Bilateral Transfer of Performance between Real and Non-Immersive Virtual Environments in Post-Stroke Individuals: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Neurological, Functional, and Mobility Evaluation
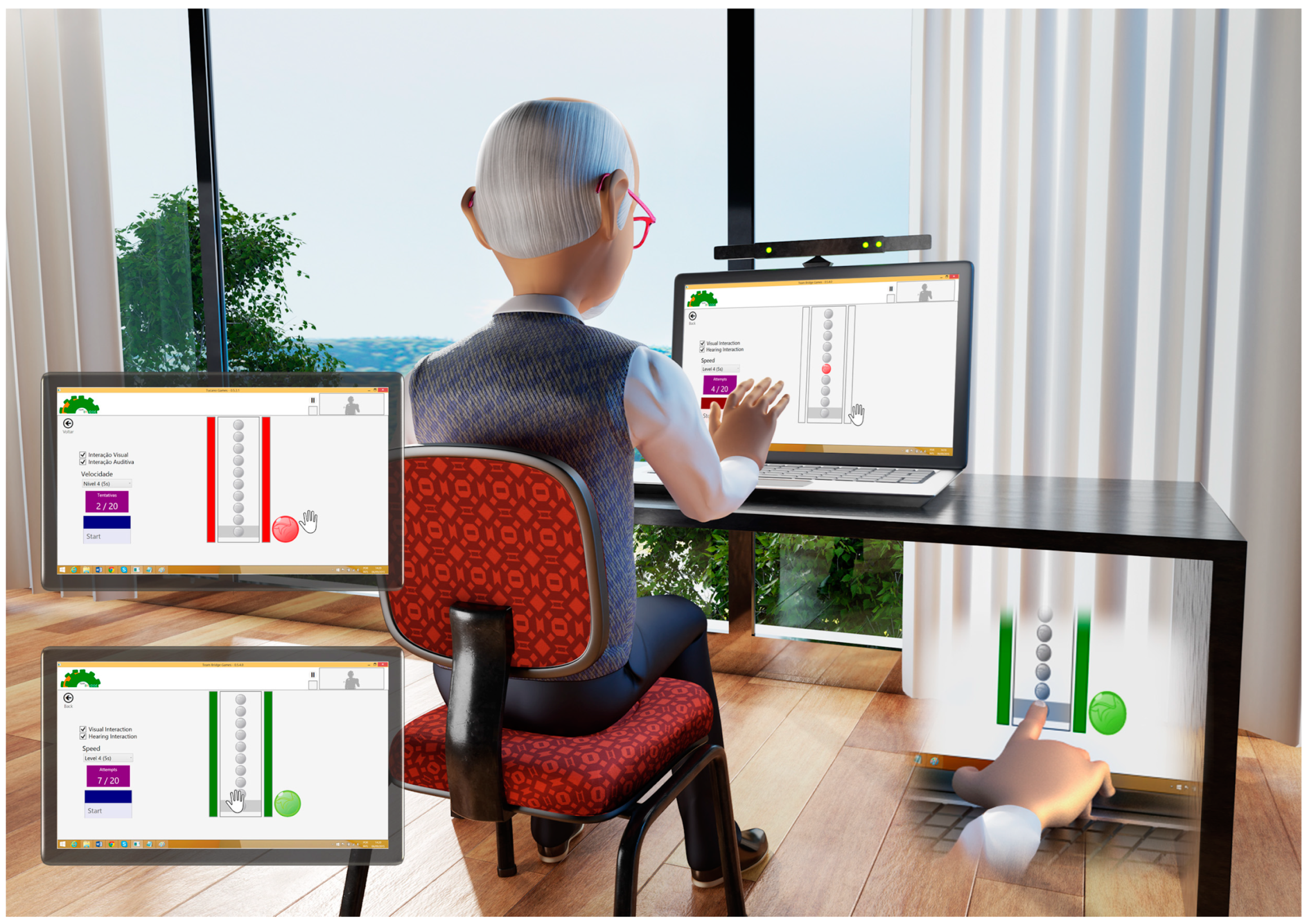
2.3. Instruments
2.4. Procedures
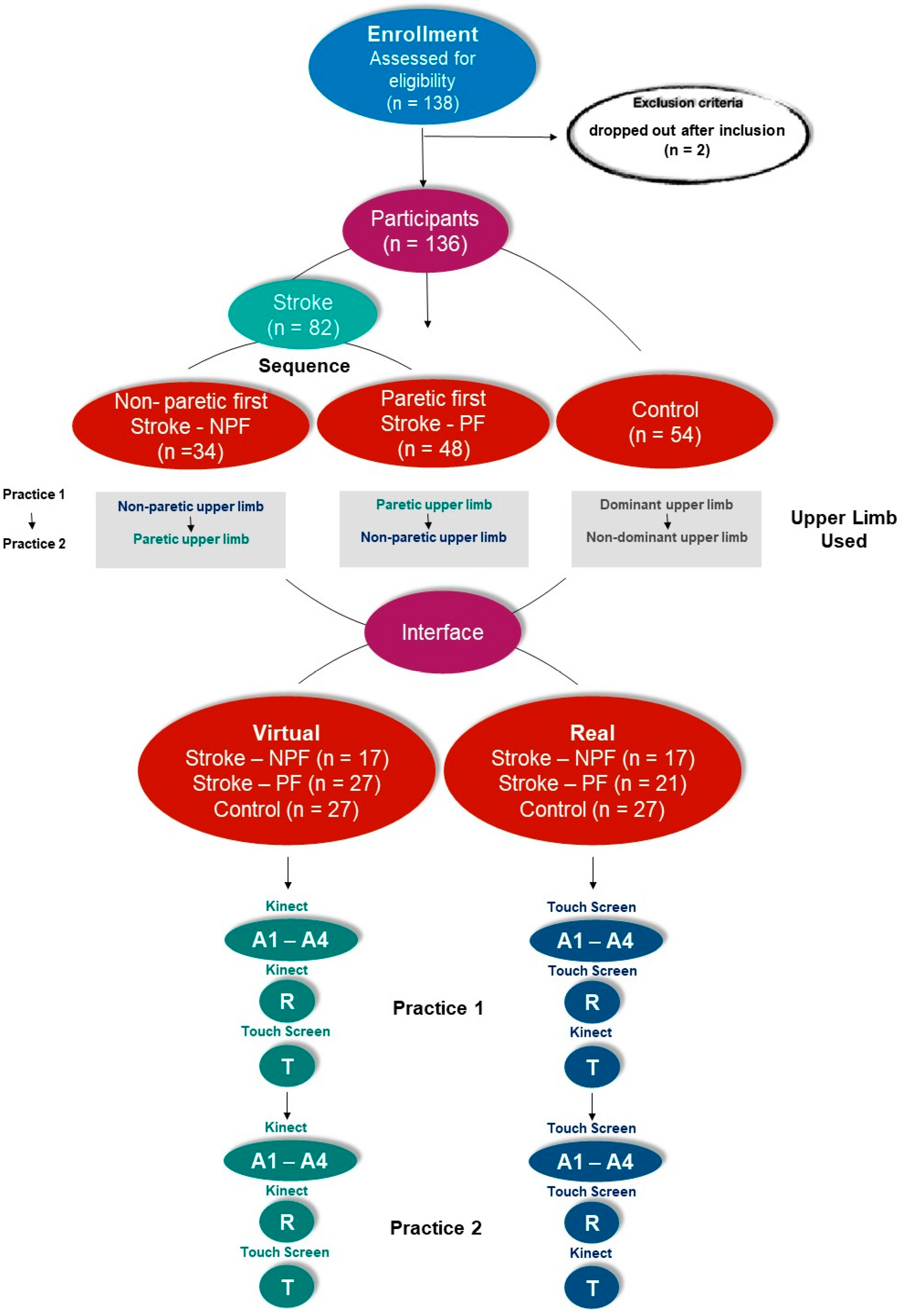
2.5. Participant Groups and Protocol
2.6. Data Analysis
3. Results
3.1. Acquisition—A1–A4
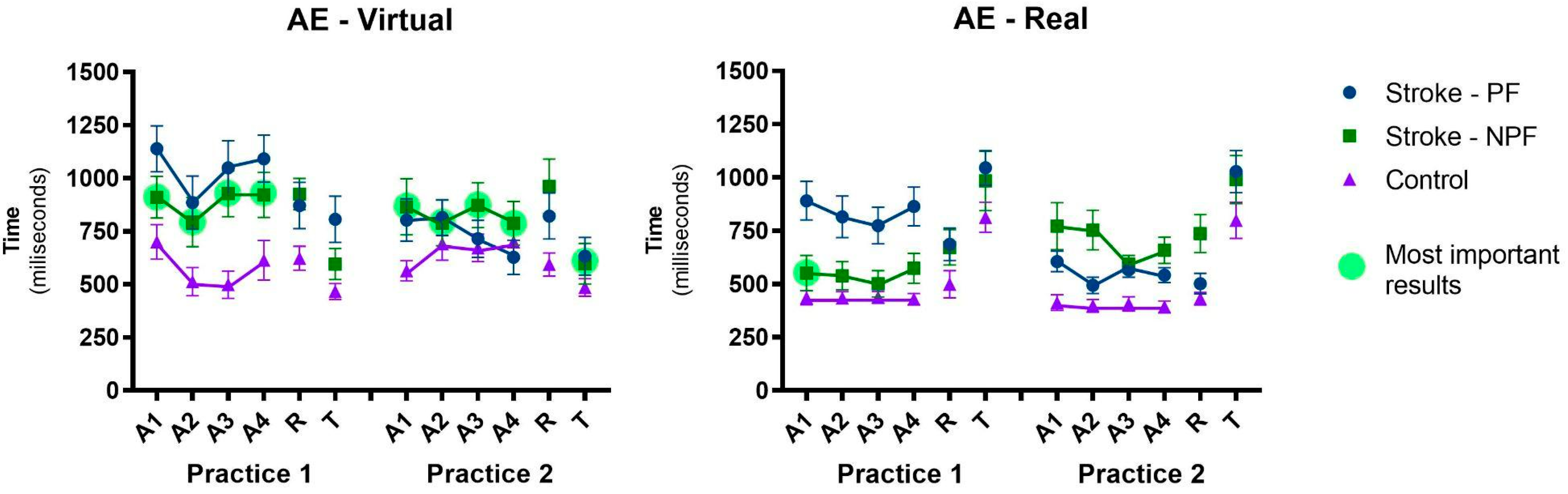
3.1.1. Absolute Error—AE
3.1.2. Variable Error—VE
3.2. Retention
3.2.1. Transfer Changing Interaction Device
3.2.2. Absolute Error—AE
3.2.3. Variable Error—VE
4. Discussion
4.1. Differences between Group (Post-Stroke and Control Group) and Upper Limb Used
4.2. Improvement in Performance Considering Interface (Virtual and Real)
4.3. Improvement Performance Considering Bilateral Transference between Environments (Virtual and Real)
- (a)
- Transference from paretic to non-paretic upper limb: we found bilateral transference in the group that started with the paretic upper limb, i.e., the group that practiced with the paretic upper limb first presented better performance when practicing with the non-paretic limb posteriorly, independently of interface (virtual or real). According to Land et al. [75], the training carried out with a limb in post-stroke individuals can have a positive effect on the performance of the same task during the use of the untrained limb, which further reinforces that the most important factor is its potential to be an effective therapeutic technique for rehabilitation of individuals with motor disabilities as stroke survivors.
- (b)
- Transference from non-paretic to paretic upper limb: another interesting result is that the group which started with the non-paretic upper limb presented bilateral transfer for the paretic upper limb, showing similar results between paretic and non-paretic. However, this result was confirmed only in the virtual interface (i.e., when participants practiced the virtual task first with the non-paretic upper limb, they transferred skill to the paretic upper limb). This result is very interesting (see Figure 3—most important result): after practicing the virtual task, the non-paretic upper limb presented the best result in the transfer to the real interface (Transfer, Practice 2). This can be reinforced by the comparison between the result of this transference with the acquisition in the first sequence with the paretic upper limb in the real interface (i.e., Transfer in Practice 2 versus A1 in Practice 1—real interface).
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sacco, R.L.; Kasner, S.E.; Broderick, J.P.; Caplan, L.R.; Connors, J.J.; Culebras, A.; Elkind, M.S.V.; George, M.G.; Hamdan, A.D.; Higashida, R.T.; et al. An Updated Definition of Stroke for the 21st Century: A Statement for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2013, 44, 2064–2089. [Google Scholar] [CrossRef] [PubMed]
