Relationship of Depression, Anxiety, and Bipolar Disease with Burning Mouth Syndrome: A Nationwide Cohort Study
Abstract
1. Introduction
2. Materials and Methods
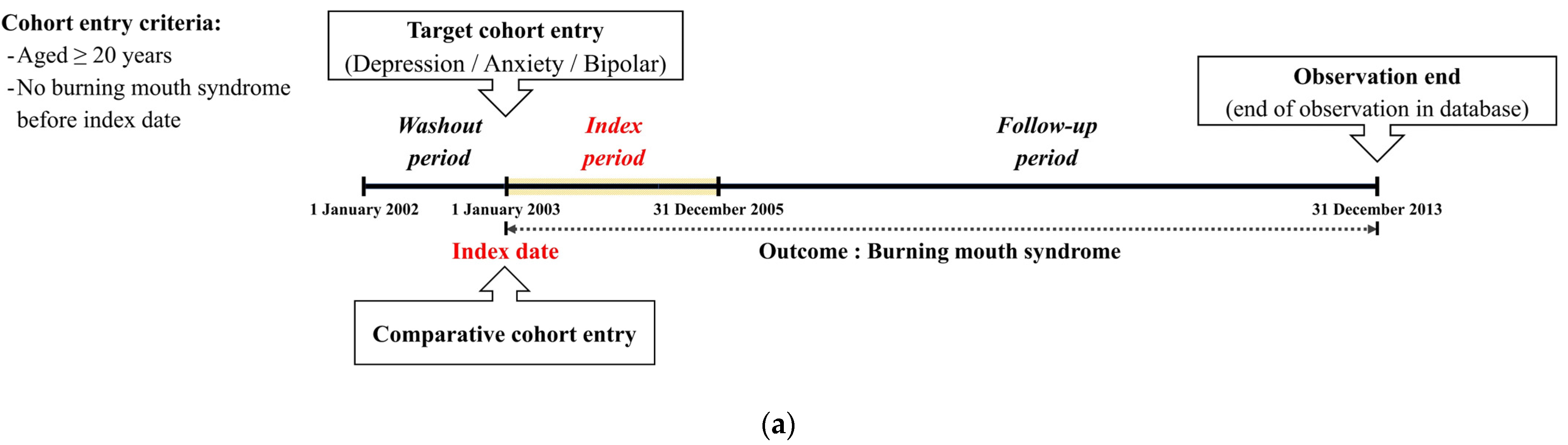
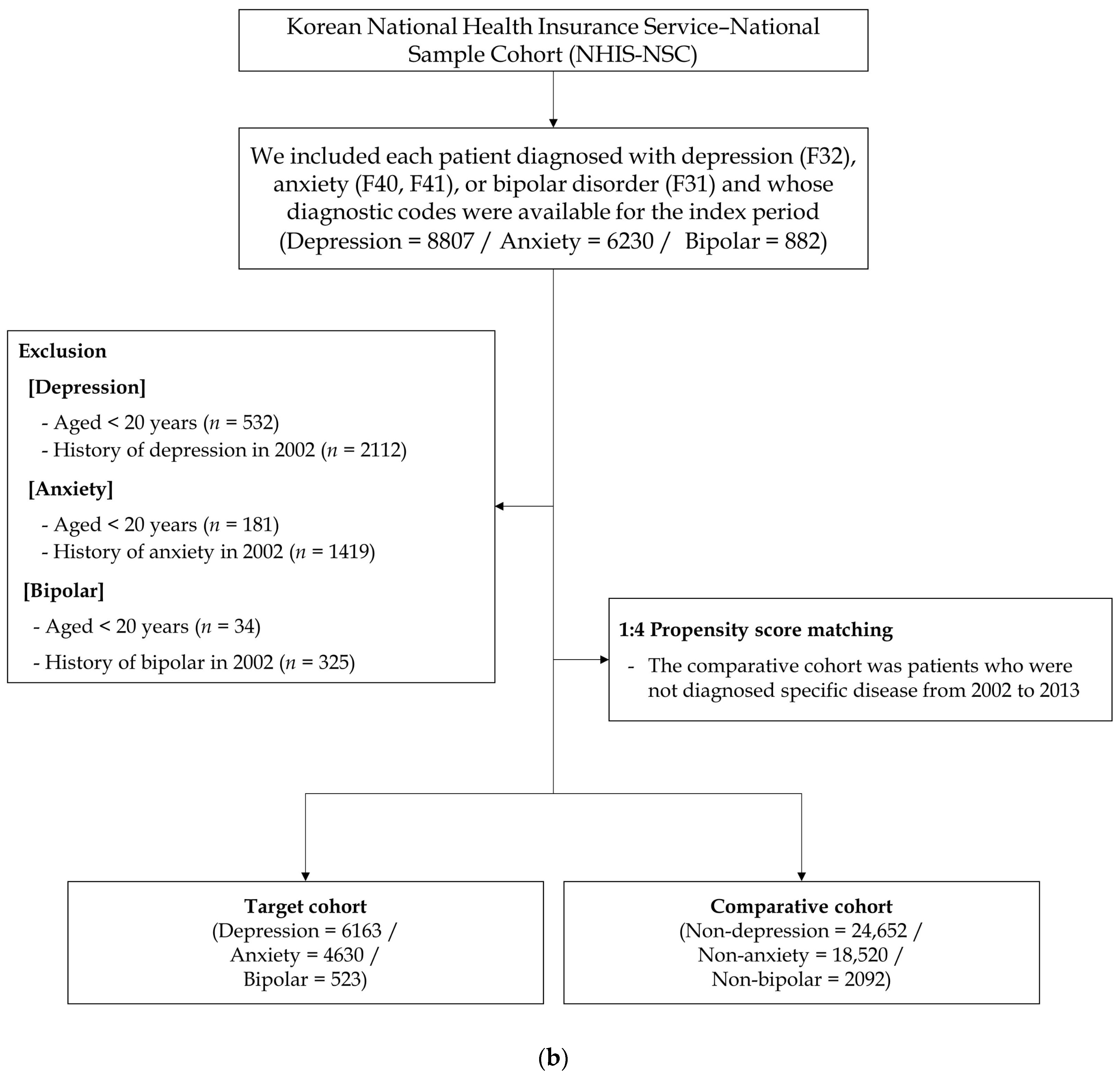
2.1. Study Design and Participant Selection
2.2. Statistical Analysis
3. Results
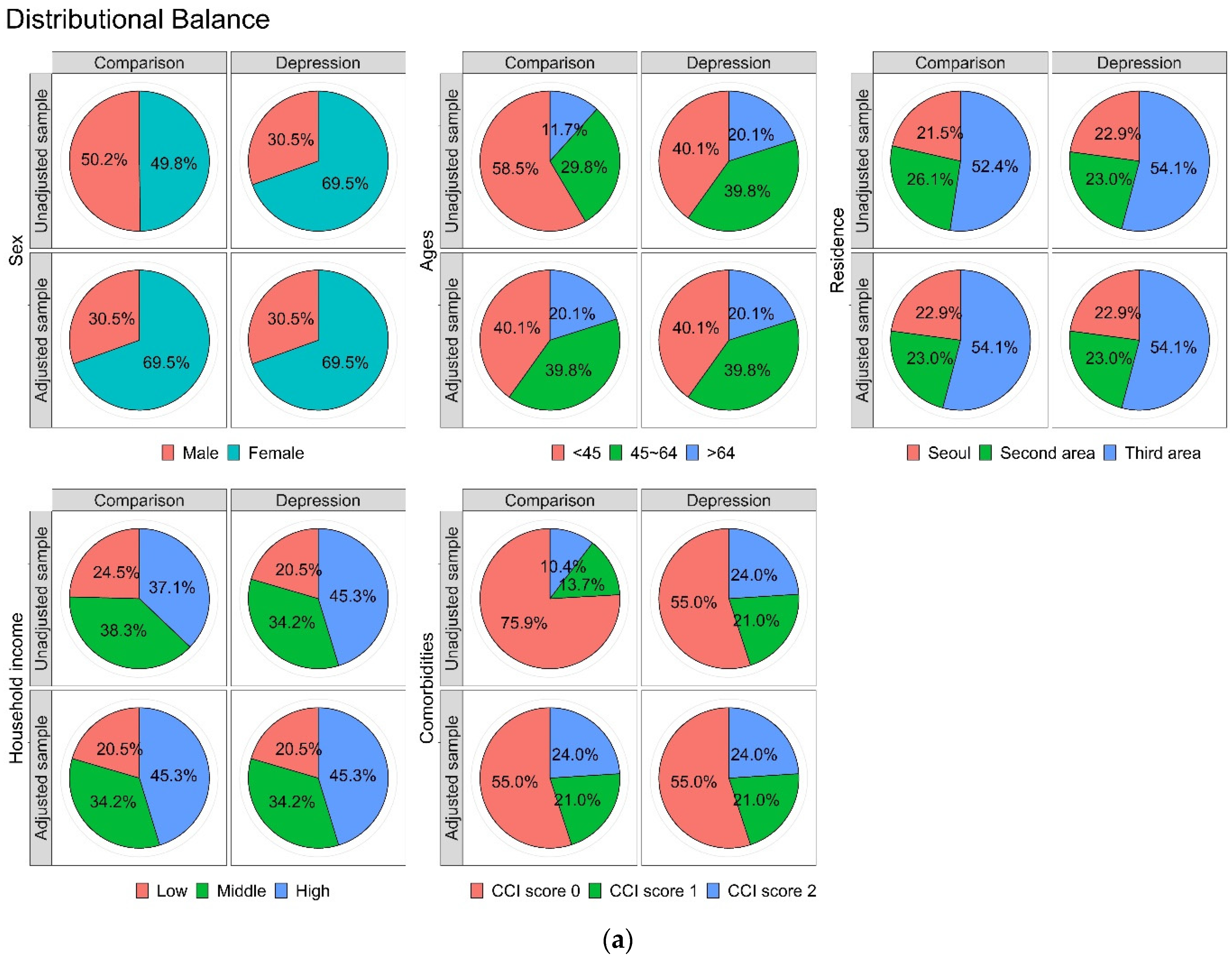
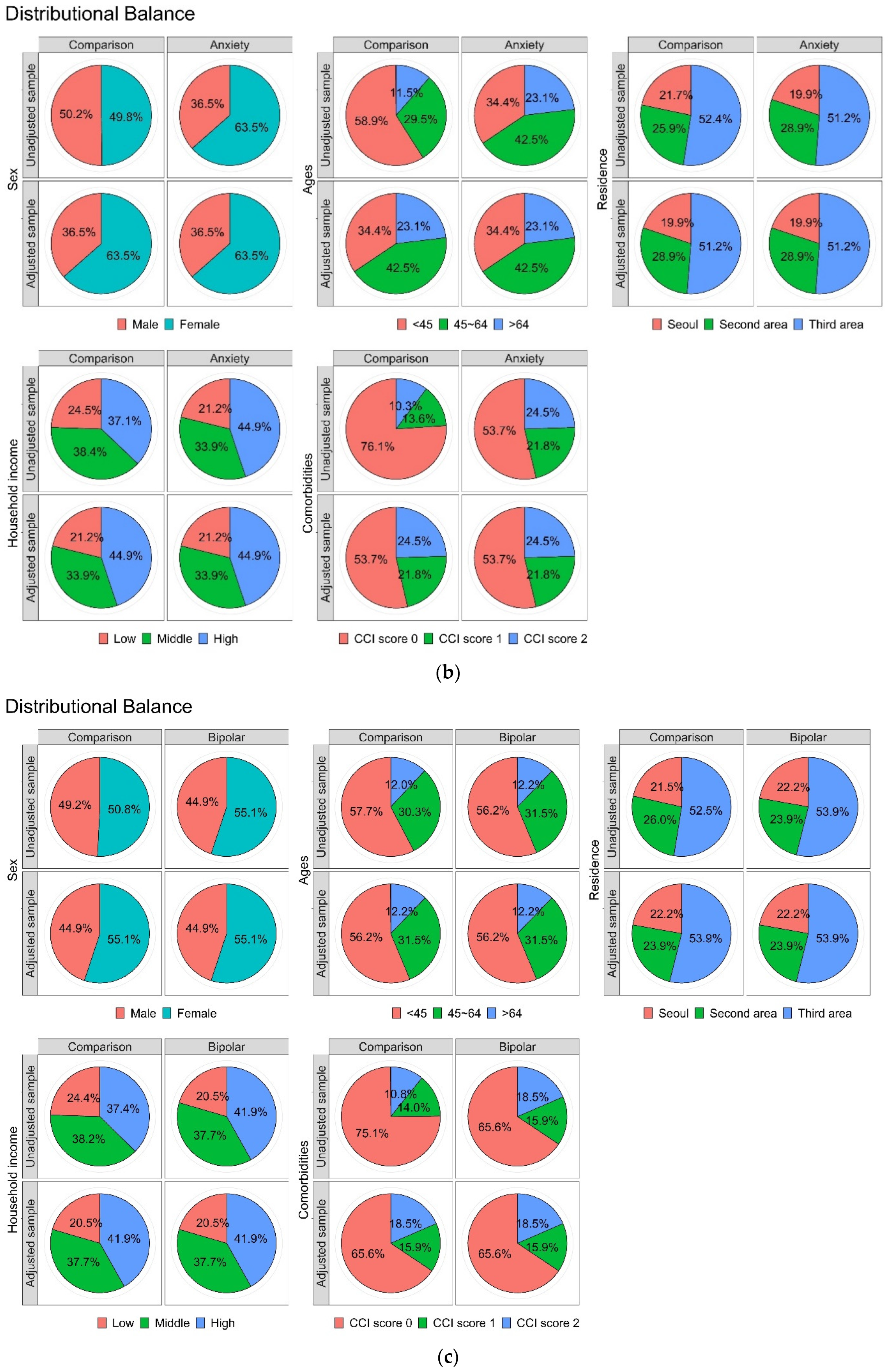
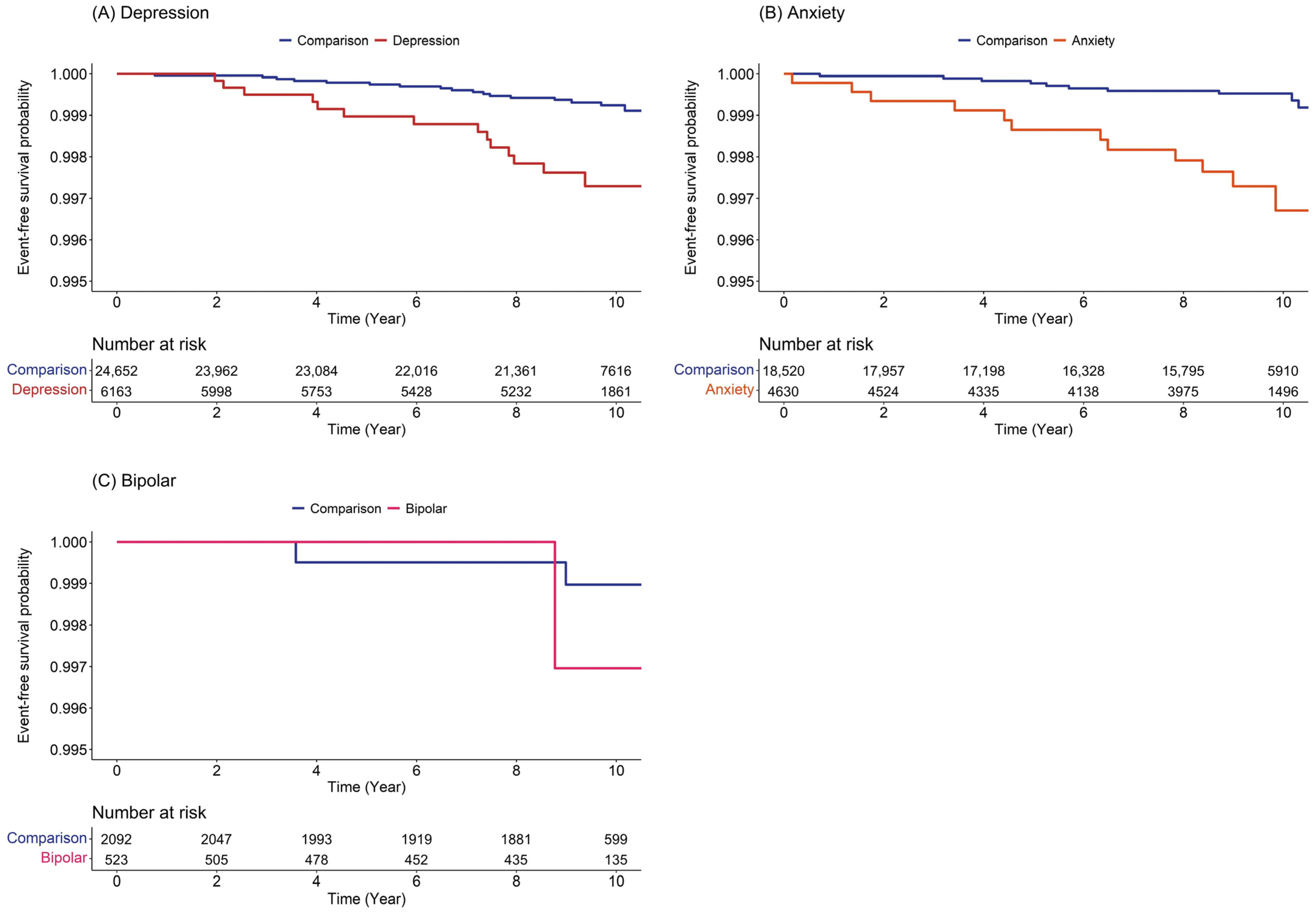
3.1. Cohort Sample Characteristics
3.2. Risk of Subsequent Development of Burning Mouth Syndrome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klasser, G.D.; Fischer, D.J.; Epstein, J.B. Burning mouth syndrome: Recognition, understanding, and management. Oral Maxillofac. Surg. Clin. N. Am. 2008, 20, 255–271. [Google Scholar] [CrossRef] [PubMed]
- Scala, A.; Checchi, L.; Montevecchi, M.; Marini, I.; Giamberardino, M.A. Update on burning mouth syndrome: Overview and patient management. Crit. Rev. Oral Biol. Med. 2003, 14, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Bender, S.D. Burning mouth syndrome. Dent. Clin. N. Am. 2018, 62, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Patton, L.L.; Siegel, M.A.; Benoliel, R.; De Laat, A. Management of burning mouth syndrome: Systematic review and management recommendations. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2007, 103, S39.e1–S39.e13. [Google Scholar] [CrossRef] [PubMed]
- Forssell, H.; Teerijoki-Oksa, T.; Kotiranta, U.; Kantola, R.; Bäck, M.; Vuorjoki-Ranta, T.R.; Siponen, M.; Leino, A.; Puukka, P.; Estlander, A.M. Pain and pain behavior in burning mouth syndrome: A pain diary study. J. Orofac. Pain 2012, 26, 117–125. [Google Scholar] [PubMed]
