The Effects of Heavy Metal Pollution on Collembola in Urban Soils and Associated Recovery Using Biochar Remediation: A Review
Abstract
1. Introduction
2. Heavy Metal Contamination in Urban Soils from Around the World
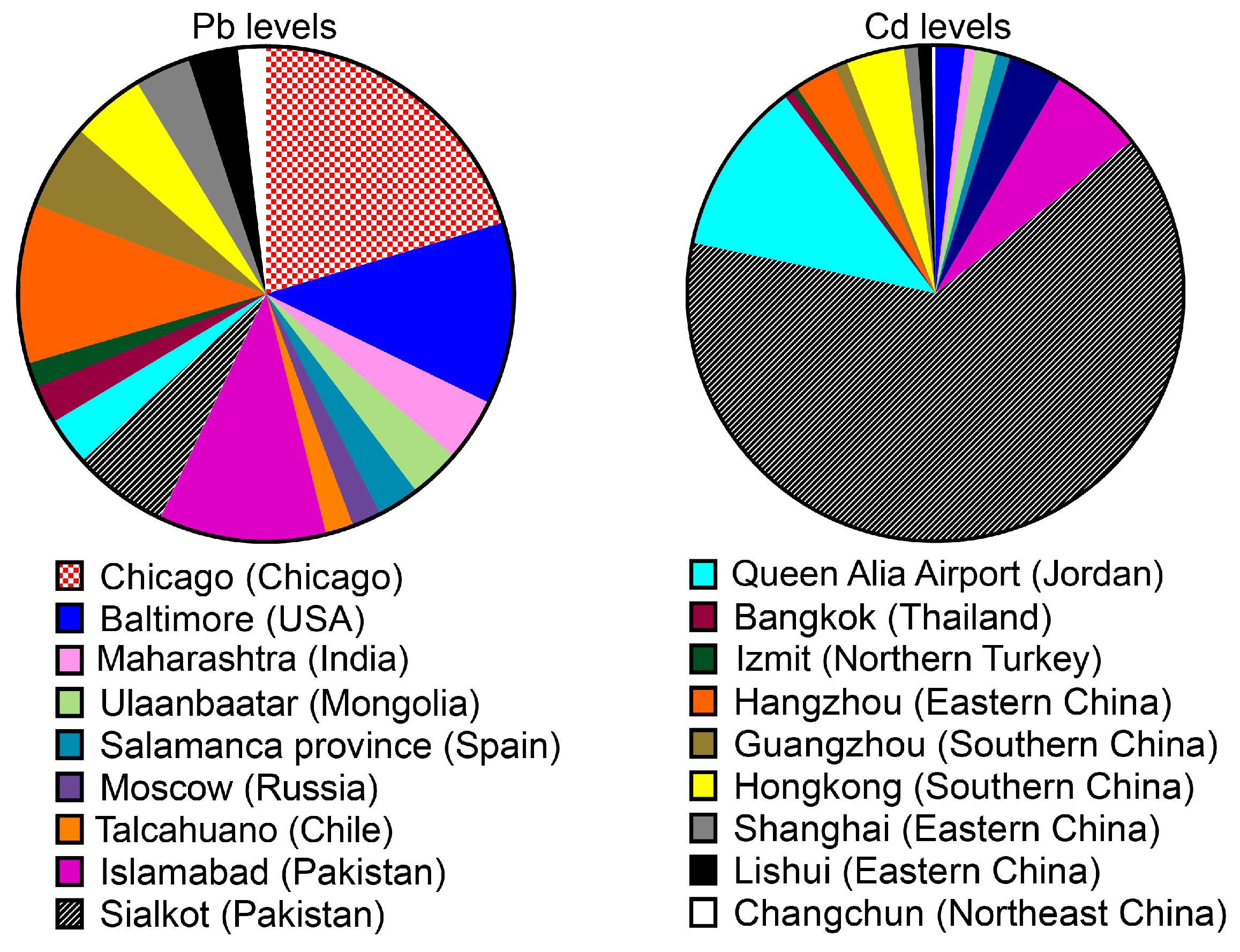
2.1. Levels of Pb and Cd Pollution in the Urban Soil
| Location | Sample | Pb | Cd | References | ||
|---|---|---|---|---|---|---|
| Mean | Range | Mean | Range | |||
| 34 European cities | 9954 | 102.00 | - | 0.95 | - | [56] |
| Salamanca province (Spain) | 16 | 53.10 | 20.10–96.20 | 0.53 | 0.20–0.95 | [58] |
| Maharashtra (India) | 12 | 80.00 | 35.90–49.20 | 0.40 | 21.60–38.6 | [59] |
| Queen Alia Airport (Jordan) | 32 | 60.20 ± 10.30 | 20.50–117.00 | 6.55 ± 1.29 | 2.50–1.50 | [60] |
| Talcahuano, Chile (South America) | 35.20 ± 43.3 | 8.00–129.00 | - | - | [61] | |
| Baltimore (USA) | 122 | 231.00 | 54.60–1013.70 | 1.1 | 0.54–1.83 | [66] |
| Chicago (Chicago) | 57 | 395.00 | - | - | - | [66] |
| Bangkok (Thailand) | 30 | 47.8 0 ± 52.70 | 12.10–269.30 | 0.34 ± 0.21 | 0.05–0.81 | [67] |
| Moscow (Russia) | 36 | 37.00 | - | 2.00 | - | [68] |
| Islamabad (Pakistan) | 307 | 212.34 ± 1.87 | - | 3.54 ± 0.06 | - | [69] |
| Sialkot (Pakistan) | 82 | 121.40 ± 18.05 | - | 36.80 ± 11.99 | - | [70] |
| Izmit industrial city (Northern Turkey) | 41 | 32.00 ± 11.00 | 8–45 | 0.21 ± 0.09 | 0.07–0.35 | [72] |
| Ulaanbaatar (Mongolia) | 22 | 64.00 | - | 0.8 | - | [71] |
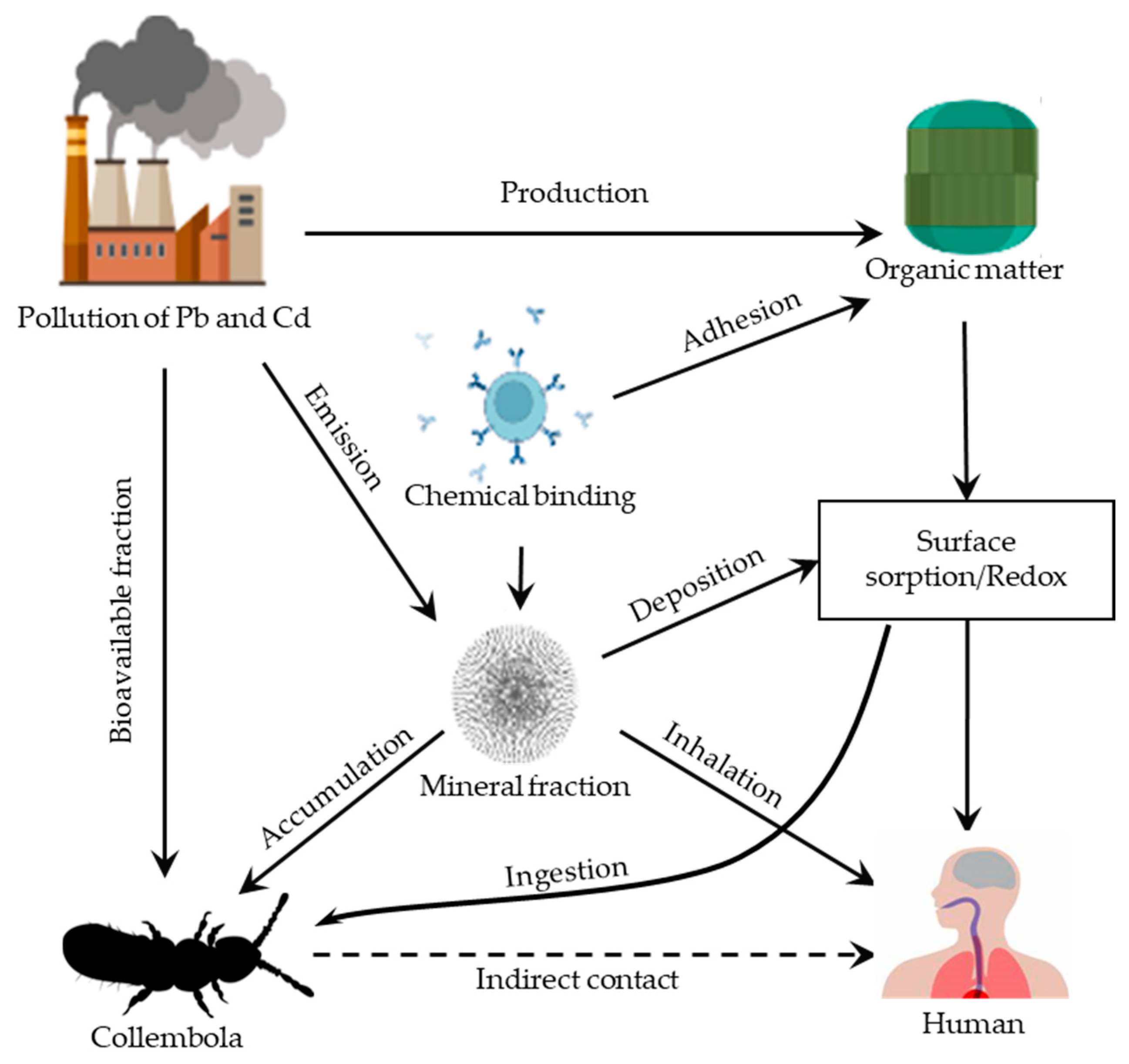
2.2. The Fate of Pb and Cd in Urban Soils
3. Toxicity of Pb and Cd on Collembolans in Contaminated Soil and Laboratory Tests
3.1. Performance of Collembolans in the Field
3.2. Performance of Collembolan Exposure Test in Laboratory
4. Factors Influencing the Toxicity of Pb and Cd to Collembolan Species
4.1. Interaction between Collembolans, Pb, and Cd
4.2. Bioavailable Fractions Predicting Metal Toxicity to Collembolans
4.3. Aging Time and Leaching
5. The Use of Biochar as a Response to Urban Soil Pollution
5.1. Biochar Production
5.2. Response of Biochar Application to Collembolan Species
6. Conclusion and Future Perspective
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, S.; Cao, Q.; Zheng, Y.M.; Huang, Y.Z.; Zhu, Y.G. Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in Beijing, China. Environ. Pollut. 2008, 152, 686–692. [Google Scholar] [CrossRef]
- Edwards, C.A. Assessing the effects of environmental pollutants on soil organisms, communities, processes and ecosystems. Eur. J. Soil Biol. 2002, 38, 225–231. [Google Scholar] [CrossRef]
- Xiao, W.; Lin, G.; He, X.; Yang, Z.; Wang, L. Interactions among heavy metal bioaccessibility, soil properties and microbial community in phyto-remediated soils nearby an abandoned realgar mine. Chemosphere 2021, 286 Pt 1, 131638. [Google Scholar] [CrossRef] [PubMed]
- Vareda, J.P.; Valente, A.J.M.; Duraes, L. Assessment of heavy metal pollution from anthropogenic activities and remediation strategies: A review. J. Environ. Manag. 2019, 246, 101–118. [Google Scholar] [CrossRef] [PubMed]
- Panagos, P.; Liedekerke, M.V.; Yigin, I.Y.; Montanarella, L. Contaminated sites in Europe: Review of the current situation based on data collected through a European network. J. Environ. Public Health 2013, 2013, 158764. [Google Scholar] [CrossRef]
- Wu, S.; Peng, S.; Zhang, X.; Wu, D.; Luo, W.; Zhang, T.; Zhou, S.; Yang, G.; Wan, H.; Wu, L. Levels and health risk assessments of heavy metals in urban soils in Dongguan, China. J. Geochem. Explor. 2015, 148, 71–78. [Google Scholar] [CrossRef]
- Rai, P.K. Heavy metal pollution in aquatic ecosystems and its phytoremediation using wetland plants: An ecosustainable approach. Int. J. Phytoremediation 2008, 10, 131–158. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhao, M.; Ma, X.; Song, Y.; Zuo, S.; Li, H.; Deng, W. A critical review on the interactions of microplastics with heavy metals: Mechanism and their combined effect on organisms and humans. Sci. Total Environ. 2021, 788, 147620. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.G.M.; Snow, E.T.; Tanaka, A. Arsenic and heavy metal contamination of vegetables grown in Samta village, Bangladesh. Sci. Total Environ. 2003, 308, 83–96. [Google Scholar] [CrossRef]