- Mendis, S. Stroke Disability and Rehabilitation of Stroke: World Health Organization Perspective. Int. J. Stroke 2013, 8, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, A.M.; Klimkiewicz, R.; Kubsik, A.; Klimkiewicz, P.; Okońska, M.W.; Śmigielski, J. Location of the ischemic focus in rehabilitated stroke patients with impairment of executive functions. Adv. Clin. Exp. Med. 2017, 26, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Schinwelski, M.J.; Sitek, E.J.; Wąż, P.; Sławek, J.W. Prevalence and predictors of post-stroke spasticity and its impact on daily living and quality of life. Neurol. Neurochir. Pol. 2019, 53, 449–457. [Google Scholar] [CrossRef]
- Charfi, N.; Trabelsi, S.; Turki, M.; Bouali, M.M.; Zouari, L.; Dammak, M.; Ben Thabet, J.; Mhiri, C.; Mâalej, M. Impact du handicap physique et des troubles émotionnels concomitants sur la qualité de vie en post-AVC. L’Encéphale 2017, 43, 429–434. [Google Scholar] [CrossRef]
- Lerdal, A.; Gay, C.L. Acute-Phase Fatigue Predicts Limitations with Activities of Daily Living 18 Months after First-Ever Stroke. J. Stroke Cerebrovasc. Dis. 2017, 26, 523–531. [Google Scholar] [CrossRef]
- Brouns, R.; Espinoza, A.V.; Goudman, L.; Moens, M.; Verlooy, J. Interventions to promote work participation after ischaemic stroke: A systematic review. Clin. Neurol. Neurosurg. 2019, 185, 105458. [Google Scholar] [CrossRef]
- Bonner, B.; Pillai, R.; Sarma, P.S.; Lipska, K.J.; Pandian, J.; Sylaja, P.N. Factors predictive of return to work after stroke in patients with mild−moderate disability in India. Eur. J. Neurol. 2015, 23, 548–553. [Google Scholar] [CrossRef]
- Ramos-Lima, M.J.M.; Brasileiro, I.D.C.; de Lima, T.L.; Braga-Neto, P. Quality of life after stroke: Impact of clinical and sociodemographic factors. Clinics 2018, 73, e418. [Google Scholar] [CrossRef]
- Rudberg, A.-S.; Berge, E.; Gustavsson, A.; Näsman, P.; Lundström, E. Long-term health-related quality of life, survival and costs by different levels of functional outcome six months after stroke. Eur. Stroke J. 2018, 3, 157–164. [Google Scholar] [CrossRef]
- Bullier, B.; Cassoudesalle, H.; Villain, M.; Cogné, M.; Mollo, C.; De Gabory, I.; Dehail, P.; Joseph, P.-A.; Sibon, I.; Glize, B. New factors that affect quality of life in patients with aphasia. Ann. Phys. Rehabilitation Med. 2019, 63, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Hatem, S.M.; Saussez, G.; della Faille, M.; Prist, V.; Zhang, X.; Dispa, D.; Bleyenheuft, Y. Rehabilitation of Motor Function after Stroke: A Multiple Systematic Review Focused on Techniques to Stimulate Upper Extremity Recovery. Front. Hum. Neurosci. 2016, 10, 442. [Google Scholar] [CrossRef] [PubMed]
- Laver, K. Virtual Reality for Stroke Rehabilitation. In Virtual Reality in Health and Rehabilitation; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar] [CrossRef]
