The Role of Web-Based Adaptive Choice-Based Conjoint Analysis Technology in Eliciting Patients’ Preferences for Osteoarthritis Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Population, Setting, and Study Design
2.2. The ACBC Questionnaire Development
2.3. Data Collection
2.4. Data Analysis
3. Results
3.1. Participants’ Characteristics
3.2. Participants’ Feedback about the ACBC Questionnaire
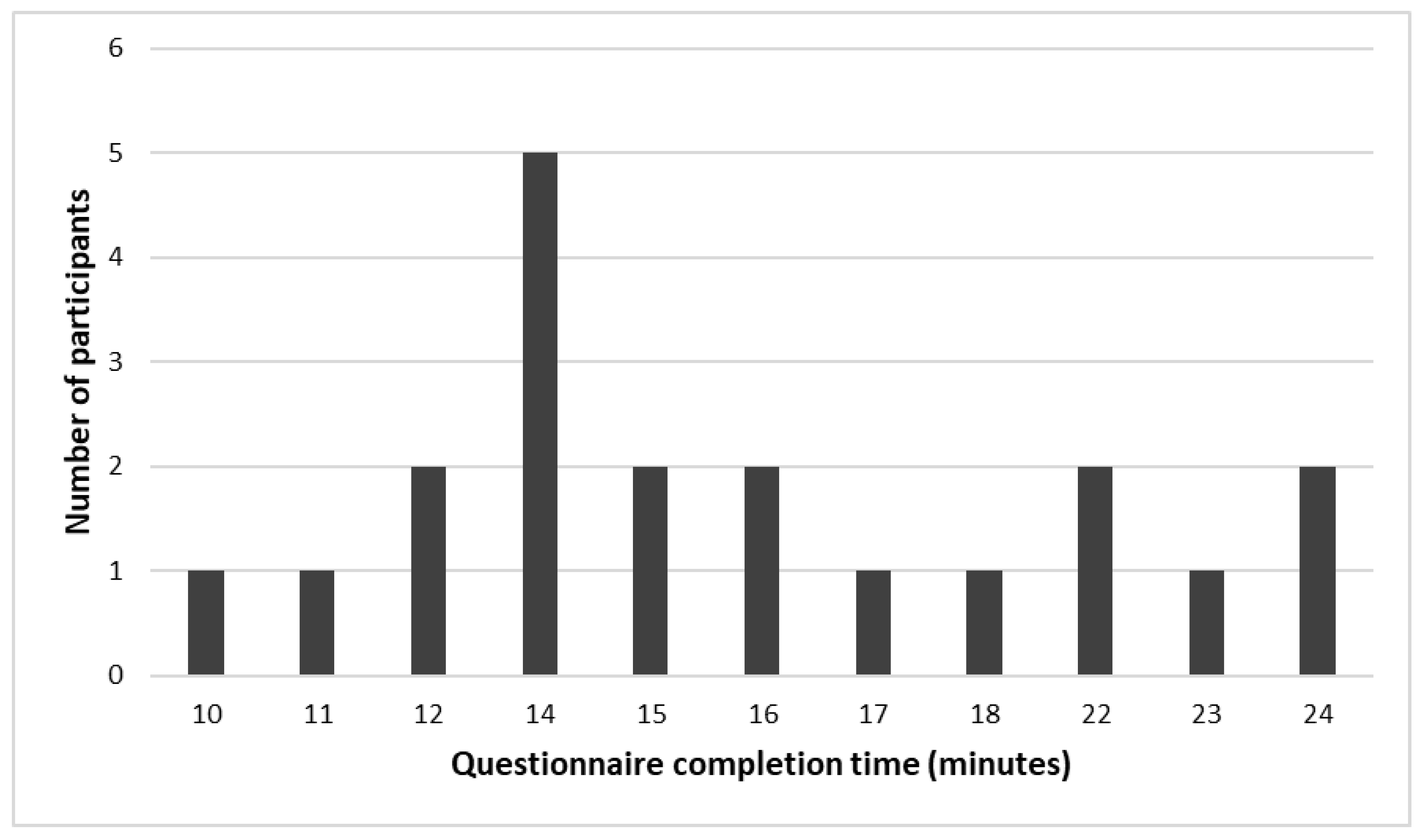
3.3. Questionnaire Completion Time
3.4. Factor Associated with the Questionnaire Completion Time
4. Discussion
4.1. Strengths and Limitations
4.2. Clinical and Research Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Attribute | Levels |
|---|---|
| Availability | Prescription drug |
| Over-the-counter drug | |
| Internet purchase drug | |
| Way of taking the medication | Cream/Gel |
| Oral | |
| Frequency | Once a day |
| Twice a day | |
| 3–4 times a day | |
| As needed | |
| How much you would expect mobility improvement | Expect 25% mobility improvement |
| Expect 50% mobility improvement | |
| Expect 75% mobility improvement | |
| How much you would expect pain reduction | Expect 25% pain reduction |
| Expect 50% pain reduction | |
| Expect 75% pain reduction | |
| Risk of gastric ulcer | No risk of gastric ulcer |
| Low risk of gastric ulcer | |
| Moderate risk of gastric ulcer | |
| High risk of gastric ulcer | |
| Risk of addiction | No risk of addiction |
| Low risk of addiction | |
| Moderate risk of addiction | |
| High risk of addiction | |
| Risk of kidney and liver impairment | No risk of kidney and liver impairment |
| Low risk of kidney and liver impairment | |
| Moderate risk of kidney and liver impairment | |
| High risk of kidney and liver impairment | |
| Risk of heart attacks and strokes | No risk of heart attacks and strokes |
