Salinity Moderated the Toxicity of Zinc Oxide Nanoparticles (ZnO NPs) towards the Early Development of Takifugu obscurus
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Embryos and Larvae
2.2. Preparation of ZnO NPs
2.3. Experimental Procedure
2.4. Determination of Biochemical Parameters
2.5. Statistical Analysis
3. Results
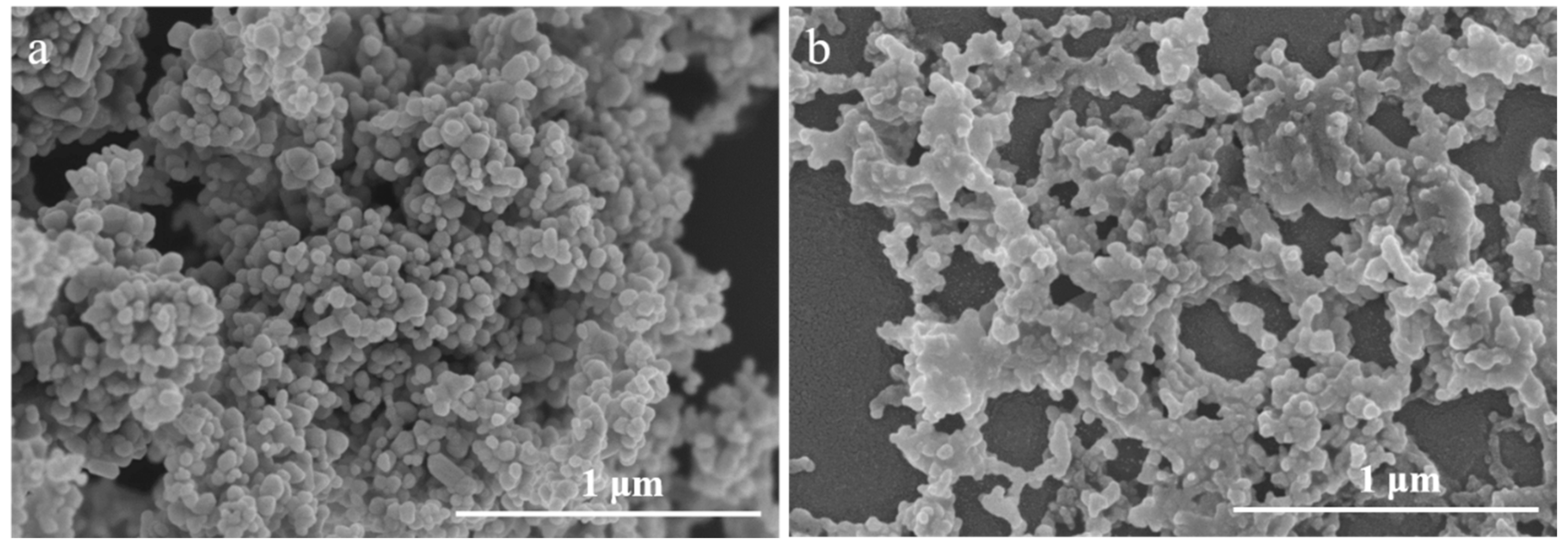
3.1. Characterization of ZnO NPs in Salinity Solution
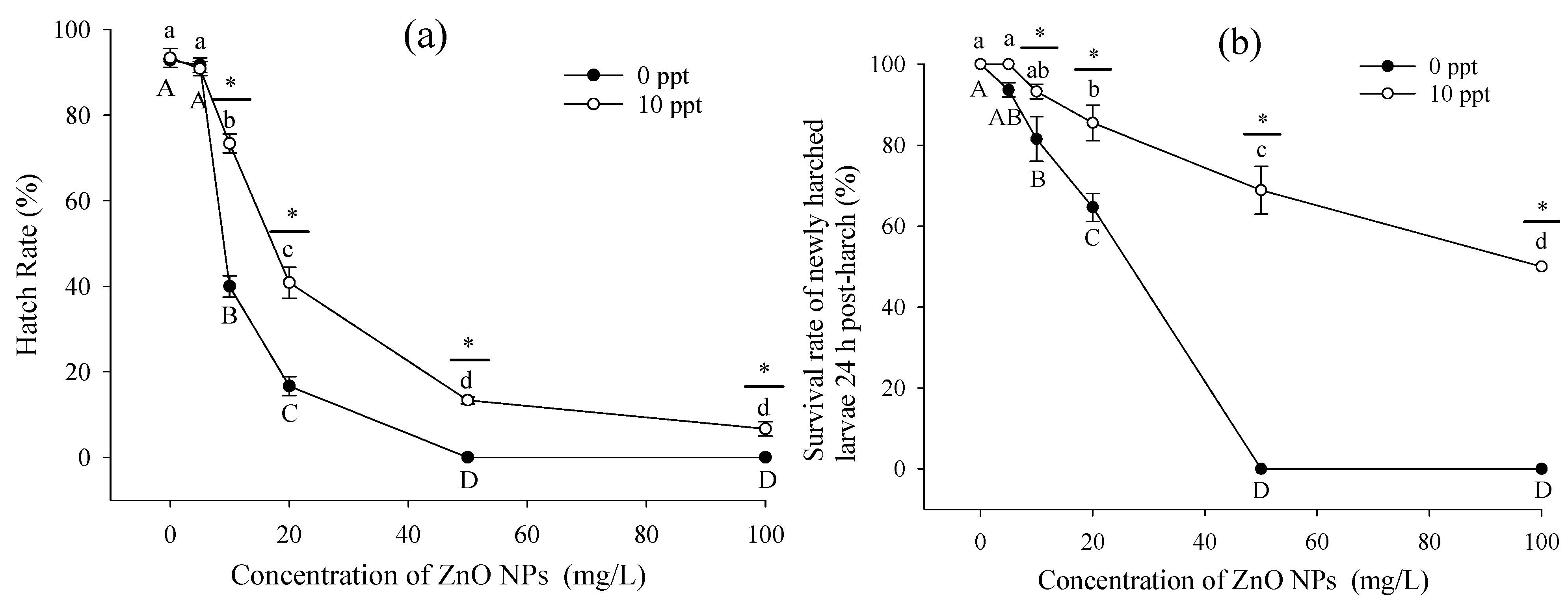
3.2. Toxic Effects of ZnO NPs on Embryos
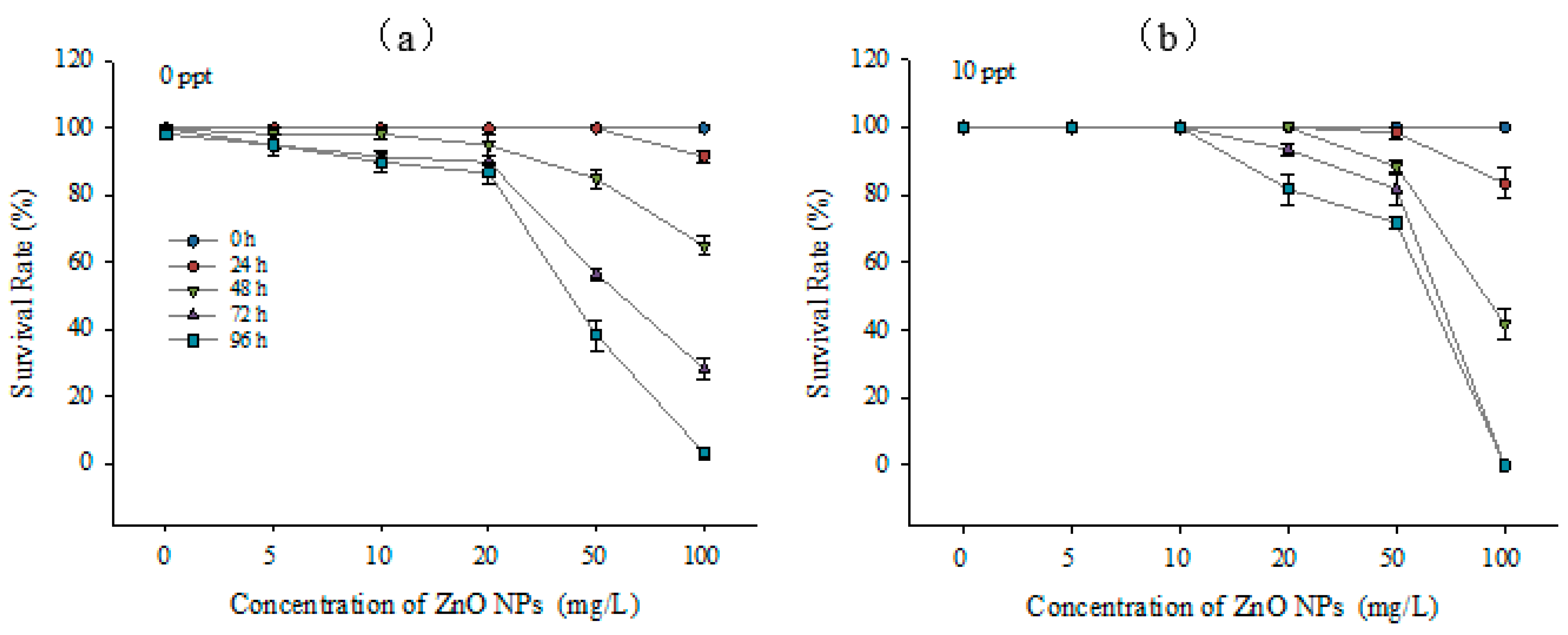
3.3. Toxic Effects on Larvae
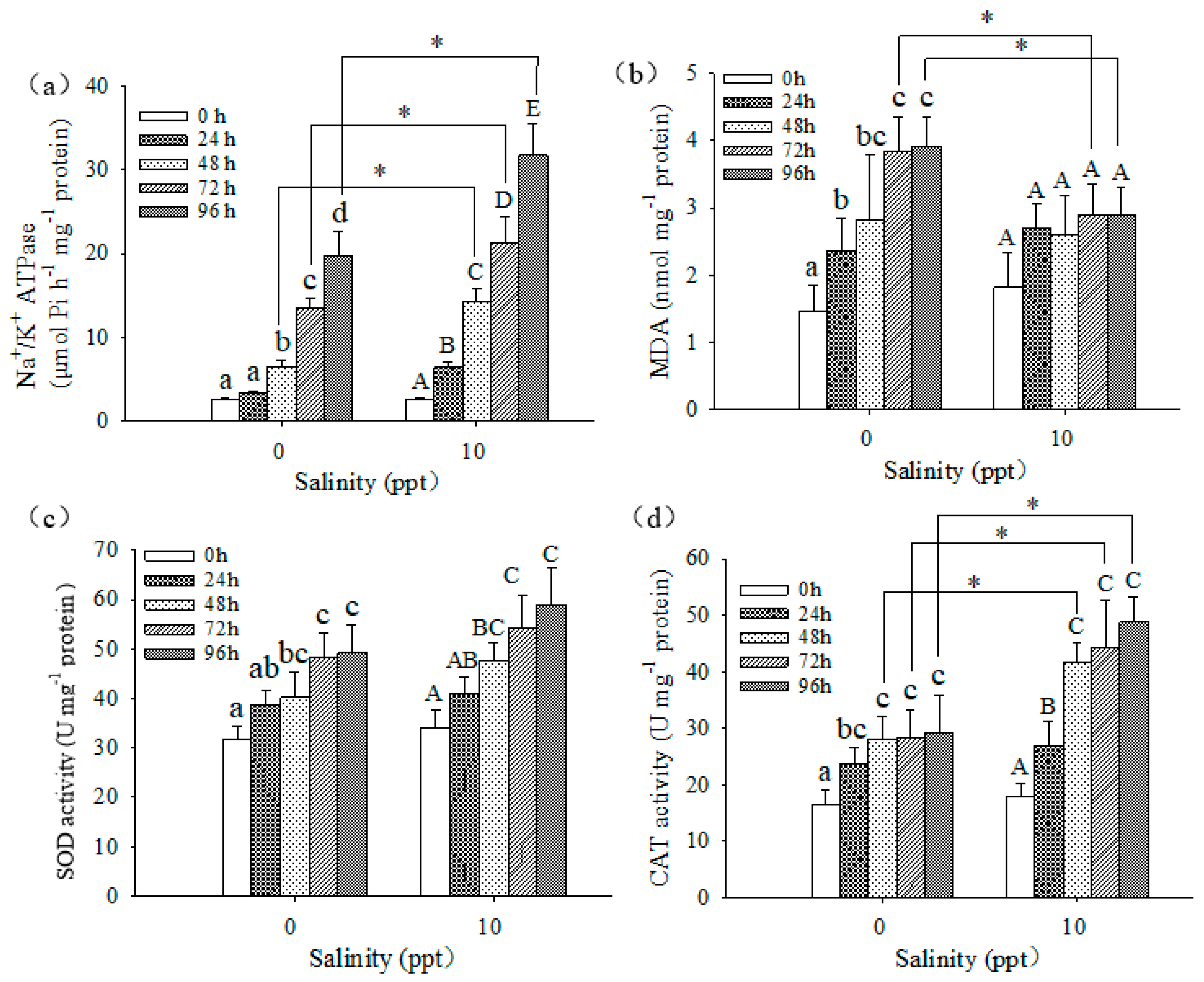
3.4. Biochemical Responses of Larvae
4. Discussion
4.1. Impacts of Salinity on the Characterization and Toxicity of ZnO NPs
4.2. Embryos and Newly Hatched Larvae
4.3. Biochemical Responses of Larvae
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vert, M.; Doi, Y.; Hellwich, K.; Hess, M.; Hodge, P.; Kubisa, P.; Rinaudo, M.; Schué, F. Terminology for biorelated polymers and applications (IUPAC Recommendations 2012). Pure Appl. Chem. 2012, 84, 377–410. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Moedler, L.; Li, N. Toxic Potential of Materials at the Nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Bouwmeester, H.; Dekkers, S.; Noordam, M.; Hagens, W.; Bulder, A.; de Heer, C.; ten Voorde, S.; Wijnhoven, S.; Marvin, H.; Sips, A. Review of health safety aspects of nanotechnologies in food production. Regul. Toxicol. Pharmacol. 2009, 53, 52–62. [Google Scholar] [CrossRef]
- Joo, S.; Zhao, D. Environmental dynamics of metal oxide nanoparticles in heterogeneous systems: A review. J. Hazard. Mater. 2017, 322, 29–47. [Google Scholar] [CrossRef]
- Szabó, T.; Németh, J.; Dékány, I. Zinc oxide nanoparticles incorporated in ultrathin layer silicate films and their photocatalytic properties. Colloids Surf. A Physicochem. Eng. Asp. 2003, 230, 23–35. [Google Scholar] [CrossRef]
- Keller, A.; McFerran, S.; Lazareva, A.; Suh, S. Global life cycle releases of engineered nanomaterials. J. Nanopart. Res. 2013, 15, 1692. [Google Scholar] [CrossRef]