- Kourtesis, P.; Collina, S.; Doumas, L.A.A.; MacPherson, S.E. Validation of the Virtual Reality Neuroscience Questionnaire: Maximum Duration of Immersive Virtual Reality Sessions Without the Presence of Pertinent Adverse Symptomatology. Front. Hum. Neurosci. 2019, 13, 417. [Google Scholar] [CrossRef] [PubMed]
- Kiper, P.; Szczudlik, A.; Agostini, M.; Opara, J.; Nowobilski, R.; Ventura, L.; Tonin, P.; Turolla, A. Virtual Reality for Upper Limb Rehabilitation in Subacute and Chronic Stroke: A Randomized Controlled Trial. Arch. Phys. Med. Rehabilitation 2018, 99, 834–842.e4. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Singh, D.K.A.; Nordin, N.A.M.; Nee, K.H.; Ibrahim, N. Virtual Reality Games as an Adjunct in Improving Upper Limb Function and General Health among Stroke Survivors. Int J Environ Res Public Health 2019, 16, 5144. [Google Scholar] [CrossRef]
- Norouzi-Gheidari, N.; Hernandez, A.; Archambault, P.S.; Higgins, J.; Poissant, L.; Kairy, D. Feasibility, Safety and Efficacy of a Virtual Reality Exergame System to Supplement Upper Extremity Rehabilitation Post-Stroke: A Pilot Randomized Clinical Trial and Proof of Principle. Int. J. Environ. Res. Public Health 2019, 17, 113. [Google Scholar] [CrossRef]
- Lefebvre, S.; Laloux, P.; Peeters, A.; Desfontaines, P.; Jamart, J.; Vandermeeren, Y. Dual-tDCS Enhances Online Motor Skill Learning and Long-Term Retention in Chronic Stroke Patients. Front. Hum. Neurosci. 2013, 6, 343. [Google Scholar] [CrossRef]
- Kitago, T.; Krakauer, J.W. Motor learning principles for neurorehabilitation. Handb. Clin. Neurol. 2013, 110, 93–103. [Google Scholar] [CrossRef]
- Krakauer, J.W. Motor learning: Its relevance to stroke recovery and neurorehabilitation. Curr. Opin. Neurol. 2006, 19, 84–90. [Google Scholar] [CrossRef]
- Bray, C.W. Transfer of Learning. J. Exp. Psychol. 1928, 11, 443–467. [Google Scholar] [CrossRef]
- Ausenda, C.D.; Carnovali, M. Transfer of Motor Skill Learning from the Healthy Hand to the Paretic Hand in Stroke Patients: A Randomized Controlled Trial. Eur. J. Phys. Rehabil. Med. 2011, 47, 417–425. [Google Scholar] [PubMed]
- Sainburg, R.L.; Schaefer, S.Y.; Yadav, V. Lateralized motor control processes determine asymmetry of interlimb transfer. Neuroscience 2016, 334, 26–38. [Google Scholar] [CrossRef]
- Kumar, S.; Mandal, M. Bilateral transfer of skill in left- and right-handers. Laterality 2005, 10, 337–344. [Google Scholar] [CrossRef]
- Ausenda, C.D. A New Idea for Stroke Rehabilitation: Bilateral Transfer Analysis from Healthy Hand to the Paretic One with a Randomized and Controlled Trial. Int. J. Phys. Med. Rehabil. 2014, s3, 1–8. [Google Scholar] [CrossRef]
- Iosa, M.; Morone, G.; Ragaglini, M.R.; Fusco, A.; Paolucci, S. Motor Strategies and Bilateral Transfer in Sensorimotor Learning of Patients with Subacute Stroke and Healthy Subjects. A Randomized Controlled Trial. Eur. J. Phys. Rehabil. Med. 2013, 49, 291–299. [Google Scholar]