| Low risk of heart attacks and strokes | |
| Moderate risk of heart attacks and strokes | |
| High risk of heart attacks and strokes |
Appendix B
| Processors | Quad Core Processor (or equivalent) |
| Operating System | Windows Server 2012 R2 Standard 64-bit |
| Network Speed | Higher than 100 Mbps |
| Network LAN and IP Address: | Provided by Server and Storage |
| Port Assignments | Defaults—Assigned by the relevant specialist |
| Installed Memory (RAM) | 8.00 GB |
| Hard Disk | 100 GB (System) 100 GB (Apps) |
| Disk Partitions—Drive Mappings | Defaults—For Applications |
| Additional software | IIS and PERL |

References
- NICE Overview|Osteoarthritis: Care and Management|Guidance|NICE. Available online: https://www.nice.org.uk/guidance/cg177 (accessed on 7 November 2020).
- Li, A.; Zhang, Y.; Lao, L.; Xin, J.; Ren, K.; Berman, B.M.; Zhang, R.-X. Serotonin receptor 2A/C is involved in electroacupuncture inhibition of pain in an osteoarthritis rat model. Evid. Based Complement. Alternat. Med. 2011, 2011, 619650. [Google Scholar] [CrossRef] [PubMed]
- Al-Omari, B.; Hill, B. Nursing people with osteoarthritis. Br. J. Nurs. 2020, 29, 1060–1063. [Google Scholar] [CrossRef] [PubMed]
- Koeberle, A.; Werz, O. Inhibitors of the microsomal prostaglandin E(2) synthase-1 as alternative to non steroidal anti-inflammatory drugs (NSAIDs)—A critical review. Curr. Med. Chem. 2009, 16, 4274–4296. [Google Scholar] [CrossRef] [PubMed]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef] [PubMed]
- Department of Health and Social Care. Action on Medicine Wastage and Improving Medicine Use. Available online: https://www.gov.uk/government/news/action-on-medicine-wastage-and-improving-medicine-use (accessed on 28 January 2023).
