Management of Chronic Atrophic Candidiasis (Denture Stomatitis)—A Narrative Review
Abstract
1. Introduction
1.1. Pathogenesis
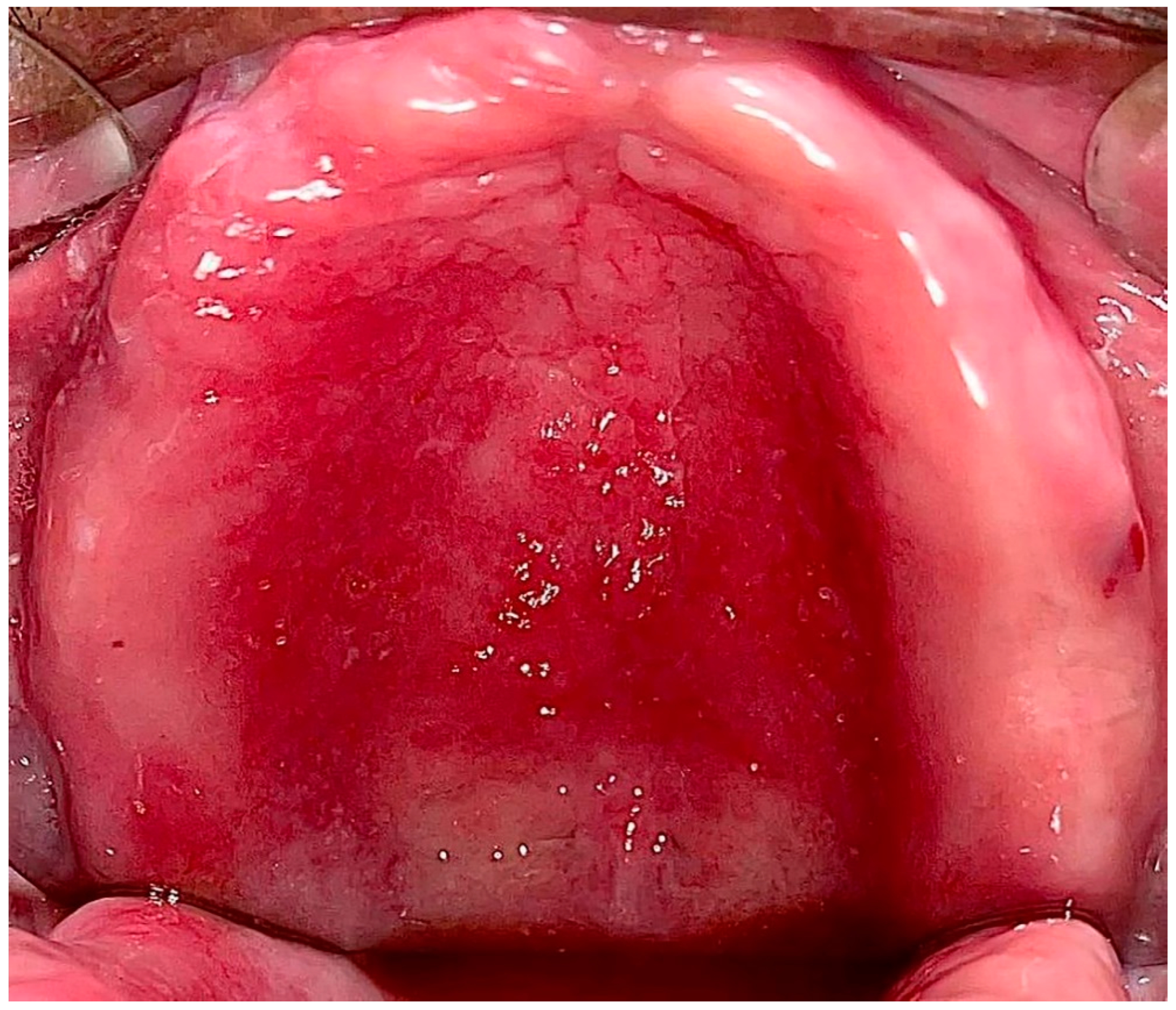
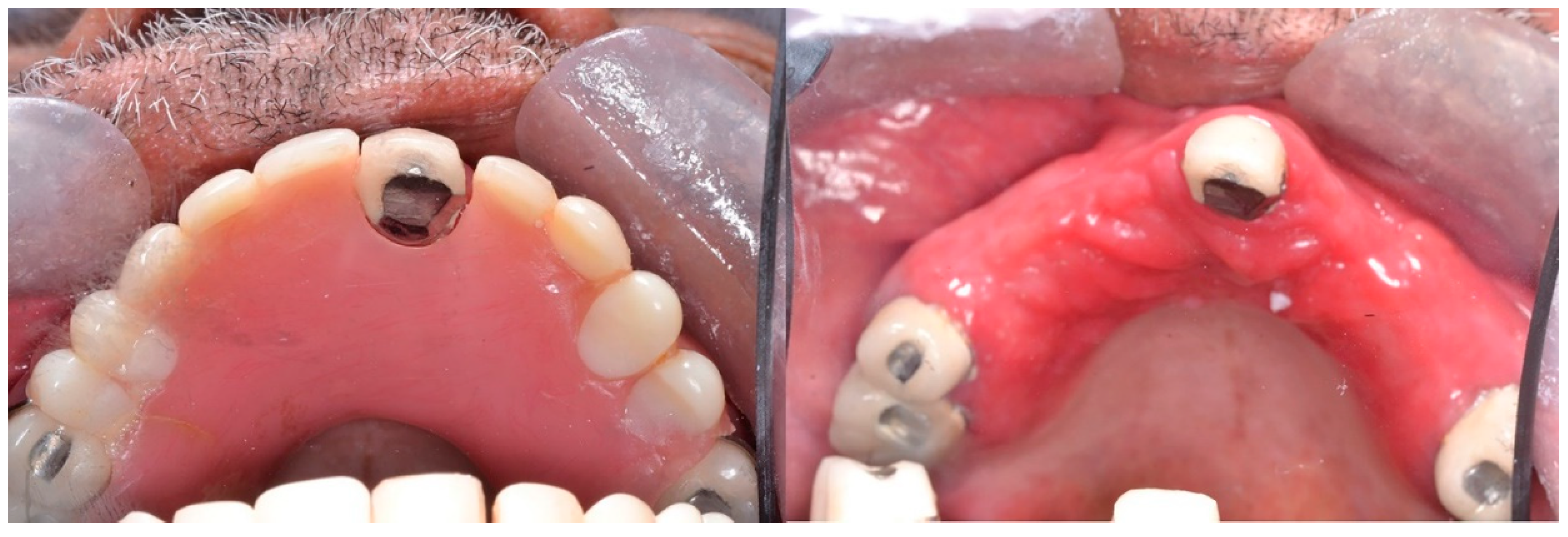
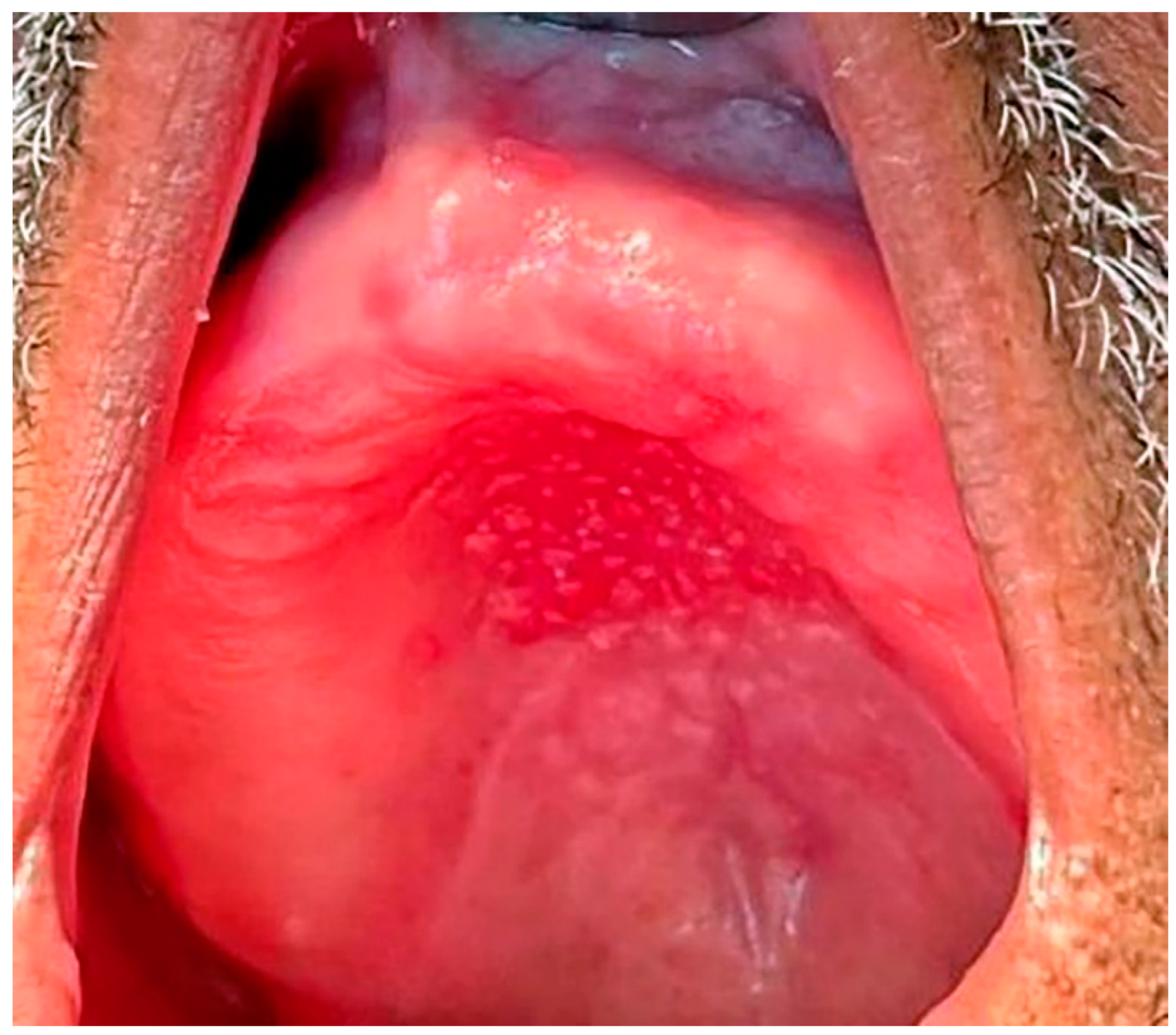
1.2. Classification, Prevalence, and Clinical Presentation
2. Methods
2.1. Eligibility Criteria
- Type of study: Randomized controlled clinical trials (RCTs) and systematic reviews; (based on RCTs) on the management of DS in human subjects;