- Bernhardt, J.; Hayward, K.; Kwakkel, G.; Ward, N.; Wolf, S.L.; Borschmann, K.; Krakauer, J.W.; Boyd, L.A.; Carmichael, S.T.; Corbett, D.; et al. Agreed definitions and a shared vision for new standards in stroke recovery research: The Stroke Recovery and Rehabilitation Roundtable taskforce. Int. J. Stroke 2017, 12, 444–450. [Google Scholar] [CrossRef]
- Kalra, L.; Crome, P. The Role of Prognostic Scores in Targeting Stroke Rehabilitation in Elderly Patients. J. Am. Geriatr. Soc. 1993, 41, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Singer, B.; Garcia-Vega, J. The Fugl-Meyer Upper Extremity Scale. J. Physiother. 2017, 63, 53. [Google Scholar] [CrossRef] [PubMed]
- Fugl Meyer, A.R.; Jaasko, L.; Leyman, I. The Post Stroke Hemiplegic Patient. I. A Method for Evaluation of Physical Performance. Scand. J. Rehabil. Med. 1975, 7, 13–31. [Google Scholar]
- Bertolucci, P.; Brucki, S.; Campacci, S.R.; Juliano, Y. O Mini-Exame do Estado Mental em uma população geral: Impacto da escolaridade. Arq. Neuro-Psiquiatr. 1994, 52, 1–7. [Google Scholar] [CrossRef]
- Folstein, S.; Mchugh, P.; Psychiatric, J. Key Papers in Geriatric Psychiatry Mini-Mental State: A Practical Method for Grading the Cognitive State of Patients for the Clinician. Int. J. Geriatr. Psychiatry 1998, 13, 285–294. [Google Scholar]
- Bohannon, R.W. Reference values for the timed up and go test: A descriptive meta-analysis. J. Geriatr. Phys. Ther. 2006, 29, 64–68. [Google Scholar] [CrossRef]
- Morlin, A.C.G.; Delattre, A.M.; Cacho, E.W.A.; Oberg, T.D.; De Oliveira, R. Concordância e tradução para o português do Teste de Habilidade Motora do Membro Superior. Rev. Neurociências 2006, 14, 6–9. [Google Scholar] [CrossRef]
- Kopp, B.; Kunkel, A.; Flor, H.; Platz, T.; Rose, U.; Mauritz, K.-H.; Gresser, K.; McCulloch, K.L.; Taub, E. The arm motor ability test: Reliability, validity, and sensitivity to change of an instrument for assessing disabilities in activities of daily living. Arch. Phys. Med. Rehabil. 1997, 78, 615–620. [Google Scholar] [CrossRef] [PubMed]
- de Mello Monteiro, C.B.; Massetti, T.; da Silva, T.D.; van der Kamp, J.; de Abreu, L.C.; Leone, C.; Savelsbergh, G.J. Transfer of motor learning from virtual to natural environments in individuals with cerebral palsy. Res. Dev. Disabil. 2014, 35, 2430–2437. [Google Scholar] [CrossRef]
- de Mello Monteiro, C.B.; da Silva, T.D.; de Abreu, L.C.; Fregni, F.; de Araujo, L.V.; Ferreira, F.H.I.B.; Leone, C. Short-Term Motor Learning through Non-Immersive Virtual Reality Task in Individuals with down Syndrome. BMC Neurol. 2017, 17, 71. [Google Scholar] [CrossRef]
- Crocetta, T.B.; de Araújo, L.V.; Guarnieri, R.; Massetti, T.; Ferreira, F.H.I.B.; de Abreu, L.C.; de Mello Monteiro, C.B. Virtual Reality Software Package for Implementing Motor Learning and Rehabilitation Experiments. Virtual Real. 2018, 22, 199–209. [Google Scholar] [CrossRef]