- Casas-Beltran, D.; Hernández-Pedraza, M.; Alvarado-Flores, J. Estimation of the Discharge of Sunscreens in Aquatic Environments of the Mexican Caribbean. Environments 2020, 7, 15. [Google Scholar] [CrossRef]
- Bhatt, I.; Tripathi, B. Interaction of engineered nanoparticles with various components of the environment and possible strategies for their risk assessment. Chemosphere 2011, 82, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Wu, Y.; Li, X.; Wei, B.; Li, S.; Wang, X. Toxic effects of different types of zinc oxide nanoparticles on algae, plants, invertebrates, vertebrates and microorganisms. Chemosphere 2018, 193, 852–860. [Google Scholar] [CrossRef]
- García-Gómez, C.; Babin, M.; Obrador, A.; Álvarez, J.; Fernández, M. Toxicity of ZnO Nanoparticles, ZnO Bulk, and ZnCl2 on Earthworms in a Spiked Natural Soil and Toxicological Effects of Leachates on Aquatic Organisms. Arch. Environ. Contam. Toxicol. 2014, 67, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Williams, P.; Diamond, S. Ecotoxicity of manufactured ZnO nanoparticles–A review. Environ. Pollut. 2013, 172, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Bai, W.; Zhang, Z.; Tian, W.; He, X.; Ma, Y.; Zhao, Y.; Chai, Z. Toxicity of zinc oxide nanoparticles to zebrafish embryo: A physicochemical study of toxicity mechanism. J. Nanopart. Res. 2009, 12, 1645–1654. [Google Scholar] [CrossRef]
- Clarkson, T. Environmental contaminants in the food chain. Am. J. Clin. Nutr. 1995, 61, 682S–686S. [Google Scholar] [CrossRef] [PubMed]
- Golbamaki, N.; Rasulev, B.; Cassano, A.; Marchese Robinson, R.; Benfenati, E.; Leszczynski, J.; Cronin, M. Genotoxicity of metal oxide nanomaterials: Review of recent data and discussion of possible mechanisms. Nanoscale 2015, 7, 2154–2198. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, J.; Zhang, X.; Chang, Y.; Chen, Y. The impact of ZnO nanoparticle aggregates on the embryonic development of zebrafish (Danio rerio). Nanotechnology 2009, 20, 195103. [Google Scholar] [CrossRef]
- Yu, L.; Fang, T.; Xiong, D.; Zhu, W.; Sima, X. Comparative toxicity of nano-ZnO and bulk ZnO suspensions to zebrafish and the effects of sedimentation, OH production and particle dissolution in distilled water. J. Environ. Monit. 2011, 13, 1975. [Google Scholar] [CrossRef] [PubMed]