- Feller, L.; Fourie, J.; Bouckaert, M.; Khammissa, R.A.G.; Ballyram, R.; Lemmer, J. Burning Mouth Syndrome: Aetiopathogenesis and Principles of Management. Pain Res. Manag. 2017, 2017, 1926269. [Google Scholar] [CrossRef]
- Kenchadze, R.; Iverieli, M.; Okribelashvili, N.; Geladze, N.; Khachapuridze, N. The psychological aspects of burning mouth syndrome. Georgian Med. News 2011, 194, 24–28. [Google Scholar]
- de Souza, F.T.; Teixeira, A.L.; Amaral, T.M.; dos Santos, T.P.; Abreu, M.H.; Silva, T.A.; Kummer, A. Psychiatric disorders in burning mouth syndrome. J. Psychosom. Res. 2012, 72, 142–146. [Google Scholar] [CrossRef]
- Carlson, C.R.; Miller, C.S.; Reid, K.I. Psychosocial profiles of patients with burning mouth syndrome. J. Orofac. Pain 2000, 14, 59–64. [Google Scholar]
- Kim, J.Y.; Kim, Y.S.; Ko, I.; Kim, D.K. Association between burning mouth syndrome and the development of depression, anxiety, dementia, and Parkinson disease. JAMA Otolaryngol. Head Neck Surg. 2020, 146, 561–569. [Google Scholar] [CrossRef]
- Malik, R.; Goel, S.; Misra, D.; Panjwani, S.; Misra, A. Assessment of anxiety and depression in patients with burning mouth syndrome: A clinical trial. J. Midlife Health 2012, 3, 36–39. [Google Scholar] [CrossRef]
- Galli, F.; Lodi, G.; Sardella, A.; Vegni, E. Role of psychological factors in burning mouth syndrome: A systematic review and meta-analysis. Cephalalgia 2017, 37, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Forssell, H.; Teerijoki-Oksa, T.; Puukka, P.; Estlander, A.M. Symptom severity in burning mouth syndrome associates with psychological factors. J. Oral Rehabil. 