- Miller, J.R.; Hudson-Edwards, K.A.; Lechler, P.J.; Preston, D.; Macklin, M.G. Heavy metal contamination of water, soil and produce within riverine communities of the Rio Pilcomayo basin, Bolivia. Sci. Total Environ. 2004, 320, 189–209. [Google Scholar] [CrossRef]
- Mani, D.; Kumar, C.; Patel, N.K. Hyperaccumulator oilcake manure as an alternative for chelate-induced phytoremediation of heavy metals contaminated alluvial soils. Int. J. Phytoremediation 2015, 17, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Yang, N.; Li, Y.; Ren, B.; Ding, X.; Bian, H.; Yao, X. Total concentrations and sources of heavy metal pollution in global river and lake water bodies from 1972 to 2017. Glob. Ecol. Conserv. 2020, 22, e00925. [Google Scholar] [CrossRef]
- Zhang, H.; Pap, S.; Taggart, M.A.; Boyd, K.G.; James, N.A.; Gibb, S.W. A review of the potential utilisation of plastic waste as adsorbent for removal of hazardous priority contaminants from aqueous environments. Environ. Pollut. 2020, 258, 113698. [Google Scholar] [CrossRef]
- Luo, Z.; Ma, J.; Chen, F.; Li, X.; Zhang, S. Effects of Pb smelting on the soil bacterial community near a secondary lead plant. Int. J. Environ. Res. Public Health 2018, 15, 1030. [Google Scholar] [CrossRef] [PubMed]
- Enuneku, A.A.; Abhulimen, P.; Isibor, P.O.; Asemota, C.O.; Okpar, B.; Imoobe, T.O.; Ezemonye, L. Interactions of trace metals with bacteria and fungi in selected agricultural soils of Egbema Kingdom, Warri North, Delta state, Nigeria. Heliyon 2020, 6, e04477. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Yu, Z.; Liu, H.; Tan, T.; Yao, J.; Zhang, Y.; Wu, J. Effects of Cd and Pb on diversity of microbial community and enzyme activity in soil. Ecotoxicology 2020, 29, 551–558. [Google Scholar] [CrossRef]
- Kudo, A.; Miyahara, S. A case history; Minamata mercury pollution in Japan from loss of human lives to decontamination. Water Sci. Technol. 1991, 23, 283–290. [Google Scholar] [CrossRef]
- Li, X.; Huang, C. Environment impact of heavy metals on urban soil in the vicinity of industrial area of Baoji city, P.R. China. Environ. Geol. 2006, 52, 1631–1637. [Google Scholar] [CrossRef]
- Wei, B.; Yang, L. review of heavy metal contaminations in urban soils, urban road dusts and agricultural soils from China. Microchem. J. 2010, 94, 99–107. [Google Scholar] [CrossRef]
- Cheng, S. Heavy metal pollution in China: Origin, pattern and control. Environ. Sci. Pollut. Res. Int. 2003, 10, 192–198. [Google Scholar] [CrossRef]
- Huffer, T.; Weniger, A.K.; Hofmann, T. Sorption of organic compounds by aged polystyrene microplastic particles. Environ. Pollut. 2018, 236, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, T.S.; Holmstrup, M.A. Comparative analysis of the toxicity of eight common soil contaminants and their effects on drought tolerance in the collembolan Folsomia candida. Ecotoxicol. Environ. Saf. 2005, 60, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Crouau, Y.; Moia, C. The relative sensitivity of growth and reproduction in the springtail, Folsomia candida, exposed to xenobiotics in the laboratory: An indicator of soil toxicity. Ecotoxicol. Environ. Saf. 2006, 64, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Deharveng, L.; Chen, J.-X. New species and rediagnosis of Coecobrya (Collembola: Entomobryidae), with a key to the species of the genus. J. Nat. Hist. 2009, 43, 2597–2615. [Google Scholar] [CrossRef]
- Li, Y.; Pang, H.-D.; He, L.-Y.; Wang, Q.; Sheng, X.-F. Cd immobilization and reduced tissue Cd accumulation of rice (Oryza sativa wuyun-23) in the presence of heavy metal-resistant bacteria. Ecotoxicol. Environ. Saf. 2017, 138, 56–63. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Y.; Chen, Q.; Li, Y.; Guo, D.; Nie, X.; Peng, X. Assessment of heavy metal pollution and the effect on bacterial community in acidic and neutral soils. Ecol. Indic. 2020, 117, 106626. [Google Scholar] [CrossRef]
