L.; Salamunic, I. Iron metabolism: Current facts and future directions. Biochem. Med. 2012, 22, 311–328. [Google Scholar] [CrossRef]
- Wilkinson, N.; Pantopoulos, K. The IRP/IRE system in vivo: Insights from mouse models. Front. Pharmacol. 2014, 5, 1–15. [Google Scholar] [CrossRef]
- Álvarez-Sánchez, M.E.; Ávila-González, L.; Becerril-García, C.; Fattel-Facenda, L.V.; Ortega-López, J.; Arroyo, R. A novel cysteine proteinase (CP65) of Trichomonas vaginalis involved in cytotoxicity. Microb. Pathog. 2000, 28, 193–202. [Google Scholar] [CrossRef]
- Park, S.J.; Lee, S.M.; Lee, J.; Yong, T.S. Differential gene expression by iron-limitation in Entamoeba histolytica. Mol. Biochem. Parasitol. 2001, 114, 257–260. [Google Scholar] [CrossRef]
- Hernandez-Gutierrez, R.; Ortega-López, J.; Arroyo, R. A 39 kDa cysteine proteinase CP39 from Trichomonas vaginalis, which is negatively affected by iron may be envolved in trichomonal cititoxicity. J. Eukaryot. Microbiol. 2003, 50, 696–698. [Google Scholar] [CrossRef]
- Hernández-Gutiérrez, R.; Avila-González, L.; Ortega-López, J.; Cruz-Talonia, F.; Gómez-Gutierrez, G.; Arroyo, R. Trichomonas vaginalis: Characterization of a 39-kDa cysteine proteinase found in patient vaginal secretions. Exp. Parasitol. 2004, 107, 125–135. [Google Scholar] [CrossRef]
- Lee, J.; Park, S.J.; Yong, T.S. Effect of iron on adherence and cytotoxicity of Entamoeba histolytica to CHO cell monolayers. Korean J. Parasitol. 2008, 46, 37–40. [Google Scholar] [CrossRef] [PubMed]
- León-Sicairos, C.R.; León-Félix, J.; Arroyo, R. tvcp12, a novel Trichomonas vaginalis cathepsin L-like cysteine proteinase- encoding gene. Microbiology 2004, 150 Pt 5, 1131–1138. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Solano-González, E.; Burrola-Barraza, E.; León-Sicairos, C.R.; Ávila-González, L.; Gutiérrez-Escolano, L.; Ortega-López, J.; Arroyo, R. The trichomonad cysteine proteinase TVCP4 transcript contains an iron responsive element. FEBS Lett. 2007, 581, 2919–2928. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Cruz, C.; López-Casamichana, M.; Cruz-Castañeda, A.; Olivares-Trejo, J.J. Transferrin regulates mRNA levels of gene involved in iron utilization in Entamoeba histolytica. Mol. Biol. Rep. 2012, 39, 4545–4551. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Cuevas, N.A.; Weber, C.; Hon, C.C.; Guillen, N. Gene expression profiling in Entamoeba histolytica identifies key components in iron uptake y metabolism. PLoS ONE 2014, 9, e107102. [Google Scholar] [CrossRef]
- Soto-Castro, L.; Plata-Guzmán, L.Y.; Figueroa-Angulo, E.E.; Calla-Choque, J.S.; Reyes-López, M.; De la Garza, M.; León-Sicairos, N.; Garzón-Tiznado, J.A.; Arroyo, R.; León-Sicairos, C. Iron Responsive-like Elements in the parasite Entamoeba histolytica. Microbiology 2019, 165, 366. [Google Scholar] [CrossRef]
- Torres-Romero, J.C.; Arroyo, R. Responsiveness of Trichomonas vaginalis to iron concentrations: Evidence for a post-transcriptional iron regulation by an IRE-IRP-like system. Infect Genet. Evol. 2009, 9, 1065–1074. [Google Scholar] [CrossRef]
- Figueroa-Angulo, E.E.; Calla-Choque, J.S.; Mancilla-Olea, M.I.; Arroyo, R. RNA-Binding Proteins in Trichomonas vaginalis: Atypical Multifunctional Proteins. Biomolecules 2015, 5, 3354–3395. [Google Scholar] [CrossRef]
- Calla-Choque, J.S.; Figueroa-Angulo, E.E.; Ávila-González, L.; Arroyo, R. α -Actinin TvACTN3 of Trichomonas vaginalis is an RNA-binding protein that could participate in its posttranscriptional iron regulatory mechanism. Biomed. Res. Int. 2014, 2014, 424767. [Google Scholar] [CrossRef]
- Keister, D.B. Axenic culture of Giardia lamblia in TYI-S-33 medium supplemented with bile. Trans. R. Soc. Trop. Med. Hyg. 1983, 77, 487–488. [Google Scholar] [CrossRef]