2020, 47, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.V.; Normando, A.G.C.; Rodrigues-Fernandes, C.I.; Rivera, C.; Santos-Silva, A.R.; Lopes, M.A. The impact on quality of life in patients with burning mouth syndrome: A systematic review and meta-analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021, 131, 186–194. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, M.J.; Kho, H.S. Oral Health-related quality of life and associated factors in patients with burning mouth syndrome. J. Oral Rehabil. 2021, 48, 150–159. [Google Scholar] [CrossRef] [PubMed]
- López-Jornet, P.; Camacho-Alonso, F.; Lucero-Berdugo, M. Quality of life in patients with burning mouth syndrome. J. Oral Pathol. Med. 2008, 37, 389–394. [Google Scholar] [CrossRef]
- Rojo, L.; Silvestre, F.J.; Bagan, J.V.; De Vicente, T. Psychiatric morbidity in burning mouth syndrome. Psychiatric interview versus depression and anxiety scales. Oral Surg. Oral Med. Oral Pathol. 1993, 75, 308–311. [Google Scholar] [CrossRef]
- Trikkas, G.; Nikolatou, O.; Samara, C.; Bazopoulou-Kyrkanidou, E.; Rabavilas, A.D.; Christodoulou, G.N. Glossodynia: Personality characteristics and psychopathology. Psychother. Psychosom. 1996, 65, 163–168. [Google Scholar] [CrossRef]
- Yoo, H.S.; Jin, S.H.; Lee, Y.J.; Song, C.M.; Ji, Y.B.; Tae, K. The role of psychological factors in the development of burning mouth syndrome. Int. J. Oral Maxillofac. Surg. 2018, 47, 374–378. [Google Scholar] [CrossRef]
- Maina, G.; Albert, U.; Gandolfo, S.; Vitalucci, A.; Bogetto, F. Personality disorders in patients with burning mouth syndrome. J. Pers. Disord. 2005, 19, 84–93. [Google Scholar] [CrossRef]
- Kim, D.K.; Lee, H.J.; Lee, I.H.; Lee, J.J. Risk of burning mouth syndrome in patients with migraine: A nationwide cohort study. J. Pers. Med. 2022, 12, 620. [Google Scholar] [CrossRef] [PubMed]
