Effectiveness of Paliperidone Palmitate in Reducing Acute Psychiatric Service Use for Patients Suffering from Psychosis—A Retrospective Mirror-Image Study
Abstract
1. Introduction
2. Methods
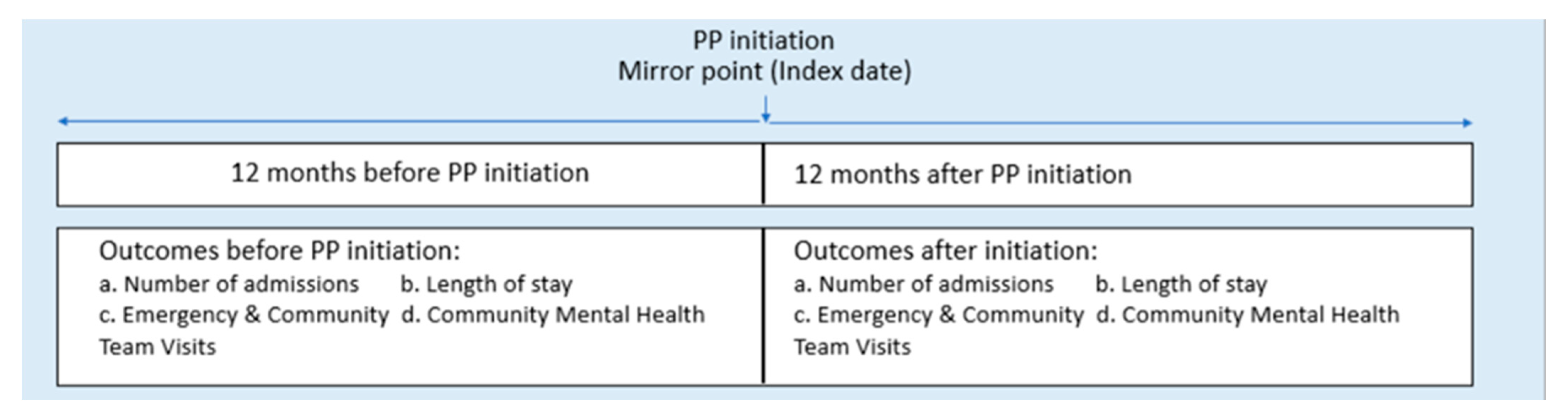
2.1. Study Design
2.2. Study Population
2.3. Study Measures
2.4. Statistical Analysis
3. Results
3.1. Mean Total Length of Hospitalization
3.2. Mean Number of Hospitalization
3.3. Mean Number of Emergency Room Visits
3.4. Mean Number of Visits by the Community Mental Health Team (CMHT)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perkins, D.O. Adherence to antipsychotic medications. J. Clin. Psychiatry 1999, 60 (Suppl. S21), 25–30. [Google Scholar] [PubMed]
- Acosta, F.J.; Hernández, J.L.; Pereira, J.; Herrera, J.; Rodríguez, C.J. Medication adherence in schizophrenia. World J. Psychiatry 2012, 2, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Lauriello, J. Prevalence and Impact of Relapse in Patients With Schizophrenia. J. Clin. Psychiatry 2020, 81, MS19053BR1C. [Google Scholar] [CrossRef] [PubMed]
- Leucht, S.; Tardy, M.; Komossa, K.; Heres, S.; Kissling, W.; Salanti, G.; Davis, J.M. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: A systematic review and meta-analysis. Lancet 2012, 379, 2063–2071. [Google Scholar] [CrossRef] [PubMed]
- Kane, J.M. Treatment Adherence and Long-Term Outcomes. CNS Spectr. 2007, 12, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Marder, S.R.; Hubbard, J.W.; Putten, V.; Midha, T.K. Pharmacokinetics of long-acting injectable neuroleptic drugs: Clinical implications. Psychopharmacology 1989, 98, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Lacro, J.; Dunn, L.; Dolder, C.; Leckband, S.; Jeste, D. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: A comprehensive review of recent literature. J. Clin. Psychiatry 2002, 63, 892–909. [Google Scholar] [CrossRef] [PubMed]
- Tiihonen, J.; Haukka, J.; Taylor, A.M.; Haddad, P.; Patel, M.X.; Korhonen, P. A Nationwide Cohort Study of Oral and Depot Antipsychotics After First Hospitalization for Schizophrenia. Am. J. Psychiatry 2011, 168, 603–609. [Google Scholar] [CrossRef]
- Adams, C.; Fenton, M.; Quraishi, S.; David, A. Systematic meta-review of depot antipsychotic drugs for people with schizophrenia. Br. J. Psychiatry 2001, 179, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Brissos, S.; Veguilla, M.; Taylor, D.; Balanzá-Martinez, V. The role of long-acting injectable antipsychotics in schizophrenia: A critical appraisal. Ther. Adv. Psychopharmacol. 2014, 4, 198–219. [Google Scholar] [CrossRef] [PubMed]
- Correll, C.; Citrome, L.; Haddad, P.; Lauriello, J.; Olfson, M.; Calloway, S.; Kane, J. The Use of Long-Acting Injectable Antipsychotics in Schizophrenia: Evaluating the Evidence. J. Clin. Psychiatry 2016, 77 (Suppl. S3), 1–24. [Google Scholar] [CrossRef]