- Kumar, K.P.; Kumawat, P. A review on the effect of herbicides on the earthworms. Int. J. Zool. Stud. 2018, 3, 120–125. [Google Scholar]
- Cao, X.; Yang, C.; Liu, J.; Hui, X.; Yang, W.; Li, S.; Tian, Y.; Cai, L. DNA damage and effects on antioxidative enzymes in earthworm (Eisenia fetida) induced by flumorph. Appl. Biochem. Biotechnol. 2014, 172, 2276–2285. [Google Scholar] [CrossRef]
- Liu, J.; Luo, X.; Sun, Y.; Tsang, D.C.W.; Qi, J.; Zhang, W.; Li, N.; Yin, M.; Wang, J.; Lippold, H.; et al. Thallium pollution in China and removal technologies for waters: A review. Environ. Int. 2019, 126, 771–790. [Google Scholar] [CrossRef]
- Sun, X.; Marian, F.; Bluhm, C.; Maraun, M.; Scheu, S. Response of Collembola to the addition of nutrients along an altitudinal gradient of tropical montane rainforests. Appl. Soil Ecol. 2020, 147, 103382. [Google Scholar] [CrossRef]
- Sterzyńska, M.; Nicia, P.; Zandrozny, P.; Fiera, C.; Shrobovych, J. Urban springtail species richness decreases with increasing air pollution. Ecol. Indic. 2018, 94, 328–335. [Google Scholar] [CrossRef]
- Austruy, A.; Laplanche, C.; Mombo, S.; Dumat, C.; Deola, F.; Gers, C. Ecological changes in historically polluted soils: Metal(loid) bioaccumulation in microarthropods and their impact on community structure. Geoderma 2016, 271, 181–190. [Google Scholar] [CrossRef]
- Addison, J.A.; Trofymow, J.A.; Marshall, V.G. Abundance, species diversity, and community structure of Collembola in successional coastal temperate forests on Vancouver Island, Canada. Appl. Soil Ecol. 2003, 24, 233–246. [Google Scholar] [CrossRef]
- Syrek, D.; Weiner, W.M.; Wojtylak, M.; Olszowska, G.; Kwapis, Z. Species abundance distribution of collembolan communities in forest soils polluted with heavy metals. Appl. Soil Ecol. 2006, 31, 239–250. [Google Scholar] [CrossRef]
- Rizhiya, E.; Bertora, C.; Van Vliet, P.C.J.; Kuikman, P.J.; Faber, J.H.; Groenigen, J.W.V. Earthworm activity as a determinant for N2O emission from crop residue. Soil Biol. Biochem. 2007, 39, 2058–2069. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, J.; Ren, L.; Zhou, Y.; Gao, J.; Luo, L.; Yang, Y.; Peng, Q.; Huang, H.; Chen, A. Diagnosis of soil contamination using microbiological indices: A review on heavy metal pollution. J. Environ. Manag. 2019, 242, 121–130. [Google Scholar] [CrossRef]
- Šalamún, P.; Hanzerova, V.; Miklisova, D.; Brazova, D. Effect of heavy metals on soil nematode communities in the vicinity of a metallurgical plant in North Slovakia. Helminthologia 2015, 52, 252–260. [Google Scholar] [CrossRef]
- Pereira, L.B.; Vicentini, R.; Ottoboni, L.M. Changes in the bacterial community of soil from a neutral mine drainage channel. PLoS ONE 2014, 9, e96605. [Google Scholar] [CrossRef]
- Liu, M.; Xu, J.; Krogh, P.H.; Song, J.; Wu, L.; Luo, W.; Xin, K. Assessment of toxicity of heavy metal-contaminated soils toward Collembola in the paddy fields supported by laboratory tests. Environ. Sci. Pollut. Res. Int. 2018, 25, 16969–16978. [Google Scholar] [CrossRef]
- Wang, J.H.; Zhu, L.S.; Meng, Y.; Wang, J.; Xie, H.; Zhang, Q.M. The combined stress effects of atrazine and cadmium on the earthworm Eisenia fetida. Environ. Toxicol. Chem. 2012, 31, 2035–2040. [Google Scholar] [CrossRef]
- Uwizeyimana, H.; Wang, M.; Chen, W.; Khan, K. The eco-toxic effects of pesticide and heavy metal mixtures towards earthworms in soil. Environ. Toxicol. Pharmacol. 2017, 55, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.B.; Zheng, Y.M.; Lei, M.; Huang, Z.C.; Wu, H.T.; Chen, H.; Fan, K.K.; Yu, K.; Wu, X.; Tian, Q.Z. Assessment of heavy metal pollution in surface soils of urban parks in Beijing, China. Chemosphere 2005, 60, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Mahar, A.; Wang, P.; Li, R.; Zhang, Z. Immobilization of lead and cadmium in contaminated soil using amendments: A review. Pedosphere 2015, 25, 555–568. [Google Scholar] [CrossRef]