- Su, N.Y.; Wang, Y.H.; Chang, Y.C. A nationwide register-based study of the prevalence of burning mouth syndrome in Taiwan from 2004 to 2013. J. Dent. Sci. 2021, 16, 1074–1079. [Google Scholar] [CrossRef]
- Kohorst, J.J.; Bruce, A.J.; Torgerson, R.R.; Schenck, L.A.; Davis, M.D.P. The prevalence of burning mouth syndrome: A population-based study. Br. J. Dermatol. 2015, 172, 1654–1656. [Google Scholar] [CrossRef] [PubMed]
- Kohorst, J.J.; Bruce, A.J.; Torgerson, R.R.; Schenck, L.A.; Davis, M.D. A population-based study of the incidence of burning mouth syndrome. Mayo Clin. Proc. 2014, 89, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Chen, L.; Zhou, J.; Peng, J. A case-control study on etiological factors involved in patients with burning mouth syndrome. J. Oral Pathol. Med. 2009, 38, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Leimola-Virtanen, R.; Salo, T.; Toikkanen, S.; Pulkkinen, J.; Syrjänen, S. Expression of estrogen receptor (ER) in oral mucosa and salivary glands. Maturitas 2000, 36, 131–137. [Google Scholar] [CrossRef]
- Santoro, V.; Caputo, G.; Peluso, F. Clinical and therapeutic experience in twenty eight patients with burning mouth syndrome. Minerva Stomatol. 2005, 54, 489–496. [Google Scholar]
- Son, D.S.; Cho, M.S.; Kim, D.K. Chronic rhinosinusitis and the increased incidence of atopic dermatitis. Am. J. Rhinol. Allergy 2022, 36, 574–582. [Google Scholar] [CrossRef]
- Kwon, Y.S.; Lee, S.H.; Kim, C.; Yu, H.; Sohn, J.H.; Lee, J.J.; Kim, D.K. Risk of dementia according to surgery type: A nationwide cohort study. J. Pers. Med. 2022, 12, 468. [Google Scholar] [CrossRef]