- Bacchetta, R.; Maran, B.; Marelli, M.; Santo, N.; Tremolada, P. Role of soluble zinc in ZnO nanoparticle cytotoxicity in Daphnia magna: A morphological approach. Environ. Res. 2016, 148, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Pokhrel, S.; Bandyopadhyay, S.; Mädler, L.; Peralta-Videa, J.; Gardea-Torresdey, J. A soil mediated phyto-toxicological study of iron doped zinc oxide nanoparticles (Fe@ZnO) in green peas (Pisum sativum L.). Chem. Eng. J. 2014, 258, 394–401. [Google Scholar] [CrossRef]
- Alexander, D.; Fairbridge, R. Encyclopedia Of Environmental Science; Springer Science & Business Media: Berlin, Germany, 1999; pp. 1–23. [Google Scholar]
- Wagner, S.; Gondikas, A.; Neubauer, E.; Hofmann, T.; von der Kammer, F. Spot the Difference: Engineered and Natural Nanoparticles in the Environment-Release, Behavior, and Fate. Angew. Chem. Int. Ed. 2014, 53, 12398–12419. [Google Scholar] [CrossRef]
- Yung, M.; Kwok, K.; Djurišić, A.; Giesy, J.; Leung, K. Influences of temperature and salinity on physicochemical properties and toxicity of zinc oxide nanoparticles to the marine diatom Thalassiosira pseudonana. Sci. Rep. 2017, 7, 3662. [Google Scholar] [CrossRef]
- Lai, R.; Yung, M.; Zhou, G.; He, Y.; Ng, A.; Djurišić, A.; Shih, K.; Leung, K. Temperature and salinity jointly drive the toxicity of zinc oxide nanoparticles: A challenge to environmental risk assessment under global climate change. Environ. Sci. Nano 2020, 7, 2995–3006. [Google Scholar] [CrossRef]
- Park, J.; Kim, S.; Yoo, J.; Lee, J.; Park, J.; Jung, J. Effect of salinity on acute copper and zinc toxicity to Tigriopus japonicus: The difference between metal ions and nanoparticles. Mar. Pollut. Bull. 2014, 85, 526–531. [Google Scholar] [CrossRef]
- Chang, S.; Kou, G.; Liao, I. Salinity Adaptation and Subsidence Attributes in the Early Stages of Japanese Eel (Anguilla japonica). Acta Zool. Taiwanica 2003, 14, 33–44. [Google Scholar]