- Fraenkel, L.; Bogardus, S.T.; Concato, J.; Wittink, D.R. Treatment options in knee osteoarthritis: The patient’s perspective. Arch. Intern. Med. 2004, 164, 1299–1304. [Google Scholar] [CrossRef]
- Hiligsmann, M.; Pinto, D.; Dennison, E.; Al-Daghri, N.; Beaudart, C.; Branco, J.; Bruyère, O.; Conaghan, P.G.; Cooper, C.; Herrero-Beaumont, G.; et al. Patients’ preferences for osteoarthritis treatment: The value of stated-preference studies. Aging Clin. Exp. Res. 2019, 31, 1–3. [Google Scholar] [CrossRef]
- Say, R.E.; Thomson, R. The importance of patient preferences in treatment decisions―Challenges for doctors. BMJ 2003, 327, 542–545. [Google Scholar] [CrossRef]
- Saultz, J.W.; Albedaiwi, W. Interpersonal continuity of care and patient satisfaction: A critical review. Ann. Fam. Med. 2004, 2, 445–451. [Google Scholar] [CrossRef]
- Laba, T.-L.; Brien, J.; Fransen, M.; Jan, S. Patient preferences for adherence to treatment for osteoarthritis: The MEdication Decisions in Osteoarthritis Study (MEDOS). BMC Musculoskelet. Disord. 2013, 14, 160. [Google Scholar] [CrossRef] [PubMed]
- Hauber, A.B.; Arden, N.K.; Mohamed, A.F.; Johnson, F.R.; Peloso, P.M.; Watson, D.J.; Mavros, P.; Gammaitoni, A.; Sen, S.S.; Taylor, S.D. A discrete-choice experiment of United Kingdom patients’ willingness to risk adverse events for improved function and pain control in osteoarthritis. Osteoarthr. Cartil. 2013, 21, 289–297. [Google Scholar] [CrossRef]
- Wong, D.W.; Fong, C.; Da Silva, C.E.; Lam, C.S.; Miller, S.M. Rehabilitation Counseling Students’ Attitudes Toward People with Disabilities in Three Social Contexts. Rehabil. Couns. Bull. 2004, 47, 194–204. [Google Scholar] [CrossRef]
- Lamiraud, K.; Geoffard, P.-Y. Therapeutic non-adherence: A rational behavior revealing patient preferences? Health Econ. 2007, 16, 1185–1204. [Google Scholar] [CrossRef] [PubMed]
- Al-Omari, B.; Farhat, J.; Ershaid, M. Conjoint analysis: A research method to study patients’ preferences and personalize care. J. Pers. Med. 2022, 12, 274. [Google Scholar] [CrossRef] [PubMed]
- Al-Omari, B.; McMeekin, P.; Bate, A. Systematic review of studies using conjoint analysis techniques to investigate patients’ preferences regarding osteoarthritis treatment. Patient Prefer. Adherence 2021, 15, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.M. Trade-Off Analysis of Consumer Values. J. Mark. Res. 1974, 11, 121–127. [Google Scholar] [CrossRef]
- Byrne, M.M.; Souchek, J.; Richardson, M.; Suarez-Almazor, M. Racial/ethnic differences in preferences for total knee replacement surgery. J. Clin. Epidemiol. 2006, 59, 1078–1086. [Google Scholar] [CrossRef]
- Rao, V.R. Applied Conjoint Analysis; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 978-3-540-87752-3. [Google Scholar]
- Cunningham, C.E.; Deal, K.; Chen, Y. Adaptive choice-based conjoint analysis: A new patient-centered approach to the assessment of health service preferences. Patient 2010, 3, 257–273. [Google Scholar] [CrossRef]
- Jervis, S.M.; Ennis, J.M.; Drake, M.A. A Comparison of Adaptive Choice-Based Conjoint and Choice-Based Conjoint to Determine Key Choice Attributes of Sour Cream with Limited Sample Size. J. Sens. Stud. 2012, 27, 451–462. [Google Scholar] [CrossRef]
- Gensler, S.; Hinz, O.; Skiera, B.; Theysohn, S. Willingness-to-pay estimation with choice-based conjoint analysis: Addressing extreme response behavior with individually adapted designs. Eur. J. Oper. Res. 2012, 219, 368–378. [Google Scholar] [CrossRef]
- Eggers, F.; Sattler, H. Preference Measurement with Conjoint Analysis. Overview of State-of-the-Art Approaches and Recent Developments. GfK Mark. Intell. Rev. 2011, 3, 36–47. [Google Scholar] [CrossRef]
- Brand, B.M.; Baier, D. Adaptive CBC: Are the Benefits Justifying its Additional Efforts Compared to CBC? Arch. Data Sci. Ser. A 2020, 6, 1–22. [Google Scholar] [CrossRef]
- Pieterse, A.H.; Berkers, F.; Baas-Thijssen, M.C.M.; Marijnen, C.A.M.; Stiggelbout, A.M. Adaptive Conjoint Analysis as individual preference assessment tool: Feasibility through the internet and reliability of preferences. Patient Educ. Couns. 2010, 78, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Beusterien, K.M.; Dziekan, K.; Schrader, S.; Flood, E.; Flood, R.; Shearer, A.; Davis, E.A. Patient preferences among third agent HIV medications: A US and German perspective. AIDS Care 2007, 19, 982–988. [Google Scholar] [CrossRef] [PubMed]