- Lee, I.H.; Ha, S.S.; Son, G.M.; Yang, H.G.; Kim, D.K. Could chronic rhinosinusitis increase the risk of ulcerative colitis? A nationwide cohort study. Diagnostics 2022, 12, 2344. [Google Scholar] [CrossRef]
- Son, D.S.; Cho, M.S.; Kim, D.K. Chronic rhinosinusitis could increase the risk of cholesteatoma of middle ear. Int. Forum Allergy Rhinol. 2022, 13, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.H.; Yu, H.; Ha, S.S.; Son, G.M.; Park, K.J.; Lee, J.J.; Kim, D.K. Association between late-onset Meniere’s disease and the risk of incident all-cause dementia. J. Pers. Med. 2021, 12, 19. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lee, S.; Cha, J.; Son, G.; Kim, D.K. Chronic kidney disease is associated with increased risk of sudden sensorineural hearing loss and Meniere’s disease: A nationwide cohort study. Sci. Rep. 2021, 11, 20194. [Google Scholar] [CrossRef] [PubMed]
| BMS Event (Depression) | BMS Event (Anxiety) | BMS Event (Bipolar) | |
|---|---|---|---|
| Event | 33 | 22 | 3 |
| Comparison | 19 | 10 | 2 |
| Depression, anxiety, or bipolar | 14 | 12 | 1 |
| Total censored (no event) | 30,782 | 23,128 | 2612 |
| Comparison | 24,633 | 18,510 | 2090 |
| Depression, anxiety, or bipolar | 6149 | 4618 | 522 |
| Termination of study | 26,025 | 19,298 | 2281 |
| Comparison | 20,916 | 15,402 | 1858 |
| Depression, anxiety, or bipolar | 5109 | 3896 | 423 |
| Loss to follow-up/drop-out | 4757 | 3830 | 331 |
| Comparison | 3717 | 3108 | 232 |
| Depression, anxiety, or bipolar | 1040 | 722 | 99 |
| Variables | Comparison (n = 24,652) | Depression (n = 6163) | p Value |
|---|---|---|---|
| Sex | 1.000 | ||
| Male | 7524 (30.5%) | 1881 (30.5%) | |
| Female | 17,128 (69.5%) | 4282 (69.5%) | |
| Ages (years) | 1.000 | ||
| <45 | 9896 (40.1%) | 2474 (40.1%) | |