- Tasca, T.; Bonan, C.D.; De Carli, G.A.; Sarkis, J.J.F.; Alderete, J.F. Heterogeneity in extracellular nucleotide hydrolysis among clinical isolates of Trichomonas vaginalis. Parasitology 2005, 131 Pt 1, 71–78. [Google Scholar] [CrossRef]
- Aurrecoechea, C.; Brestelli, J.; Brunk, B.P.; Carlton, J.M.; Dommer, J.; Fisher, S.; Gajria, B.; Gao, X.; Gingle, A.; Grant, G.; et al. GiardiaDB and TrichDB: Integrated genomic resources for the eukaryotic protist pathogens Giardia lamblia and Trichomonas vaginalis. Nucleic Acids Res. 2009, 37, D526–D530. [Google Scholar] [CrossRef]
- Xu, F.; Jex, A.; Svärd, S.G. A chromosome-scale reference genoma for Giardia intestinalis WB. Sci. Data 2020, 4, 38. [Google Scholar] [CrossRef] [PubMed]
- Knodler, L.A.; Svärd, S.G.; Silberman, J.D.; Davids, B.J.; Gillin, F.D. Developmental gene regulation in Giardia lamblia: First evidence for an encystations-specific promoter y differential 5’ mRNA processing. Mol. Microbiol. 1999, 34, 327–340. [Google Scholar] [CrossRef]
- Elmendorf, H.G.; Singer, S.M.; Pierce, J.; Cowan, J.; Nash, T.E. Initiator and upstream elements in the α2-tubulin promoter of Giardia lamblia. Mol. Biochem. Parasitol. 2000, 113, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Tolba, M.E.M.; Kobayashi, S.; Imada, M.; Suzuki, Y.; Sugano, S. Giardia lamblia Transcriptome Analysis Using TSS-Seq and RNA-Seq. PLoS ONE 2013, 8, e76184. [Google Scholar] [CrossRef] [PubMed]
- Peattie, D.A.; Alonso, R.A.; Hein, A.; Caulfield, J.P. Ultrastructural Localization of Giardins to the Edges of Disk Microribbons of Giardia lamblia and the Nucleotide and Deduced Protein Sequence of Alpha Giardin. J. Cell Biol. 1989, 109, 2323–2335. [Google Scholar] [CrossRef] [PubMed]
- Que, X.; Svärd, S.G.; Meng, T.C.; Hetsko, M.L.; Aley, S.B.; Gillin, F.D. Developmentally regulated transcripts and evidence of differential mRNA processing in Giardia lamblia. Mol. Biochem. Parasitol. 1996, 81, 101–110.37. [Google Scholar] [CrossRef]
- Franzén, O.; Jerlström-Hultqvist, J.; Einarsson, E.; Ankarklev, J.; Ferella, M.; Andersson, B.; Svärd, S.G. Transcriptome profiling of Giardia intestinalis using strand-specific RNA-seq. PLoS Comput. Biol. 2013, 9, e1003000. [Google Scholar] [CrossRef]
- Zuker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003, 31, 3406–3415. [Google Scholar] [CrossRef]
- Adedoja, A.N.; McMahan, T.; Neal, J.P.; Hamal Dhakal, S.; Jois, S.; Romo, D.; Hull, K.; Garlapati, S. Translation initiation factors GleIF4E2 and GleIF4A can interact directly with the components of the pre-initiation complex to facilitate translation initiation in Giardia lamblia. Mol. Biochem. Parasitol. 2020, 236, 111258. [Google Scholar] [CrossRef] [PubMed]
- Henderson, B.R.; Menotti, E.; Kühn, L.C. Iron regulatory proteins 1 and 2 bind distinct 524 sets of RNA target sequences. J. Biol. Chem. 1996, 271, 4900–4908. [Google Scholar] [CrossRef] [PubMed]
- Vanacova, S.; Liston, D.R.; Tachezy, J.; Johnson, P.J. Molecular biology of the amitochondriate parasites, Giardia intestinalis, Entamoeba histolytica y Trichomonas vaginalis. Int. J. Parasitol. 2003, 33, 235–255. [Google Scholar] [CrossRef] [PubMed]
- Mathews, D.H.; Sabina, J.; Zuker, M.; Turner, D.H. Expanded sequence dependence of thermodynamic parameters improves prediction of RNA secondary structure. J. Mol. Biol. 1999, 288, 911–940. [Google Scholar] [CrossRef] [PubMed]
- Volz, K. The functional duality of iron regulatory protein 1. Curr. Opin. Struct. Biol. 2008, 18, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Piccinelli, P.; Samuelsson, T. Evolution of the Iron-Responsive Element. RNA 2017, 13, 952–966. [Google Scholar] [CrossRef]