- Fraenkel, L.; Suter, L.; Cunningham, C.E.; Hawker, G. Understanding preferences for disease-modifying drugs in osteoarthritis. Arthritis Care Res. 2014, 66, 1186–1192. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Goos, P.; Vandebroek, M. Individually adapted sequential Bayesian conjoint-choice designs in the presence of consumer heterogeneity. Int. J. Res. Mark. 2011, 28, 378–388. [Google Scholar] [CrossRef]
- Gustafsson, A.; Herrmann, A.; Huber, F. Conjoint Measurement; Springer: Berlin/Heidelberg, Germany, 2000; ISBN 978-3-662-06395-8. [Google Scholar]
- Shin, H.-S.; Farkas, Z.A.; Nickkar, A. An analysis of attributes of electric vehicle owners’ travel and purchasing behavior: The case of maryland. In Proceedings of the International Conference on Transportation and Development 2019, Alexandria, VA, USA, 9–12 June 2019; Noyce, D.A., Ed.; American Society of Civil Engineers: Reston, VA, USA, 2019; pp. 77–90. [Google Scholar]
- Sichtmann, C.; Wilken, R.; Diamantopoulos, A. Estimating Willingness-to-pay with Choice-based Conjoint Analysis-Can Consumer Characteristics Explain Variations in Accuracy? Br. J. Manag. 2011, 22, 628–645. [Google Scholar] [CrossRef]
- Cunningham, C.E.; Chen, Y.; Vaillancourt, T.; Rimas, H.; Deal, K.; Cunningham, L.J.; Ratcliffe, J. Modeling the anti-cyberbullying preferences of university students: Adaptive choice-based conjoint analysis. Aggress. Behav. 2015, 41, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Veitch, J.; Ball, K.; Rivera, E.; Loh, V.; Deforche, B.; Timperio, A. Understanding children’s preference for park features that encourage physical activity: An adaptive choice based conjoint analysis. Int. J. Behav. Nutr. Phys. Act. 2021, 18, 133. [Google Scholar] [CrossRef]
- Louviere, J.J.; Street, D.; Burgess, L.; Wasi, N.; Islam, T.; Marley, A.A.J. Modeling the choices of individual decision-makers by combining efficient choice experiment designs with extra preference information. J. Choice Model. 2008, 1, 128–164. [Google Scholar] [CrossRef]
- de Groot, I.B.; Otten, W.; Smeets, H.J.; Marang-van de Mheen, P.J. CHOICE-2 study group Is the impact of hospital performance data greater in patients who have compared hospitals? BMC Health Serv. Res. 2011, 11, 214. [Google Scholar] [CrossRef]
- Reinisch, M.; Marschner, N.; Otto, T.; Korfel, A.; Stoffregen, C.; Wöckel, A. Patient Preferences: Results of a German Adaptive Choice-Based Conjoint Analysis (Market Research Study Sponsored by Eli Lilly and Company) in Patients on Palliative Treatment for Advanced Breast Cancer. Breast Care 2021, 16, 491–499. [Google Scholar] [CrossRef]
- Al-Omari, B.; McMeekin, P. Patients’ Preferences Regarding Osteoarthritis Medications: An Adaptive Choice-Based Conjoint Analysis Study. Patient Prefer. Adherence 2020, 14, 2501–2515. [Google Scholar] [CrossRef] [PubMed]
- Orme, B. Fine-Tuning CBC and Adaptive CBC Questionnaires. 2009. Available online: https://sawtoothsoftware.com/resources/technical-papers/fine-tuning-cbc-and-adaptive-cbc-questionnaires (accessed on 14 January 2022).
- Webb, E.J.D.; Meads, D.; Eskyte, I.; King, N.; Dracup, N.; Chataway, J.; Ford, H.L.; Marti, J.; Pavitt, S.H.; Schmierer, K.; et al. A Systematic Review of Discrete-Choice Experiments and Conjoint Analysis Studies in People with Multiple Sclerosis. Patient 2018, 11, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Al-Omari, B.; Sim, J.; Croft, P.; Frisher, M. Patient preferences for the pharmacological treatment of osteoarthritis: A feasibility study using adaptive choice-based conjoint analysis (acbca). Eur. J. Pers. Cent. Healthc. 2015, 3, 253. [Google Scholar] [CrossRef]
- About Us-Healthwatch Newcastle. Available online: https://www.healthwatchnewcastle.org.uk/about-us/ (accessed on 7 November 2020).
- Health and Social Care Act 2012. Available online: https://www.legislation.gov.uk/ukpga/2012/7/contents/enacted (accessed on 7 November 2020).