- Fooken, J.; Yeo, S.H.; Pai, D.K.; Spering, M. Eye Movement Accuracy Determines Natural Interception Strategies. J. Vis. 2016, 16, 1. [Google Scholar] [CrossRef]
- Ohta, Y. Effects of Oncoming Target Velocities on Rapid Force Production and Accuracy of Force Production Intensity and Timing. J. Sports Sci. 2016, 35, 2304–2312. [Google Scholar] [CrossRef]
- Bezerra, Í.M.P.; Crocetta, T.B.; Massetti, T.; da Silva, T.D.; Guarnieri, R.; de Miranda Meira, C., Jr.; Arab, C.; de Abreu, L.C.; de Araujo, L.V.; de Mello Monteiro, C.B. Functional Performance Comparison between Real and Virtual Tasks in Older Adults. Medicine 2018, 97, e9612. [Google Scholar] [CrossRef]
- Quadrado, V.H.; Silva, T.D.D.; Favero, F.M.; Tonks, J.; Massetti, T.; Monteiro, C.B.D.M. Motor Learning from Virtual Reality to Natural Environments in Individuals with Duchenne Muscular Dystrophy. Disabil. Rehabil. Assist. Technol. 2017, 14, 12–20. [Google Scholar] [CrossRef] [PubMed]
- de Moraes, Í.A.P.; Monteiro, C.B.D.M.; Silva, T.D.D.; Massetti, T.; Crocetta, T.B.; de Menezes, L.D.C.; Andrade, G.P.D.R.; Ré, A.H.N.; Dawes, H.; Coe, S.; et al. Motor Learning and Transfer between Real and Virtual Environments in Young People with Autism Spectrum Disorder: A Prospective Randomized Cross over Controlled Trial. Autism Res. 2019, 13, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Psychol. 2013, 4, 863. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Shin, J.Y.; Son, S.M. Deficit of Motor Skill Acquisition on the Upper Limb Ipsilesional to the Injured Hemisphere in Individuals with Stroke. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 5062–5067. [Google Scholar] [CrossRef]
- Subramaniam, S.; Varghese, R.; Bhatt, T. Influence of Chronic Stroke on Functional Arm Reaching: Quantifying Deficits in the Ipsilesional Upper Extremity. Rehabil. Res. Pract. 2019, 2019, 5182310. [Google Scholar] [CrossRef]
- Coderre, A.M.; Zeid, A.A.; Dukelow, S.P.; Demmer, M.J.; Moore, K.D.; Demers, M.J.; Bretzke, H.; Herter, T.M.; Glasgow, J.I.; Norman, K.E.; et al. Assessment of Upper-Limb Sensorimotor Function of Subacute Stroke Patients Using Visually Guided Reaching. Neurorehabilit. Neural Repair 2010, 24, 528–541. [Google Scholar] [CrossRef]
- Subramanian, S.; Knaut, L.A.; Beaudoin, C.; McFadyen, B.J.; Feldman, A.G.; Levin, M.F. Virtual Reality Environments for Post-Stroke Arm Rehabilitation. J. Neuroeng. Rehabil. 2007, 4, 20. [Google Scholar] [CrossRef]
- Hussain, N.; Murphy, M.A.; Sunnerhagen, K.S. Upper Limb Kinematics in Stroke and Healthy Controls Using Target-to-Target Task in Virtual Reality. Front. Neurol. 2018, 9, 300. [Google Scholar] [CrossRef]
- de Diego, C.; Puig, S.; Navarro, X. A sensorimotor stimulation program for rehabilitation of chronic stroke patients. Restor. Neurol. Neurosci. 2013, 31, 361–371. [Google Scholar] [CrossRef]