- Kishimoto, T.; Hagi, K.; Nitta, M.; Leucht, S.; Olfson, M.; Kane, J.M.; Correll, C.U. Effectiveness of Long-Acting Injectable vs Oral Antipsychotics in Patients With Schizophrenia: A Meta-analysis of Prospective and Retrospective Cohort Studies. Schizophr. Bull. 2017, 44, 603–619. [Google Scholar] [CrossRef]
- Taylor, M.; Dangelo-Kemp, D.; Liu, D.; Kisely, S.; Graham, S.; Hartmann, J.; Colman, S. Antipsychotic utilisation and persistence in Australia: A nationwide 5-year study. Aust. N. Z. J. Psychiatry 2021, 56, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Schneider-Thoma J, C.K. Comparative efficacy and tolerability of 32 oral and long-acting injectable antipsychotics for the maintenance treatment of adults with schizophrenia: A systematic review and network meta-analysis. Lancet 2022, 399, 824–836. [Google Scholar] [CrossRef]
- Kishimoto, T.; Nitta, M.; Borenstein, M.; Kane, J.; Correll, C. Long-acting injectable versus oral antipsychotics in schizophrenia: A systematic review and meta-analysis of mirror-image studies. J. Clin. Psychiatry 2013, 74, 957–965. [Google Scholar] [CrossRef]
- Kishimoto, T.; Hagi, K.; Kurokawa, S.; Kane, J.M.; Correll, C.U. Long-acting injectable versus oral antipsychotics for the maintenance treatment of schizophrenia: A systematic review and comparative meta-analysis of randomised, cohort, and pre–post studies. Lancet Psychiatry 2021, 8, 387–404. [Google Scholar] [CrossRef]
- Ostuzzi, G.; Bertolini, F.; Del Giovane, C.; Tedeschi, F.; Bovo, C.; Gastaldon, C.; Nosé, M.; Ogheri, F.; Papola, D.; Purgato, M.; et al. Maintenance Treatment With Long-Acting Injectable Antipsychotics for People With Nonaffective Psychoses: A Network Meta-Analysis. Am. J. Psychiatry 2021, 178, 424–436. [Google Scholar] [CrossRef]
- Emsley, R.; Kilian, S. Efficacy and safety profile of paliperidone palmitate injections in the management of patients with schizophrenia: An evidence-based review. Neuropsychiatr. Dis. Treat. 2018, 14, 205–223. [Google Scholar] [CrossRef]
- Karow, A.; Schnedler, D.; Naber, D. What Would The Patient Choose? Subjective Comparison of Atypical and Typical Neuroleptics. Pharmacopsychiatry 2006, 39, 47–51. [Google Scholar] [CrossRef]
- Alphs, L.; Bossie, C.A.; Sliwa, J.K.; Ma, Y.-W.; Turner, N. Onset of efficacy with acute long-acting injectable paliperidone palmitate treatment in markedly to severely ill patients with schizophrenia: Post hoc analysis of a randomized, double-blind clinical trial. Ann. Gen. Psychiatry 2011, 10, 12. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Feng, Y.; Zhuo, J.M.; Turkoz, I.; Mathews, M.; Tan, W. Impact of time of initiation of once-monthly paliperidone palmitate in hospitalized Asian patients with acute exacerbation of schizophrenia: A post hoc analysis from the PREVAIL study. Neuropsychiatr. Dis. Treat. 2018, 14, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.; Sparshatt, A.; O’Hagan, M.; Dzahini, O. Effect of paliperidone palmitate on hospitalisation in a naturalistic cohort—a four-year mirror image study. Eur. Psychiatry 2016, 37, 43–48. [Google Scholar] [CrossRef]