- Luo, Z.; Kayiranga, A.; Uwiringiyimana, E.; Zhnag, Q.; Yan, C.; Guo, J.; Xing, B. Thallium contamination in agricultural soils and associated potential remediation via biochar utilization. Biochar 2020, 2, 33–46. [Google Scholar] [CrossRef]
- Zemanova, V.; Traka, L.L.; OcheCova, P.; Száková, J.; Pavlíková, D. The sorption behaviour of Cd, Cu, Pb and Zn: A model experiment. In Proceedings of the 18th International Conference on Reasonable Use of Fertilizers, Modesto, CA, USA, 30–31 October 2012; pp. 204–209. [Google Scholar]
- He, L.; Zhong, H.; Liu, G.; Dai, Z.H.; Brooks, P.C.; Xu, J. Remediation of heavy metal contaminated soils by biochar: Mechanisms, potential risks and applications in China. Environ. Pollut. 2019, 252, 846–855. [Google Scholar] [CrossRef]
- Kayiranga, A.; Luo, Z.; Ndayishimiye, J.; Nkinahamira, F.; Cyubahiro, E.; Habumugisha, T.; Yan, C.; Guo, J.; Zhen, Z.; Tuyishimire, A.; et al. Insights into thallium adsorption onto the soil, bamboo-derived biochar, and biochar amended soil in Pomelo orchard. Biochar 2021, 3, 315–328. [Google Scholar] [CrossRef]
- Reibe, K.; Götz, K.P.; Roß, C.L.; Döring, T.F.; Ellmer, F.; Ruess, L. Impact of quality and quantity of biochar and hydrochar on soil Collembola and growth of spring wheat. Soil Biol. Biochem. 2015, 83, 84–87. [Google Scholar] [CrossRef]
- Fountain, M.T.; Hopkin, S.P. Folsomia candida (Collembola): A “standard” soil arthropod. Annu. Rev. Entomol. 2005, 50, 201–222. [Google Scholar] [CrossRef]
- Shi, G.; Chen, Z.; Xu, S.; Zhang, J.; Wang, L.; Bi, C.; Teng, J. Potentially toxic metal contamination of urban soils and roadside dust in Shanghai, China. Environ. Pollut. 2008, 156, 251–260. [Google Scholar] [CrossRef]
- Li, X.; Poon, C.S.; Liu, P.S. Heavy metal contamination of urban soils and street dusts in Hong Kong. Appl. Geochem. 2001, 16, 1361–1368. [Google Scholar] [CrossRef]
- Lu, Y.; Zhu, F.; Chen, J.; Gan, H.; Guo, Y. Chemical fractionation of heavy metals in urban soils of Guangzhou, China. Environ. Monit. Assess 2007, 134, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, H. Concentrations and chemical forms of potentially toxic metals in road-deposited sediments from different zones of Hangzhou, China. J. Environ. Sci. 2009, 21, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lu, S. Spatial distribution, source identification and affecting factors of heavy metals contamination in urban-suburban soils of Lishui city, China. Environ. Earth Sci. 2011, 64, 1921–1929. [Google Scholar] [CrossRef]
- Yang, Z.; Lu, W.; Long, W.; Bao, X.; Yang, Q. Assessment of heavy metals contamination in urban topsoil from Changchun City, China. J. Geochem. Explor. 2011, 108, 27–38. [Google Scholar] [CrossRef]
- Luo, X.S.; Yu, S.; Zhu, Y.G.; Li, X.D. Trace metal contamination in urban soils of China. Sci. Total Environ. 2012, 421–422, 17–30. [Google Scholar] [CrossRef]
- Antrop, M. Landscape change and the urbanization process in Europe. Landsc. Urban Plan. 2004, 67, 9–26. [Google Scholar] [CrossRef]
- Sfinchez, C.M.; Martin, M.J.S.; Lorenzo, L.F. Lead and cadmium in soils and vegetables from urban gardens of Salamanca (Spain). Sci. Total Environ. 1994, 146, 163–168. [Google Scholar]
- Bhagure, G.R.; Mirgane, S.R. Heavy metal concentrations in groundwaters and soils of Thane Region of Maharashtra, India. Environ. Monit. Assess 2011, 173, 643–652. [Google Scholar] [CrossRef]
- Al-Khashman, O.A.; Shawabkeh, R.A. Metal distribution in urban soil around steel industry beside Queen Alia Airport, Jordan. Environ. Geochem. Health 2009, 31, 717–726. [Google Scholar] [CrossRef]
- Tume, P.; Bech, J.; Sepulveda, B.; Tume, L.; Bech, J. Concentrations of heavy metals in urban soils of Talcahuano (Chile): A preliminary study. Environ. Monit. Assess 2008, 140, 91–98. [Google Scholar] [CrossRef]