- Sun, Y.; Ledwell, N.M.H.; Boyd, L.A.; Zehr, E.P. Unilateral wrist extension training after stroke improves strength and neural plasticity in both arms. Exp. Brain Res. 2018, 236, 2009–2021. [Google Scholar] [CrossRef]
- Lang, C.E.; Bland, M.D.; Bailey, R.R.; Schaefer, S.Y.; Birkenmeier, R.L. Assessment of upper extremity impairment, function, and activity after stroke: Foundations for clinical decision making. J. Hand Ther. 2013, 26, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Sathian, K.; Buxbaum, L.J.; Cohen, L.G.; Krakauer, J.W.; Lang, C.E.; Corbetta, M.; Fitzpatrick, S.M. Neurological Principles and Rehabilitation of Action Disorders. Neurorehabilit. Neural Repair 2011, 25, 21S–32S. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, C.D.; Reinkensmeyer, D.J. Hemiparetic stroke impairs anticipatory control of arm movement. Exp. Brain Res. 2003, 149, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Cameirão, M.S.; i Badia, S.B.; Oller, E.D.; Verschure, P.F. Neurorehabilitation using the virtual reality based Rehabilitation Gaming System: Methodology, design, psychometrics, usability and validation. J. Neuroeng. Rehabil. 2010, 7, 48. [Google Scholar] [CrossRef]
- Torriani-Pasin, C.; Bonuzzi, G.M.G.; dos Santos Palma, G.C.; Freudenheim, A.M.; Corrêa, U.C. Motor Learning in Post Stroke Subjects: The Effects of Practice Conditions on the Temporal Synchronization. Mot. Rev. De Educ. Física 2018, 24, 1–8. [Google Scholar] [CrossRef]
- Moliterno, A.H.; Bezerra, F.V.; Pires, L.A.; Roncolato, S.S.; da Silva, T.D.; Massetti, T.; Fernani, D.C.G.L.; Magalhães, F.H.; de Mello Monteiro, C.B.; Dantas, M.T.A.P. Effect of Contextual Interference in the Practicing of a Computer Task in Individuals Poststroke. BioMed. Res. Int. 2020, 2020, 2937285. [Google Scholar] [CrossRef] [PubMed]
- Semrau, J.A.; Herter, T.M.; Kenzie, J.M.; Findlater, S.E.; Scott, S.H.; Dukelow, S.P. Robotic Characterization of Ipsilesional Motor Function in Subacute Stroke. Neurorehabilit. Neural Repair 2017, 31, 571–582. [Google Scholar] [CrossRef]
- Winstein, C.J.; Merians, A.S.; Sullivan, K.J. Motor learning after unilateral brain damage. Neuropsychologia 1999, 37, 975–987. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.F.; Weiss, P.L.; Keshner, E.A. Emergence of Virtual Reality as a Tool for Upper Limb Rehabilitation: Incorporation of Motor Control and Motor Learning Principles. Phys. Ther. 2015, 95, 415–425. [Google Scholar] [CrossRef]
- Kate Paquin, S.L. Non-Immersive Virtual Reality for Fine Motor Rehabilitation of Functional Activities in Individuals with Chronic Stroke: A Review. J. Aging Sci. 2013, 1, 2. [Google Scholar] [CrossRef]