- Al-Omari, B. Patient preferences for the pharmacological treatment of osteoarthritis using adaptive choice-based conjoint (ACBC) analysis: A pilot study. Eur. J. Pers. Cent. Healthc. 2017, 5, 220. [Google Scholar] [CrossRef]
- Al-Omari, B.; Sim, J.; Croft, P.; Frisher, M. Generating Individual Patient Preferences for the Treatment of Osteoarthritis Using Adaptive Choice-Based Conjoint (ACBC) Analysis. Rheumatol. Ther. 2017, 4, 167–182. [Google Scholar] [CrossRef]
- Hsu, H.; Siwiec, R.M. Knee Osteoarthritis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Liu, C.; Du, Z.; Zhou, Q.; Hu, B.; Li, Z.; Yu, L.; Xu, T.; Fan, X.; Yang, J.; Li, J. Microscopic examination of intracellular organisms in bronchoalveolar lavage fluid for the diagnosis of ventilator-associated pneumonia: A prospective multi-center study. Chin. Med. J. 2014, 127, 1808–1813. [Google Scholar]
- Song, H.J.; Dennis, S.; Levesque, J.-F.; Harris, M.F. What do consumers with chronic conditions expect from their interactions with general practitioners? A qualitative study of Australian consumer and provider perspectives. Health Expect. 2020, 23, 707–716. [Google Scholar] [CrossRef]
- de Wit, M.P.T.; Elberse, J.E.; Broerse, J.E.W.; Abma, T.A. Do not forget the professional--the value of the FIRST model for guiding the structural involvement of patients in rheumatology research. Health Expect. 2015, 18, 489–503. [Google Scholar] [CrossRef]
- Bailey, C.M. New Adaptive Choice-Based Conjoint Technique Shows Promise|GreenBook|GreenBook.org. Available online: https://www.greenbook.org/marketing-research/choice-based-conjoint-technique (accessed on 14 January 2022).
- ACBC Technical Paper. 2014. Available online: https://www.sawtoothsoftware.com/support/technical-papers/adaptive-cbc-papers/acbc-technical-paper-2009 (accessed on 7 November 2020).
- Rochon, D.; Eberth, J.M.; Fraenkel, L.; Volk, R.J.; Whitney, S.N. Elderly patients’ experiences using adaptive conjoint analysis software as a decision aid for osteoarthritis of the knee. Health Expect. 2014, 17, 840–851. [Google Scholar] [CrossRef]
- Stacey, D.; Hawker, G.; Dervin, G.; Tomek, I.; Cochran, N.; Tugwell, P.; O’Connor, A.M. Management of Chronic Pain: Improving shared decision making in osteoarthritis. BMJ 2008, 336, 954–955. [Google Scholar] [CrossRef] [PubMed]
- Austin, C.A.; Mohottige, D.; Sudore, R.L.; Smith, A.K.; Hanson, L.C. Tools to promote shared decision making in serious illness: A systematic review. JAMA Intern. Med. 2015, 175, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
| Variable | Number (%) |
|---|---|
| Age group (years) | |
| 40–59 | 5 (25) |
| 60–79 | 10 (50) |
| >79 | 5 (25) |
| Gender | |
| Female | 13 (65) |
| Male | 7 (35) |
| Years suffering from OA | |
| <5 | 3 (15) |
| 5–10 | 6 (30) |
| >10 | 10 (50) |
| Do not know | 1 (5) |
| Pain interference with normal life | |
| Not at all | 1 (5) |
| A little bit | 4 (20) |
| Moderately | 7 (35) |
| Quite a bit | 6 (30) |
| Extremely | 2 (10) |
| Site of OA * | |
| Hip | 7 (35) |
| Knee | 15 (75) |
| Back and Neck | 3 (15) |
| Foot or hand | 1 (5) |
| Other | 2 (10) |
| Medication * | |
| Paracetamol | 18 (90) |
| Capsaicin | 0 (0) |
| Glucosamine | 8 (40) |
| Diclofenac | 9 (45) |
| Etodolac | 2 (10) |
| Ibuprofen | 10 (50) |
| Meloxicam | 0 (0) |
| Naproxen | 1 (5) |
| Codeine or Dihydrocodeine | 11 (55) |
| Tramadol | 6 (30) |
| Fentanyl | 0 (0) |
| Morphine | 2 (10) |
| Oxycodone | 2 (10) |
| Others | 3 (15) |
| Computer usage per week | |
| Every day | 7 (35) |