- Maas, S.R.; Benslama, M.; Crini, N.; Lucot, E.; Brahmia, Z.; Benyacoub, S.; Giraudoux, P. Spatial distribution of heavy metal concentrations in urban, suburban and agricultural soils in a Mediterranean city of Algeria. Environ. Pollut. 2010, 158, 2294–2301. [Google Scholar] [CrossRef] [PubMed]
- Magni, L.F.; Castro, L.N.; Rendina, A.E. Evaluation of heavy metal contamination levels in river sediments and their risk to human health in urban areas: A case study in the Matanza-Riachuelo Basin, Argentina. Environ. Res. 2021, 197, 110979. [Google Scholar] [CrossRef] [PubMed]
- Anthony, K.G.; Singh, B. Heavy metals contamination in vegetables grown in urban and metal smelter contaminated sites in Australia. Water Air Soil Pollut. 2006, 169, 101–123. [Google Scholar]
- Milenkovic, B.; Stajic, J.M.; Gulan, L.J.; Zeremski, T.; Nikezic, D. Radioactivity levels and heavy metals in the urban soil of Central Serbia. Environ. Sci. Pollut. Res. Int. 2015, 22, 16732–16741. [Google Scholar] [CrossRef]
- Cannon, W.F.; Horton, J.D. Soil geochemical signature of urbanization and industrialization—Chicago, Illinois, USA. Appl. Geochem. 2009, 24, 1590–1601. [Google Scholar] [CrossRef]
- Wilcke, W.; Muller, S.; Kanchanakool, N.; Zech, W. Urban soil contamination in Bangkok: Heavy metaland aluminium partitioning in topsoils. Geoderma 1998, 86, 211–228. [Google Scholar] [CrossRef]
- Plyaskina, O.V.; Ladonin, D.V. Heavy metal pollution of urban soils. Eurasian Soil Sci. 2009, 42, 816–823. [Google Scholar] [CrossRef]
- Ali, S.M.; Malik, R.N. Spatial distribution of metals in top soils of Islamabad City, Pakistan. Environ. Monit. Assess 2011, 172, 1–16. [Google Scholar] [CrossRef]
- Malik, R.N.; Jadoon, W.A.; Husain, S.Z. Metal contamination of surface soils of industrial city Sialkot, Pakistan: A multivariate and GIS approach. Environ. Geochem. Health 2010, 32, 179–191. [Google Scholar] [CrossRef]
- Batjargal, T.; Otgonjargal, E.; Baek, K.; Yang, J.S. Assessment of metals contamination of soils in Ulaanbaatar, Mongolia. J. Hazard. Mater. 2010, 184, 872–876. [Google Scholar] [CrossRef]
- Canbay, M.; Aydin, A.; Kurtulus, C. Magnetic susceptibility and heavy-metal contamination in topsoils along the Izmit Gulf coastal area and IZAYTAS (Turkey). J. Appl. Geophy. 2010, 70, 46–57. [Google Scholar] [CrossRef]
- Yang, W.J.; Ding, K.B.; Zhang, P.; Qiu, H.; Cloquet, C.; Wen, H.J.; Morel, J.L.; Qiu, R.L.; Tang, Y.T. Cadmium stable isotope variation in a mountain area impacted by acid mine drainage. Sci. Total Environ. 2019, 646, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Chi, T.; Zuo, J.; Liu, F. Performance and mechanism for cadmium and lead adsorption from water and soil by corn straw biochar. Front. Environ. Sci. Eng. 2017, 11, 15. [Google Scholar] [CrossRef]
- Ford, R.G.; Wilkin, R.T. Monitored Natural Attenuation of Inorganic Contaminants in Ground Water; EPA/600/R-07/139; U.S. Environmental Protection Agency: Washington, DC, USA, 2007. [Google Scholar]
- Kubier, A.; Wilkin, R.T.; Pichler, T. Cadmium in soils and groundwater: A review. Appl. Geochem. 2019, 108, 104388. [Google Scholar] [CrossRef]
- Lovering, T.G. Lead in the Environment; Professional Paper 957; USGS: Washington, DC, USA, 1976. [Google Scholar]
- Dai, W.; Holmstrup, M.; Slotsbo, S.; Ke, X.; Li, Z.; Gao, M.; Wu, L. Compartmentation and effects of lead (Pb) in the collembolan, Folsomia candida. Environ. Sci. Pollut. Res. Int. 2020, 27, 43638–43645. [Google Scholar] [CrossRef]
- Ding, Y.; Li, Z.; Ke, X.; Wu, L.; Zuo, S. Toxicity of lead pollution to the collembolan Folsomia candida in Ferri-Udic Cambosols. Pedosphere 2021, 31, 627–637. [Google Scholar] [CrossRef]
- Menta, C.; Maggiani, A.; Vattuone, Z. Effects of Cd and Pb on the survival and juvenile production of Sinella coeca and Folsomia candida. Eur. J. Soil Biol. 2006, 42, 181–189. [Google Scholar] [CrossRef]