- Yang, Z.; Chen, Y. Induced ovulation using LHRHa in anadromous obscure puffer Takifugu obscurus cultured entirely in freshwater. Fish Physiol. Biochem. 2003, 29, 323–326. [Google Scholar] [CrossRef]
- Kwok, K.; Leung, K. Toxicity of antifouling biocides to the intertidal harpacticoid copepod Tigriopus japonicus (Crustacea, Copepoda): Effects of temperature and salinity. Mar. Pollut. Bull. 2005, 51, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Dahms, H.; Rhee, J.; Lee, Y.; Lee, J.; Han, K.; Lee, J. Expression profiles of seven glutathione S-transferase (GST) genes in cadmium-exposed river pufferfish (Takifugu obscurus). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2010, 151, 99–106. [Google Scholar] [CrossRef]
- Katusic, Z.; Vanhoutte, P. Superoxide anion is an endothelium-derived contracting factor. Am. J. Physiol-Heart C 1989, 257, H33–H37. [Google Scholar] [CrossRef]
- Ighodaro, O.; Akinloye, O. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Frankič, T.; Pajk, T.; Rezar, V.; Levart, A.; Salobir, J. The role of dietary nucleotides in reduction of DNA damage induced by T-2 toxin and deoxynivalenol in chicken leukocytes. Food Chem. Toxicol. 2006, 44, 1838–1844. [Google Scholar] [CrossRef] [PubMed]
- Rombough, P. The functional ontogeny of the teleost gill: Which comes first, gas or ion exchange? Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2007, 148, 732–742. [Google Scholar] [CrossRef]
- Sun, H.; Lü, K.; Minter, E.; Chen, Y.; Yang, Z.; Montagnes, D. Combined effects of ammonia and microcystin on survival, growth, antioxidant responses, and lipid peroxidation of bighead carp Hypophthalmythys nobilis larvae. J. Hazard. Mater 2012, 221–222, 213–219. [Google Scholar] [CrossRef]
- Yung, M.; Wong, S.; Kwok, K.; Liu, F.; Leung, Y.; Chan, W.; Li, X.; Djurišić, A.; Leung, K. Salinity-dependent toxicities of zinc oxide nanoparticles to the marine diatom Thalassiosira pseudonana. Aquat. Toxicol. 2015, 165, 31–40. [Google Scholar] [CrossRef]
- Kim, I.; Taghavy, A.; DiCarlo, D.; Huh, C. Aggregation of silica nanoparticles and its impact on particle mobility under high-salinity conditions. J. Pet. Sci. Eng. 2015, 133, 376–383. [Google Scholar] [CrossRef]