- Vincent, P.D.; Demers, M.-F.; Doyon-Kemp, V.; Duchesneau, J.; Halme, A.; Masson, V. One year mirror-image study using paliperidone palmitate for relapse prevention of schizophrenia in four university hospitals in Canada. Schizophr. Res. 2017, 185, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Marder, S.R. Overview of partial compliance. J. Clin. Psychiatry 2003, 64 (Suppl. S16), 3–9. [Google Scholar] [PubMed]
- Oh, S.Y.; Jon, D.-I.; Hong, H.J.; Hong, N.; Yi, J.-S.; Roh, D.; Jung, M.H. The Impact of Paliperidone Palmitate on Hospitalization in Patients with Schizophrenia: A Retrospective Mirror-image Study. Clin. Psychopharmacol. Neurosci. 2019, 17, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Crossley, N.; Sweeney, B. Patient and service-level factors affecting length of inpatient stay in an acute mental health service: A retrospective case cohort study. BMC Psychiatry 2020, 20, 438. [Google Scholar] [CrossRef] [PubMed]
- McEvoy JP, B.M.; Hamer, R.; Dominik, R.; Swartz, M.; Rosenheck, R.; Ray, N.; Lamberti, J.S.; Buckley, P.F.; Wilkins, T.M.; Stroup, T. Effectiveness of paliperidone palmitate vs haloperidol decanoate for maintenance treatment of schizophrenia: A randomized clinical trial. JAMA 2014, 311, 1978–1987. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Rui, Q.; Ning, X.; Xu, H.; Gu, N. A comparative study of paliperidone palmitate and risperidone long-acting injectable therapy in schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 1002–1008. [Google Scholar] [CrossRef]
- Di Lorenzo, R.; Ferri, P.; Cameli, M.; Rovesti, S.; Piemonte, C. Effectiveness of 1-year treatment with long-acting formulation of aripiprazole, haloperidol, or paliperidone in patients with schizophrenia: Retrospective study in a real-world clinical setting. Neuropsychiatr. Dis. Treat. 2019, 15, 183–198. [Google Scholar] [CrossRef]

| n | % | |||
|---|---|---|---|---|
| Gender | ||||
| Female | 78 | 49.37 | ||
| Male | 80 | 50.63 | ||
| Ethnicity | ||||
| Chinese | 113 | 71.52 | ||
| Indian | 16 | 10.13 | ||
| Malay | 20 | 12.66 | ||
| Others | 9 | 5.7 | ||
| Marital status | ||||
| Single | 82 | 51.9 | ||
| Married | 29 | 18.35 | ||
| Divorced | 5 | 3.16 | ||
| Unknown | 42 | 26.58 | ||
| Employment | ||||
| Employed | 2 | 1.27 | ||
| Student | 4 | 2.53 | ||
| Unemployed | 6 | 3.8 | ||
| homemaker | 2 | 1.27 | ||
| Unknown | 144 | 91.14 | ||
| Deceased | ||||
| No | 155 | 98.1 | ||
| Yes | 3 | 1.9 | ||
| Mean | SD | Min | Max | |
| Age | 46.28 | 11.33 | 25 | 66 |