- Bur, T.; Crouau, Y.; Bianco, A.; Gandois, L.; Probst, A. Toxicity of Pb and of Pb/Cd combination on the springtail Folsomia candida in natural soils: Reproduction, growth and bioaccumulation as indicators. Sci. Total Environ. 2012, 414, 187–197. [Google Scholar] [CrossRef]
- Fountain, M.T.; Hopkin, S.P. Continuous monitoring of Folsomia candida (Insecta: Collembola) in a metal exposure test. Ecotoxicol. Environ. Saf. 2001, 48, 275–286. [Google Scholar] [CrossRef]
- Xu, J.; Ke, X.; Krogh, P.H.; Wang, Y.; Luo, Y.M.; Song, J. Evaluation of growth and reproduction as indicators of soil metal toxicity to the Collembolan, Sinella curviseta. Insect Sci. 2009, 16, 57–63. [Google Scholar] [CrossRef]
- Greenslade, P.; Vaughan, G.T. A comparison of Collembola species for toxicity testing of Australian soils. Pedobiologia 2003, 47, 171–179. [Google Scholar] [CrossRef]
- Vijver, M.; Jager, T.; Posthuma, L.; Peijnenburg, W. Impact of metal pools and soil properties on metal accumulation in Folsomia candida (Collembola). Environ. Toxicol. Chem. 2001, 20, 712–720. [Google Scholar] [CrossRef]
- Menta, C.; Siniscarlco, C.; Bonati, B.; Remelli, S. Food choice and fitness of Folsomia candida (Collembola, Isotomidae) fed on twelve species of truffle. Front. Environ. Sci. 2019, 7, 114. [Google Scholar] [CrossRef]
- Langdon, C.J.; Piearce, T.G.; Meharg, A.A.; Semple, K.T. Interactions between earthworms and arsenic in the soil environment: A review. Environ. Pollut. 2003, 124, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Sun, Z.; Zhao, L.; Zhou, C.; Wu, Z.; Hou, H. The toxicity thresholds of metal(loid)s to soil-dwelling springtail Folsomia candida: A review. Ecotoxicol. Environ. Saf. 2019, 180, 632–645. [Google Scholar] [CrossRef]
- Criel, P.; Lock, K.; Eeckhout, H.V.; Oorts, K.; Smolders, E.; Janssen, C.R. influence of soil properties on copper toxicity for two soil invertebrates. Environ. Toxicol. Chem. 2008, 27, 1748–1755. [Google Scholar] [CrossRef]
- Speir, T.W.; Kettles, H.A.; Percival, H.J.; Parshotam, A. Is soil acidification the cause of biochemical responses when soils are amended with heavy metal salts? Soil Biol. Biochem. 1999, 31, 1953–1961. [Google Scholar] [CrossRef]
- Sandifer, R.D.; Hopkin, S.P. Effects of pH on the toxicity of cadmium, copper, lead and zinc to Folsomia candida Willem, 1902 (Collembola) in a standard laboratory test system. Chemosphere 1996, 33, 2475–2486. [Google Scholar] [CrossRef]
- Jansen, M.P.M.; Bergem, W.F. The effect of temperature on cadmium kinetics and oxygen consumption in soil arthropods. Environ. Toxicol. Chem. 1991, 10, 1493–1501. [Google Scholar] [CrossRef]
- Giller, K.E.; Witter, E.; Mcgrath, S.P. Toxicity of heavy metals to microorganisms and microbial processes in agricultural soils: A review. Soil Biol. Biochem. 1998, 30, 1389–1414. [Google Scholar] [CrossRef]
- Shahid, M.; Shamshad, S.; Rafiq, M.; Khalid, S.; Bibi, I.; Niazi, N.K.; Dumat, C.; Rashid, M.I. Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: A review. Chemosphere 2017, 178, 513–533. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, M.; Konvalina, P.; Neugschwandtner, R.W.; Kopecký, M.; Amirahmadi, E.; Moudrý, J.; Menšík, L. Preliminary findings on cadmium bioaccumulation and photosynthesis in rice (Oryza sativa L.) and maize (Zea mays L.) using biochar made from C3- and C4-originated straw. Plants 2022, 11, 1424. [Google Scholar] [CrossRef] [PubMed]
- Van Gestel, C.A.M.; Mol, S. The influence of soil characteristics on cadmium toxicity for Folsomia candida (Collembola: Isotomidae). Pedobiologia 2003, 47, 387–395. [Google Scholar] [CrossRef]
- Jiang, C.; Sun, H.; Sun, T.; Zhang, Q.; Zhang, Y. Immobilization of cadmium in soils by uv-mutated bacillus subtilis 38 bioaugmentation and novogro amendment. J. Hazard. Mater. 2009, 167, 1170–1177. [Google Scholar] [CrossRef]