- Mach, J.; Sutak, R. Iron in parasitic protists—From uptake to storage and where we can interfere. Metallomics 2020, 12, 1335–1347. [Google Scholar] [CrossRef]
- Arroyo, R.; Ochoa, T.; Tai, J.H.; de la Garza, M. Iron and Parasites. Biomed. Res. Int. 2015, 2015, 291672. [Google Scholar] [CrossRef]
- Gastelum-Martínez, A.; León-Sicairos, C.; Plata-Guzmán, L.; Soto-Castro, L.; León-Sicairos, N.; De La Garza, M. Iron-modulated virulence factors of Entamoeba histolytica. Future Microbiol. 2018, 13, 1329–1341. [Google Scholar] [CrossRef]
- Aguilar-Diaz, H.; Canizalez-Roman, A.; Nepomuceno-Mejia, T.; Gallardo-Vera, F.; Hornelas-Orozco, Y.; Nazmi, K.; Bolscher, J.G.; Carrero, J.C.; Leon-Sicairos, C.; Leon-Sicairos, N. Parasiticidal effect of synthetic bovine lactoferrin peptides on the enteric parasite Giardia intestinalis. Biochem. Cell Biol. 2017, 95, 82–90. [Google Scholar] [CrossRef]
- Paredez, A.R.; Nayeri, A.; Xu, J.W.; Krtková, J.; Cande, W.Z. Identification of Obscure yet Conserved Actin-Associated Proteins in Giardia lamblia. Eukaryot. Cell 2014, 13, 776–784. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Castillo-Romero, A.; León-Ávila, G.; Pérez-Rangel, A.; Cortes-Zárate, R.; García-Tovar, C.; Hernández, J.M. Participation of Actin on Giardia lamblia Growth and Encystation. PLoS ONE 2009, 4, e7156. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowska-Semrau, K.; Czarnecka, J.; Wojciechowski, M.; Milewski, S. Heterogeneity of quaternary structure of glucosamine-6-phosphate deaminase from G. lamblia. Parasitol. Res. 2015, 114, 175–184. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lopez, A.B.; Hossan, M.T.; Keulen, H.V. Giardia intestinalis glucosamine 6-phosphate isomerase: The key enzyme to encystment appears to be controlled by ubiquitin attachment. J. Eukaryot. Microbiol. 2002, 49, 134–136. [Google Scholar] [CrossRef]
- Jarroll, E.L.; Manning, P.; Berrada, A.; Hare, D.; Lindmark, D.G. Biochemistry and Metabolism of Giardia. J. Protozool. 1989, 36, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Rafferty, S.P.; Dayer, G. Heme proteins of Giardia intestinalis. Exp. Parasitol. 2015, 159, 13–23. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plata-Guzmán, L.Y.; Arroyo, R.; León-Sicairos, N.; Canizález-Román, A.; López-Moreno, H.S.; Chávez-Ontiveros, J.; Garzón-Tiznado, J.A.; León-Sicairos, C. Stem–Loop Structures in Iron-Regulated mRNAs of Giardia duodenalis. Int. J. Environ. Res. Public Health 2023, 20, 3556. https://doi.org/10.3390/ijerph20043556
Plata-Guzmán LY, Arroyo R, León-Sicairos N, Canizález-Román A, López-Moreno HS, Chávez-Ontiveros J, Garzón-Tiznado JA, León-Sicairos C. Stem–Loop Structures in Iron-Regulated mRNAs of Giardia duodenalis. International Journal of Environmental Research and Public Health. 2023; 20(4):3556. https://doi.org/10.3390/ijerph20043556
Chicago/Turabian StylePlata-Guzmán, Laura Y., Rossana Arroyo, Nidia León-Sicairos, Adrián Canizález-Román, Héctor S. López-Moreno, Jeanett Chávez-Ontiveros, José A. Garzón-Tiznado, and Claudia León-Sicairos. 2023. "Stem–Loop Structures in Iron-Regulated mRNAs of Giardia duodenalis" International Journal of Environmental Research and Public Health 20, no. 4: 3556. https://doi.org/10.3390/ijerph20043556
APA StylePlata-Guzmán, L. Y., Arroyo, R., León-Sicairos, N., Canizález-Román, A., López-Moreno, H. S., Chávez-Ontiveros, J., Garzón-Tiznado, J. A., & León-Sicairos, C. (2023). Stem–Loop Structures in Iron-Regulated mRNAs of Giardia duodenalis. International Journal of Environmental Research and Public Health, 20(4), 3556. https://doi.org/10.3390/ijerph20043556