- VandeSteeg, H.; Cohen Stuart, M.; De Keizer, A.; Bijsterbosch, B. Polyelectrolyte adsorption: A subtle balance of forces. Langmuir 1992, 8, 2538–2546. [Google Scholar] [CrossRef]
- Dong, Z.; Zhang, W.; Qiu, Y.; Yang, Z.; Wang, J.; Zhang, Y. Cotransport of nanoplastics (NPs) with fullerene (C60) in saturated sand: Effect of NPs/C60 ratio and seawater salinity. Water Res. 2019, 148, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Verslycke, T.; Vangheluwe, M.; Heijerick, D.; De Schamphelaere, K.; Van Sprang, P.; Janssen, C. The toxicity of metal mixtures to the estuarine mysid Neomysis integer (Crustacea: Mysidacea) under changing salinity. Aquat. Toxicol. 2003, 64, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Loza, K.; Diendorf, J.; Sengstock, C.; Ruiz-Gonzalez, L.; Gonzalez-Calbet, J.; Vallet-Regi, M.; Köller, M.; Epple, M. The dissolution and biological effects of silver nanoparticles in biological media. J. Mater. Chem. B 2014, 2, 1634. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.; Leung, P.; Djurišić, A.; Leung, K. Toxicities of nano zinc oxide to five marine organisms: Influences of aggregate size and ion solubility. Anal. Bioanal. Chem. 2009, 396, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Adam, N.; Schmitt, C.; Galceran, J.; Companys, E.; Vakurov, A.; Wallace, R.; Knapen, D.; Blust, R. The chronic toxicity of ZnO nanoparticles and ZnCl2 to Daphnia magna and the use of different methods to assess nanoparticle aggregation and dissolution. Nanotoxicology 2014, 8, 709–717. [Google Scholar]
- Zander, D.; Thompson, J.; Lane, M. Perturbations in Mouse Embryo Development and Viability Caused by Ammonium Are More Severe after Exposure at the Cleavage Stages1. Biol. Reprod. 2006, 74, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Browning, L.; Lee, K.; Huang, T.; Nallathamby, P.; Lowman, J.; Nancy Xu, X. Random walk of single gold nanoparticles in zebrafish embryos leading to stochastic toxic effects on embryonic developments. Nanoscale 2009, 1, 138. [Google Scholar] [CrossRef] [PubMed]
- Bar-Ilan, O.; Albrecht, R.; Fako, V.; Furgeson, D. Toxicity Assessments of Multisized Gold and Silver Nanoparticles in Zebrafish Embryos. Small 2009, 5, 1897–1910. [Google Scholar] [CrossRef]