- Period: Studies published from January 2012 to December 2022;
- Language: Studies published in English were considered.
- o
- Animal or cadaveric studies;
- o
- In vitro studies;
- o
- Studies conducted before 2012;
- o
- Studies published in languages other than English;
- o
- Unpublished studies.
2.2. Information Sources
2.3. Search Strategy
2.4. Data Screening
3. Results
Study Selection
4. Discussion
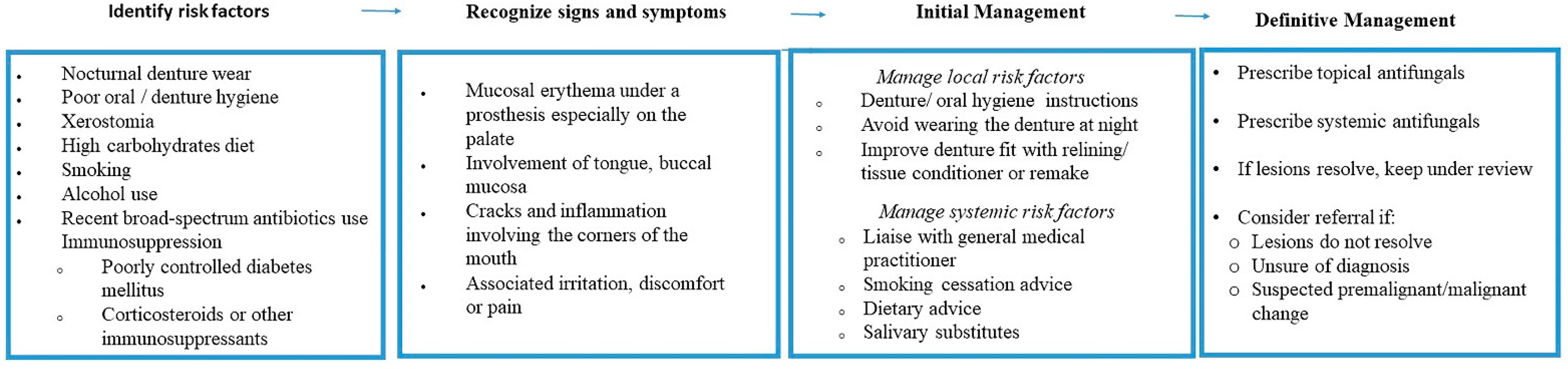
Identification of Risk Factors
- (a)
- Oral and denture hygiene protocol
- Brushing the hard palate for 2 min, three times a day with soft toothbrush bristles and water;
- During the day, brush the dentures for 2 min, three times a day, with a denture brush and neutral, non-abrasive liquid soap;
- Before going to bed at night, soak the dentures in 150 mL of 0.25–0.5% sodium hypochlorite for 10–20 min, followed by the overnight storage of the dentures in fresh, clean water at room temperature.