- Levin, M.F. Can virtual reality offer enriched environments for rehabilitation? Expert Rev. Neurother. 2011, 11, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Kleim, J.A.; Jones, T.A. Principles of Experience-Dependent Neural Plasticity: Implications for Rehabilitation After Brain Damage. J. Speech, Lang. Heart Res. 2008, 51, S225–S239. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.F. What is the potential of virtual reality for post-stroke sensorimotor rehabilitation? Expert Rev. Neurother. 2020, 20, 195–197. [Google Scholar] [CrossRef] [PubMed]
- Samuel, G.; Choo, M.; Chan, W.; Kok, S.; Ng, Y. The use of virtual reality-based therapy to augment poststroke upper limb recovery. Singap. Med. J. 2015, 56, e127–e130. [Google Scholar] [CrossRef] [PubMed]
- Cirstea, M.C.; Ptito, A.; Levin, M.F. Arm reaching improvements with short-term practice depend on the severity of the motor deficit in stroke. Exp. Brain Res. 2003, 152, 476–488. [Google Scholar] [CrossRef]
- Knorr, S.; Rice, C.L.; Garland, S.J. Perspective on neuromuscular factors in poststroke fatigue. Disabil. Rehabil. 2012, 34, 2291–2299. [Google Scholar] [CrossRef]
- Colle, F.; Bonan, I.; Gellez Leman, M.C.; Bradai, N.; Yelnik, A. Fatigue after Stroke. Ann. De Readapt. Med. Phys. 2006, 49, 361–364. [Google Scholar] [CrossRef]
- Acciarresi, M.; Bogousslavsky, J.; Paciaroni, M. Post-Stroke Fatigue: Epidemiology, Clinical Characteristics and Treatment. Eur. Neurol. 2014, 72, 255–261. [Google Scholar] [CrossRef]
- Johansson, B.; Starmark, A.; Berglund, P.; Rödholm, M.; Rönnbäck, L. A self-assessment questionnaire for mental fatigue and related symptoms after neurological disorders and injuries. Brain Inj. 2009, 24, 2–12. [Google Scholar] [CrossRef]
- Puchta, A.E. Why Am I so Tired after My Stroke? J. Vasc. Interv. Neurol. 2008, 1, 63. [Google Scholar]
- Yang, C.; Lin, Y.; Cai, M.; Qian, Z.; Kivol, J.; Zhang, W. Cognitive Fatigue Effect on Rehabilitation Task Performance in a Haptic Virtual Environment System. J. Rehabil. Assist. Technol. Eng. 2017, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pridmore, T.; Cobb, S.; Hilton, D.; Green, J.; Eastgate, R. Mixed reality environments in stroke rehabilitation: Interfaces across the real/virtual divide. Int. J. Disabil. Hum. Dev. 2007, 6, 87–96. [Google Scholar] [CrossRef]
- Trevizan, I.L.; Silva, T.D.; Dawes, H.; Massetti, T.; Crocetta, T.B.; Favero, F.M.; Oliveira, A.S.B.; De Araújo, L.V.; Santos, A.C.C.; De Abreu, L.C.; et al. Efficacy of different interaction devices using non-immersive virtual tasks in individuals with Amyotrophic Lateral Sclerosis: A cross-sectional randomized trial. BMC Neurol. 2018, 18, 209. [Google Scholar] [CrossRef] [PubMed]
- Land, W.M.; Liu, B.; Cordova, A.; Fang, M.; Huang, Y.; Yao, W.X. Effects of Physical Practice and Imagery Practice on Bilateral Transfer in Learning a Sequential Tapping Task. PLoS ONE 2016, 11, e0152228. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zehr, E.P. Training-Induced Neural Plasticity and Strength Are Amplified After Stroke. Exerc. Sport Sci. Rev. 2019, 47, 223–229. [Google Scholar] [CrossRef]