- Jiang, T.Y.; Jiang, J.; Xu, R.K.; Li, Z. Adsorption of pb(ii) on variable charge soils amended with rice-straw derived biochar. chemosphere 2012, 89, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Owojori, O.J.; Reinecke, A.J.; Rozanov, A.B. Effects of salinity on partitioning, uptake and toxicity of zinc in the earthworm Eisenia fetida. Soil Biol. Biochem. 2008, 40, 2385–2393. [Google Scholar] [CrossRef]
- Van Gestel, C.A.M.; Koolhaas, J.E.E. Water-extractability, free ion activity, and pH explain cadmium sorption and toxicity to Folsomia candida (Collembola) in seven soil-pH combinations. Environ. Toxicol. Chem. 2004, 23, 1822–1833. [Google Scholar] [CrossRef]
- Pedersen, M.B.; Van Gestel, C.A.M.; Elmegaard, N. Effects of copper on reproduction of two collembolan species exposed through soil, food, and water. Environ. Toxicol. Chem. 2000, 19, 2579–2588. [Google Scholar] [CrossRef]
- Son, J.; Ryoo, M.; Jung, J.; Cho, K. Effects of cadmium, mercury and lead on the survival and instantaneous rate of increase of Paronychiurus kimi (Lee) (Collembola). Appl. Soil Ecol. 2007, 35, 404–411. [Google Scholar] [CrossRef]
- Nikolic, T.V.; Kojić, D.; Orčić, S.; Batinić, D. The impact of sublethal concentrations of Cu, Pb and Cd on honey bee redox status, superoxide dismutase and catalase in laboratory conditions. Chemosphere 2016, 164, 98–105. [Google Scholar] [CrossRef]
- Van Gestel, C.A.M.; van Diepen, A.M.F. The influence of soil moisture content on the bioavailability and toxicity of cadmium for Folsomia candida Willem (Collembola: Isotomidae). Ecotoxicol. Environ. Saf. 1997, 36, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Lock, K.; Janssen, C.R. Ecotoxicity of zinc in spiked artificial soils versus contaminated field soils. Environ. Sci. Technol. 2001, 35, 4295–4300. [Google Scholar] [CrossRef] [PubMed]
- Smolders, E.; Oorts, K.; Peeter, S.; Lanno, R.; Cheyns, K. Toxicity in lead salt spiked soils to plants, invertebrates and microbial processes: Unraveling effects of acidification, salt stress and ageing reactions. Sci. Total. Environ. 2015, 536, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Lock, k.; Waegeneers, N.; Smolders, E.; Criel, P.; Eeckhout, H.V.; Janssen, C.R. Effect of leaching and aging on the bioavailability of lead to the springtail Folsomia candida. Environ. Toxicol. Chem. 2006, 25, 2006–2010. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Cui, L.; Lin, O.; Li, G.; Zhao, X. Efficiency of sewage sludge biochar in improving urban soil properties and promoting grass growth. Chemosphere 2017, 173, 551–556. [Google Scholar] [CrossRef]
- Liu, H.; Xu, F.; Xie, Y.; Wang, C.; Zhang, A.; Li, L.; Xu, H. Effect of modified coconut shell biochar on availability of heavy metals and biochemical characteristics of soil in multiple heavy metals contaminated soil. Sci. Total Environ. 2018, 645, 702–709. [Google Scholar] [CrossRef]
- Silva, F.C.; Borrego, C.; Keizer, J.J.; Amorim, J.H.; Verheijen, F.G.A. Effects of moisture content on wind erosion thresholds of biochar. Atmos. Environ. 2015, 123, 121–128. [Google Scholar] [CrossRef]
- Laird, D.; Fleming, F.; Wang, B.; Horton, R.; Karlen, D. Biochar impact on nutrient leaching from a Midwestern agricultural soil. Geoderma 2010, 158, 436–442. [Google Scholar] [CrossRef]
- Zama, E.F.; Reid, B.J.; Arp, H.P.H.; Sun, G.-X.; Yuan, H.-Y.; Zhu, Y.-G. Advances in research on the use of biochar in soil for remediation: A review. J. Soil Sediment. 2018, 18, 2433–2450. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, J.; Zhao, J.; Luo, Z.; Tu, S.; Yin, Y. Properties of biochar obtained from pyrolysis of bamboo shoot shell. J. Anal. Appl. Pyrolysis. 2015, 114, 172–178. [Google Scholar] [CrossRef]
- Nartey, O.D.; Zhao, B. Biochar preparation, characterization, and adsorptive capacity and its effect on bioavailability of contaminants: An overview. Adv. Mater. Sci. Eng. 2014, 2014, 71539. [Google Scholar] [CrossRef]