- Asharani, P.; Lianwu, Y.; Gong, Z.; Valiyaveettil, S. Comparison of the toxicity of silver, gold and platinum nanoparticles in developing zebrafish embryos. Nanotoxicology 2010, 5, 43–54. [Google Scholar] [CrossRef]
- Hua, J.; Vijver, M.; Richardson, M.; Ahmad, F.; Peijnenburg, W. Particle-specific toxic effects of differently shaped zinc oxide nanoparticles to zebrafish embryos (Danio rerio). Environ. Toxicol. Chem. 2014, 33, 2859–2868. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, S.; Wu, Y.; You, H.; Lv, L. Acute ZnO nanoparticles exposure induces developmental toxicity, oxidative stress and DNA damage in embryo-larval zebrafish. Aquat. Toxicol. 2013, 136–137, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Garner, W.; Garner, M.; Spector, A. H2O2-induced uncoupling of bovine lens Na+, K+-ATPase. Proc. Natl. Acad. Sci. USA 1983, 80, 2044–2048. [Google Scholar] [CrossRef] [PubMed]
- Richards, J. Na+/K+-ATPase α-isoform switching in gills of rainbow trout (Oncorhynchus mykiss) during salinity transfer. J. Exp. Biol. 2003, 206, 4475–4486. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Wang, G.; Li, S. Effects of low temperature acclimation on antioxidant defenses and ATPase activities in the muscle of mud crab (Scylla paramamosain). Aquaculture 2012, 370–371, 144–149. [Google Scholar] [CrossRef]
- Sharma, V.; Anderson, D.; Dhawan, A. Zinc oxide nanoparticles induce oxidative DNA damage and ROS-triggered mitochondria mediated apoptosis in human liver cells (HepG2). Apoptosis 2012, 17, 852–870. [Google Scholar] [CrossRef]
- Olsvik, P.; Kristensen, T.; Waagbø, R.; Rosseland, B.; Tollefsen, K.; Baeverfjord, G.; Berntssen, M. mRNA expression of antioxidant enzymes (SOD, CAT and GSH-Px) and lipid peroxidative stress in liver of Atlantic salmon (Salmo salar) exposed to hyperoxic water during smoltification. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2005, 141, 314–323. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.; Lee, I. Alteration of Phytotoxicity and Oxidant Stress Potential by Metal Oxide Nanoparticles in Cucumis sativus. Water Air Soil Pollut. 2012, 223, 2799–2806. [Google Scholar] [CrossRef]