| Stroke—NPF (n = 34) | Stroke—PF (n = 48) | Control (n = 54) | p-Value | |
|---|---|---|---|---|
| Mean (SD) [CI 95%] | Mean (SD) [CI 95%] | Mean (SD) [CI 95%] | ||
| Age (years) | 56.2 (14.1) [51, 61] | 59.0 (11.6) [56, 62] | 57.4 (12.8) [54, 61] | 0.603 |
| OPS | 2.24 (0.6) [2.05, 2.47] | 2.09 (0.38) [1.98, 2.20] | - | 0.070 |
| FMA | 53.3 (12.8) [48.8, 57.5] | 54.4 (11.9) [50.7, 57.4] | - | 0.156 |
| MMSE | 22.2 (5.2) [20.2, 23.9] | 23.6 (4.6) [22.2, 24.8] | - | 0.275 |
| TUG | 23.1 (11.9) [19.2, 27.4] | 24.0 (12.5) [20.6, 28.0] | - | 0.741 |
| Lesion time (months) | 31.5 (50.5) [16.6, 52.4] | 41.5 (52.1) [26.9, 58.3] | - | 0.330 |
| AMAT FS | 4.1 (1.4) [3.5, 4.5] | 4.0 (1.4) [3.6, 4.4] | - | 0.663 |
| AMAT MQ | 4.1 (1.4) [3.5, 4.5] | 4.0 (1.5) [3.5, 4.4] | - | 0.714 |
| n (%) | n (%) | |||
| Sex | ||||
| Male | 22 (28) | 28 (34) | 26 (38) | 0.731 |
| Female | 12 (22) | 20 (37) | 28 (41) | |
| Type of Stroke | ||||
| Ischemic | 25 (70) | 41 (30) | - | 0.088 |
| Hemorrhagic | 9 (85) | 7 (15) | - | |
| Side of hemiparesis | ||||
| Right | 17 (50) | 23 (50) | - | 0.441 |
| Left | 17 (54) | 25 (46) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mota, D.M.S.; Moraes, Í.A.P.; Papa, D.C.R.; Fernani, D.C.G.L.; Almeida, C.S.; Tezza, M.H.S.; Dantas, M.T.A.P.; Fernandes, S.M.S.; Ré, A.H.N.; Silva, T.D.; et al. Bilateral Transfer of Performance between Real and Non-Immersive Virtual Environments in Post-Stroke Individuals: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2023, 20, 3301. https://doi.org/10.3390/ijerph20043301
Mota DMS, Moraes ÍAP, Papa DCR, Fernani DCGL, Almeida CS, Tezza MHS, Dantas MTAP, Fernandes SMS, Ré AHN, Silva TD, et al. Bilateral Transfer of Performance between Real and Non-Immersive Virtual Environments in Post-Stroke Individuals: A Cross-Sectional Study. International Journal of Environmental Research and Public Health. 2023; 20(4):3301. https://doi.org/10.3390/ijerph20043301
Chicago/Turabian StyleMota, Deise M. S., Íbis A. P. Moraes, Denise C. R. Papa, Deborah C. G. L. Fernani, Caroline S. Almeida, Maria H. S. Tezza, Maria T. A. P. Dantas, Susi M. S. Fernandes, Alessandro H. N. Ré, Talita D. Silva, and et al. 2023. "Bilateral Transfer of Performance between Real and Non-Immersive Virtual Environments in Post-Stroke Individuals: A Cross-Sectional Study" International Journal of Environmental Research and Public Health 20, no. 4: 3301. https://doi.org/10.3390/ijerph20043301
APA StyleMota, D. M. S., Moraes, Í. A. P., Papa, D. C. R., Fernani, D. C. G. L., Almeida, C. S., Tezza, M. H. S., Dantas, M. T. A. P., Fernandes, S. M. S., Ré, A. H. N., Silva, T. D., & Monteiro, C. B. M. (2023). Bilateral Transfer of Performance between Real and Non-Immersive Virtual Environments in Post-Stroke Individuals: A Cross-Sectional Study. International Journal of Environmental Research and Public Health, 20(4), 3301. https://doi.org/10.3390/ijerph20043301