- Tang, J.; Zhu, W.; Kookana, R.; Katayama, A. Characteristics of biochar and its application in remediation of contaminated soil. J. Biosci. Bioeng. 2013, 116, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Rillig, M.C.; Thies, T.; Masiello, C.A.; Hockaday, W.C. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Marks, E.A.N.; Mattana, S.; Alcañiz, J.M.; Domene, X. Biochars provoke diverse soil mesofauna reproductive responses in laboratory bioassays. Eur. J. Soil Biol. 2014, 60, 104–111. [Google Scholar] [CrossRef]
- Pathy, A.; Ray, J.; Paramasivan, B. Biochar amendments and its impact on soil biota for sustainable agriculture. Biochar 2020, 2, 287–305. [Google Scholar] [CrossRef]
- Gorovtsov, A.V.; Minkina, T.M.; Mandzhieva, S.S.; Perelomov, L.V.; Soja, G.; Zamulina, I.V.; Yao, J. The mechanisms of biochar interactions with microorganisms in soil. Environ. Geochem. Health 2020, 42, 2495–2518. [Google Scholar] [CrossRef]
- Zhong, L.-B.; Yin, J.; Liu, S.G.; Liu, Q.; Yang, Y.S.; Zheng, Y.-M. Facile one-pot synthesis of urchin-like Fe-Mn binary oxide nanoparticles for effective adsorption of Cd(ii) from water. RSC Adv. 2016, 6, 103438–103445. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Sizmur, T.; Quilliam, R.; Puga, A.P.; Moreno-Jiménez, E.; Beesley, L.; Gomez-Eyles, J.L. Application of biochar for soil remediation. Agric. Environ. Appl. Biochar Adv. Barriers 2016, 63, 295–324. [Google Scholar] [CrossRef]
- Ahmad, M.; Lee, S.S.; Rajapaksha, A.U.; Vithanage, M.; Zhang, M.; Cho, J.S.; Lee, S.-U.; Ok, Y.S. Trichloroethylene adsorption by pine needle biochars produced at various pyrolysis temperatures. Bioresour. Technol. 2013, 143, 615–622. [Google Scholar] [CrossRef]
- Sizmur, T.; Fresno, T.; Akgül, G.; Frost, H.; Moreno-Jiménez, E. Biochar modification to enhance sorption of inorganics from water. Bioresour. Technol. 2017, 246, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Beesley, L.; Marmiroli, M. The immobilisation and retention of soluble arsenic, cadmium and zinc by biochar. Environ. Pollut. 2011, 159, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, M.; Konvalina, P.; Neugschwandtner, R.W.; Kopecký, M.; Amirahmadi, E.; Bucur, D.; Walkiewicz, A. Interaction of biochar with chemical, green and biological nitrogen fertilizers on nitrogen use efficiency indices. Agronomy 2022, 12, 2106. [Google Scholar] [CrossRef]
- Das, S.K.; Ghosh, G.K.; Avasthe, R. Application of biochar in agriculture and environment, and its safety issues. Biomass Convers. Biorefin. 2020, 13, 1359–1369. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kayiranga, A.; Li, Z.; Isabwe, A.; Ke, X.; Simbi, C.H.; Ifon, B.E.; Yao, H.; Wang, B.; Sun, X. The Effects of Heavy Metal Pollution on Collembola in Urban Soils and Associated Recovery Using Biochar Remediation: A Review. Int. J. Environ. Res. Public Health 2023, 20, 3077. https://doi.org/10.3390/ijerph20043077
Kayiranga A, Li Z, Isabwe A, Ke X, Simbi CH, Ifon BE, Yao H, Wang B, Sun X. The Effects of Heavy Metal Pollution on Collembola in Urban Soils and Associated Recovery Using Biochar Remediation: A Review. International Journal of Environmental Research and Public Health. 2023; 20(4):3077. https://doi.org/10.3390/ijerph20043077
Chicago/Turabian StyleKayiranga, Alexis, Zhu Li, Alain Isabwe, Xin Ke, Claudien Habimana Simbi, Binessi Edouard Ifon, Haifeng Yao, Bin Wang, and Xin Sun. 2023. "The Effects of Heavy Metal Pollution on Collembola in Urban Soils and Associated Recovery Using Biochar Remediation: A Review" International Journal of Environmental Research and Public Health 20, no. 4: 3077. https://doi.org/10.3390/ijerph20043077
APA StyleKayiranga, A., Li, Z., Isabwe, A., Ke, X., Simbi, C. H., Ifon, B. E., Yao, H., Wang, B., & Sun, X. (2023). The Effects of Heavy Metal Pollution on Collembola in Urban Soils and Associated Recovery Using Biochar Remediation: A Review. International Journal of Environmental Research and Public Health, 20(4), 3077. https://doi.org/10.3390/ijerph20043077