| Number of injections at Post-PP1M injection (Median = 4) | 6.09 | 5.18 | 1 | 29 |
| Pre-Injection | Post-Injection | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | Range | Mean | Median | Range | Bootstrap | Wilcoxon | |||
| (SD) | (IQR) | (Min) | (Max) | (SD) | (IQR) | (Min) | (Max) | Paired | Signed | |
| t-Test | Rank | |||||||||
| Test | ||||||||||
| Total of Length of Stay (days) | 106.53 (127.44) | 25.5 (210) | 0 | 419 | 19.10 (53.86) | 0 (10) | 0 | 337 | <0.001 | <0.001 |
| Number of Psychiatric Admissions | 1.47 (1.03) | 1 (1) | 0 | 5 | 0.49 (0.98) | 0 (1) | 0 | 6 | 0.001 | <0.001 |
| Inpatient | 1.05 (0.23) | 1 (0) | 1 | 2 | 0.21 (0.41) | 1 (0) | 0 | 1 | <0.001 | <0.001 |
| Outpatient | 1.71 (1.21) | 2 (1) | 0 | 5 | 0.65 (1.16) | 0 (1) | 0 | 6 | <0.001 | <0.001 |
| Number of Emergency Visits | 1.77 (1.75) | 1 (1) | 0 | 10 | 0.73 (1.46) | 0 (1) | 0 | 9 | 0.001 | <0.001 |
| Inpatient | 1.19 (0.61) | 1 (0) | 0 | 4 | 0.33 (0.72) | 0 (0) | 0 | 4 | <0.001 | <0.001 |
| Outpatient | 2.10 (2.08) | 2 (2) | 2 | 10 | 0.95 (1.71) | 0 (1) | 0 | 9 | <0.001 | <0.001 |
| Number of CMHT visits | 1.42 (3.78) | 6 (8) | 0 | 21 | 1.60 (3.80) | 6.5 (8) | 0 | 20 | 0.579 | 0.8145 |
| Inpatient | 0.89 (2.53) | 0 (0) | 0 | 11 | 1.37 (3.58) | 0 (0) | 0 | 20 | 0.335 | 0.9807 |
| Outpatient | 1.72 (4.31) | 0 (0) | 0 | 21 | 1.73 (3.93) | 0 (0) | 0 | 15 | 0.430 | 0.688 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, C.T.; Verma, S.; Subramaniam, M.; Abdin, E.; Tay, J. Effectiveness of Paliperidone Palmitate in Reducing Acute Psychiatric Service Use for Patients Suffering from Psychosis—A Retrospective Mirror-Image Study. Int. J. Environ. Res. Public Health 2023, 20, 3403. https://doi.org/10.3390/ijerph20043403
Chan CT, Verma S, Subramaniam M, Abdin E, Tay J. Effectiveness of Paliperidone Palmitate in Reducing Acute Psychiatric Service Use for Patients Suffering from Psychosis—A Retrospective Mirror-Image Study. International Journal of Environmental Research and Public Health. 2023; 20(4):3403. https://doi.org/10.3390/ijerph20043403
Chicago/Turabian StyleChan, Chun Ting, Swapna Verma, Mythily Subramaniam, Edimansyah Abdin, and Jenny Tay. 2023. "Effectiveness of Paliperidone Palmitate in Reducing Acute Psychiatric Service Use for Patients Suffering from Psychosis—A Retrospective Mirror-Image Study" International Journal of Environmental Research and Public Health 20, no. 4: 3403. https://doi.org/10.3390/ijerph20043403
APA StyleChan, C. T., Verma, S., Subramaniam, M., Abdin, E., & Tay, J. (2023). Effectiveness of Paliperidone Palmitate in Reducing Acute Psychiatric Service Use for Patients Suffering from Psychosis—A Retrospective Mirror-Image Study. International Journal of Environmental Research and Public Health, 20(4), 3403. https://doi.org/10.3390/ijerph20043403