| 45–64 | 9804 (39.8%) | 2451 (39.8%) | |
| >64 | 4952 (20.1%) | 1238 (20.1%) | |
| Residence | 1.000 | ||
| Seoul | 5648 (22.9%) | 1412 (22.9%) | |
| Second area | 5676 (23.0%) | 1419 (23.0%) | |
| Third area | 13,328 (54.1%) | 3332 (54.1%) | |
| Household income | 1.000 | ||
| Low (0–30%) | 5060 (20.5%) | 1265 (20.5%) | |
| Middle (30–70%) | 8420 (34.2%) | 2105 (34.2%) | |
| High (70–100%) | 11,172 (45.3%) | 2793 (45.3%) | |
| CCI | 1.000 | ||
| 0 | 13,568 (55.0%) | 3392 (55.0%) | |
| 1 | 5168 (21.0%) | 1292 (21.0%) | |
| ≥2 | 5916 (24.0%) | 1479 (24.0%) |
| Variables | Comparison (n = 18,520) | Anxiety (n = 4630) | p Value |
|---|---|---|---|
| Sex | 1.000 | ||
| Male | 6756 (36.5%) | 1689 (36.5%) | |
| Female | 11,764 (63.5%) | 2941 (63.5%) | |
| Ages (years) | 1.000 | ||
| <45 | 6372 (34.4%) | 1593 (34.4%) | |
| 45–64 | 7872 (42.5%) | 1968 (42.5%) | |
| >64 | 4276 (23.1%) | 1069 (23.1%) | |
| Residence | 1.000 | ||
| Seoul | 3680 (19.9%) | 920 (19.9%) | |
| Second area | 5360 (28.9%) | 1340 (28.9%) | |
| Third area | 9480 (51.2%) | 2370 (51.2%) | |
| Household income | 1.000 | ||
| Low (0–30%) | 3924 (21.2%) | 981 (21.2%) | |
| Middle (30–70%) | 6284 (33.9%) | 1571 (33.9%) | |
| High (70–100%) | 8312 (44.9%) | 2078 (44.9%) | |
| CCI | 1.000 | ||
| 0 | 9952 (53.7%) | 2488 (53.7%) | |
| 1 | 4032 (21.8%) | 1008 (21.8%) | |
| ≥2 | 4536 (24.5%) | 1134 (24.5%) |
| Variables | Comparison (n = 2092) | Bipolar (n = 523) | p Value |
|---|---|---|---|
| Sex | 1.000 | ||
| Male | 940 (44.9%) | 235 (44.9%) | |
| Female | 1152 (55.1%) | 288 (55.1%) | |
| Ages (years) | 1.000 | ||
| <45 | 1176 (56.2%) | 294 (56.2%) | |
| 45–64 | 660 (31.5%) | 165 (31.5%) | |
| >64 | 256 (12.2%) | 64 (12.2%) | |