| A few times every week | 4 (20) |
| Very rarely | 4 (20) |
| Do not use a PC | 5 (25) |
| Completion of a computerized questionnaire in the past | |
| Yes | 12 (60) |
| No | 8 (40) |
| Completion of pen and paper questionnaire in the past | |
| Yes | 14 (70) |
| No | 6 (30) |
| Feedback Statement | Frequency | ||||
|---|---|---|---|---|---|
| Strongly Agree | Agree | Neither Agree Nor Disagree | Disagree | Strongly Disagree | |
| I found the questionnaire easy to read | 15 | 5 | 0 | 0 | 0 |
| I found the questionnaire easy to understand | 14 | 6 | 0 | 0 | 0 |
| I felt that the questionnaire was adjusting the questions according to my previous answers | 14 | 6 | 0 | 0 | 0 |
| I enjoyed completing the questionnaire | 13 | 5 | 2 | 0 | 0 |
| Completing the questionnaire helped me in making a decision about my preferences | 11 | 6 | 3 | 0 | 0 |
| I would be happy to complete a similar computerized questionnaire in the future | 12 | 7 | 1 | 0 | 0 |
| Variable | B | SE | 95% CIs | p-Value |
|---|---|---|---|---|
| Gender | ||||
| Female (Ref) | ||||
| Male | −0.5 | 1.5 | −4.1, −3.2 | 0.763 |
| Age group (years) | ||||
| 40–59 (Ref) | ||||
| 60–79 | 5.3 | 2.0 | 1.4, 9.2 | 0.008 |
| >79 | 4.4 | 2.3 | −0.2, 9.0 | 0.058 |
| Number of years suffering from OA | ||||
| >10 years (Ref) | ||||
| 5–10 years | 3.1 | 2.0 | −0.8, 7.1 | 0.122 |
| <5 years | 2.3 | 2.6 | −2.8, 7.4 | 0.374 |
| Pain interference with daily life | ||||
| Not at all to a little bit (Ref) | ||||
| Moderately | −0.4 | 2.4 | −5.0, 4.2 | 0.865 |
| Quite a bit to extremely | 2.7 | 2.3 | −1.8, 7.2 | 0.235 |
| Computer/laptop use frequency per week | ||||
| Every day (Ref) | ||||
| Few times | 2.9 | 2.4 | −1.8, 7.6 | 0.225 |
| Very rarely | 3.4 | 2.4 | −1.3, 8.1 | 0.155 |
| Never | 4.9 | 2.2 | 0.6, 9.3 | 0.026 |
| Completion of computerized questionnaires | ||||
| No (Ref) | ||||
| Yes | −4.0 | 1.7 | −7.4, −0.6 | 0.021 |
| Completion of pen/paper questionnaire | ||||
| No (Ref) | ||||
| Yes | −6.2 | 1.6 | −9.2, −3.1 | <0.001 |
| I found the questionnaire easy to read | ||||
| Strongly agree (Ref) | ||||
| Agree | 0.1 | 2.2 | −4.3, 4.43 | 0.976 |
| I found the questionnaire easy to understand | ||||
| Strongly agree (Ref) | ||||
| Agree | −1.5 | 2.1 | −5.5, −2.6 | 0.482 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Omari, B.; Farhat, J.; Shraim, M. The Role of Web-Based Adaptive Choice-Based Conjoint Analysis Technology in Eliciting Patients’ Preferences for Osteoarthritis Treatment. Int. J. Environ. Res. Public Health 2023, 20, 3364. https://doi.org/10.3390/ijerph20043364
Al-Omari B, Farhat J, Shraim M. The Role of Web-Based Adaptive Choice-Based Conjoint Analysis Technology in Eliciting Patients’ Preferences for Osteoarthritis Treatment. International Journal of Environmental Research and Public Health. 2023; 20(4):3364. https://doi.org/10.3390/ijerph20043364
Chicago/Turabian StyleAl-Omari, Basem, Joviana Farhat, and Mujahed Shraim. 2023. "The Role of Web-Based Adaptive Choice-Based Conjoint Analysis Technology in Eliciting Patients’ Preferences for Osteoarthritis Treatment" International Journal of Environmental Research and Public Health 20, no. 4: 3364. https://doi.org/10.3390/ijerph20043364
APA StyleAl-Omari, B., Farhat, J., & Shraim, M. (2023). The Role of Web-Based Adaptive Choice-Based Conjoint Analysis Technology in Eliciting Patients’ Preferences for Osteoarthritis Treatment. International Journal of Environmental Research and Public Health, 20(4), 3364. https://doi.org/10.3390/ijerph20043364