- Gomes, T.; Pinheiro, J.; Cancio, I.; Pereira, C.; Cardoso, C.; Bebianno, M. Effects of Copper Nanoparticles Exposure in the Mussel Mytilus galloprovincialis. Environ. Sci. Technol. 2011, 45, 9356–9362. [Google Scholar] [CrossRef] [PubMed]
- Vinagre, C.; Madeira, D.; Narciso, L.; Cabral, H.; Diniz, M. Effect of temperature on oxidative stress in fish: Lipid peroxidation and catalase activity in the muscle of juvenile seabass, Dicentrarchus labrax. Ecol. Indic. 2012, 23, 274–279. [Google Scholar] [CrossRef]
- Xiong, D.; Fang, T.; Yu, L.; Sima, X.; Zhu, W. Effects of nano-scale TiO2, ZnO and their bulk counterparts on zebrafish: Acute toxicity, oxidative stress and oxidative damage. Sci. Total Environ. 2011, 409, 1444–1452. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.; Wang, J.; Dai, H.; Mao, F.; Chen, Q.; Yan, H.; Chen, M. Salinity Moderated the Toxicity of Zinc Oxide Nanoparticles (ZnO NPs) towards the Early Development of Takifugu obscurus. Int. J. Environ. Res. Public Health 2023, 20, 3209. https://doi.org/10.3390/ijerph20043209
Lin Y, Wang J, Dai H, Mao F, Chen Q, Yan H, Chen M. Salinity Moderated the Toxicity of Zinc Oxide Nanoparticles (ZnO NPs) towards the Early Development of Takifugu obscurus. International Journal of Environmental Research and Public Health. 2023; 20(4):3209. https://doi.org/10.3390/ijerph20043209
Chicago/Turabian StyleLin, Yuqing, Jun Wang, Huichao Dai, Feijian Mao, Qiuwen Chen, Hanlu Yan, and Mo Chen. 2023. "Salinity Moderated the Toxicity of Zinc Oxide Nanoparticles (ZnO NPs) towards the Early Development of Takifugu obscurus" International Journal of Environmental Research and Public Health 20, no. 4: 3209. https://doi.org/10.3390/ijerph20043209
APA StyleLin, Y., Wang, J., Dai, H., Mao, F., Chen, Q., Yan, H., & Chen, M. (2023). Salinity Moderated the Toxicity of Zinc Oxide Nanoparticles (ZnO NPs) towards the Early Development of Takifugu obscurus. International Journal of Environmental Research and Public Health, 20(4), 3209. https://doi.org/10.3390/ijerph20043209