| Residence | 1.000 | ||
| Seoul | 464 (22.2%) | 116 (22.2%) | |
| Second area | 500 (23.9%) | 125 (23.9%) | |
| Third area | 1128 (53.9%) | 282 (53.9%) | |
| Household income | 1.000 | ||
| Low (0–30%) | 428 (20.5%) | 107 (20.5%) | |
| Middle (30–70%) | 788 (37.7%) | 197 (37.7%) | |
| High (70–100%) | 876 (41.9%) | 219 (41.9%) | |
| CCI | 1.000 | ||
| 0 | 1372 (65.6%) | 343 (65.6%) | |
| 1 | 332 (15.9%) | 83 (15.9%) | |
| ≥2 | 388 (18.5%) | 97 (18.5%) |
| Variables | N | Case | Person-Years | Incidence Rate | Unadjusted HR (95% CI) | Adjusted HR (95% CI) |
|---|---|---|---|---|---|---|
| Comparison | 24,652 | 19 | 228,284.5 | 0.08 | 1.00 (ref) | 1.00 (ref) |
| Depression | 6163 | 14 | 54,005.2 | 0.26 | 3.40 (1.69–6.87) *** | 3.37 (1.67–6.80) *** |
| Comparison | 18,520 | 10 | 170,302.5 | 0.06 | 1.00 (ref) | 1.00 (ref) |
| Anxiety | 4630 | 12 | 41,005.8 | 0.29 | 5.14 (2.22–11.91) *** | 5.09 (2.19–11.80) *** |
| Comparison | 2092 | 2 | 19,637.2 | 0.10 | 1.00 (ref) | 1.00 (ref) |
| Bipolar | 523 | 1 | 4488.6 | 0.22 | 2.66 (0.24–29.67) | 3.17 (0.27–37.21) |
| Sex | Male | Female | ||
|---|---|---|---|---|
| Comparison | Depression | Comparison | Depression | |
| Depression | ||||
| Unadjusted HR (95% CI) | 1.00 (ref) | 3.05 (0.70–13.33) | 1.00 (ref) | 3.50 (1.57–7.80) ** |
| Adjusted HR (95% CI) | 1.00 (ref) | 3.08 (0.71–13.46) | 1.00 (ref) | 3.47 (1.5–67.72) ** |
| Anxiety | ||||
| Unadjusted HR (95% CI) | 1.00 (ref) | 7.92 (0.72–87.35) | 1.00 (ref) | 4.80 (1.95–11.85) *** |
| Adjusted HR (95% CI) | 1.00 (ref) | 7.71 (0.70–85.11) | 1.00 (ref) | 4.74 (1.92–11.69) *** |
| Time (Year) after Diagnosis | Depression | Anxiety |
|---|---|---|
| Adjusted HR (95% CI) | Adjusted HR (95% CI) | |
| 1 | 0.00 (0–Inf) | 3.96 (0.25–63.28) |
| 2 | 4.02 (0.25–64.26) | 11.85 (1.23–113.91) * |
| 3 | 5.97 (1.00–35.75) | 11.85 (1.23–113.91) * |
| 4 | 3.96 (0.99–15.82) | 5.34 (1.19–23.86) * |
| 5 | 4.78 (1.46–15.67) ** | 6.00 (1.69–21.27) ** |
| 6 | 3.98 (1.40–11.34) ** | 3.97 (1.28–12.32) * |
| 7 | 3.10 (1.15–8.32) * | 4.54 (1.65–12.53) ** |
| 8 | 3.72 (1.70–8.15) ** | 5.11 (1.90–13.72) ** |
| 9 | 3.80 (1.79–8.09) *** | 5.62 (2.26–13.97) *** |
| 10 | 3.64 (1.78–7.47) *** | 6.26 (2.55–15.33) *** |
| 11 | 3.37 (1.67–6.80) *** | 5.09 (2.19–11.80) *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.J.; Kim, C.; Yu, H.; Kim, D.-K. Relationship of Depression, Anxiety, and Bipolar Disease with Burning Mouth Syndrome: A Nationwide Cohort Study. Int. J. Environ. Res. Public Health 2023, 20, 3391. https://doi.org/10.3390/ijerph20043391
Lee SJ, Kim C, Yu H, Kim D-K. Relationship of Depression, Anxiety, and Bipolar Disease with Burning Mouth Syndrome: A Nationwide Cohort Study. International Journal of Environmental Research and Public Health. 2023; 20(4):3391. https://doi.org/10.3390/ijerph20043391
Chicago/Turabian StyleLee, Su Jung, Chulho Kim, Hyunjae Yu, and Dong-Kyu Kim. 2023. "Relationship of Depression, Anxiety, and Bipolar Disease with Burning Mouth Syndrome: A Nationwide Cohort Study" International Journal of Environmental Research and Public Health 20, no. 4: 3391. https://doi.org/10.3390/ijerph20043391
APA StyleLee, S. J., Kim, C., Yu, H., & Kim, D.-K. (2023). Relationship of Depression, Anxiety, and Bipolar Disease with Burning Mouth Syndrome: A Nationwide Cohort Study. International Journal of Environmental Research and Public Health, 20(4), 3391. https://doi.org/10.3390/ijerph20043391