- (b)
- Antifungal therapy
- Dry powder forms: Approximately ⅛ teaspoonful of dry powder is added to 4 ounces of water and stirred thoroughly. The medicine can then be used as a mouthwash/gargle;
- Lozenges (pastilles): Need to be held in the mouth and allowed to dissolve slowly over 15–30 min;
- 1–2 Tablets or lozenges may be used 3–5 times/day;
- Oral suspensions: Hold 4 to 6 milliliters (mL) in the mouth for a few minutes, then swish it around, and gargle before swallowing it. Repeat four times/day.
- (c)
- Nanoparticles and antimicrobials
- (d)
- Photodynamic therapy
- (e)
- Phytomedicine
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sivaramakrishnan, G.; Sridharan, K. Alternatives to antifungal therapy for denture stomatitis: A systematic review and meta-analysis. Saudi J. Oral Sci. 2017, 4, 67–71. [Google Scholar] [CrossRef]
- Ribeiro, A.B.; de Araújo, C.B.; Silva, L.E.V.; Fazan-Junior, R.; Salgado, H.C.; Ribeiro, A.B.; Fortes, C.V.; Bueno, F.L.; de Oliveira, V.C.; de FO Paranhos, H.; et al. Hygiene protocols for the treatment of denture-related stomatitis: Local and systemic parameters analysis—A randomized, double-blind trial protocol. Trials 2019, 20, 661. [Google Scholar] [CrossRef]
- de Souza, R.; Chaves, C.; Rohani, K.; Bouferguene, S.; Barbeau, J.; Borie, E.; Weber, B.; Fuentes, R.; Crizostomo, L.; Silva-Lovato, C.; et al. Palatal brushing for the treatment of denture stomatitis: A multicentre randomized controlled trial. J. Prosthodont. Res. 2022, 67, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Lavinia Cosmina, A.; Laura-Cristina, R.; Codruta Victoria, T. Alternative Denture Base Materials for Allergic Patients. In Oral Health Care; Lavinia Cosmina, A., Laura Cristina, R., Eds.; IntechOpen: Rijeka, Croatia, 2022. [Google Scholar] [CrossRef]
- Mansour, A.S.; Abbas, N.A.; Cheta, N. Evaluation of Candida albicans Growth on Bre-Flex Versus PEEK Denture Base in Bilateral Maxillary Bounded Partial Denture: A Randomized Clinical Trial. Adv. Dent. J. 2020, 2, 177–183. [Google Scholar] [CrossRef]
- Navabi, N.; Gholamhoseinian, A.; Baghaei, B.; Hashemipour, M.A. Risk factors associated with denture stomatitis in healthy subjects attending a dental school in southeast iran. Sultan Qaboos Univ. Med. J. 2013, 13, 574–580. [Google Scholar] [CrossRef]
- Iba, B.; Falegbe, R.K.; Iortyom, C.; Nwaohabuenyi, T.; Asa, Y.I.; Ibeobi, A.C.; Dogoh, A.F. Denture stomatitis. Orapuh Lit. Rev. 2021, 1, OR006. [Google Scholar]
- Pereira-Cenci, T.; Del Bel Cury, A.A.; Crielaard, W.; Ten Cate, J.M. Development of Candida-associated denture stomatitis: New insights. J. Appl. Oral Sci. 2008, 16, 86–94. [Google Scholar] [CrossRef]
- Morel, L.L.; Possebon, A.P.d.R.; Faot, F.; Pinto, L.d.R. Prevalence of risk factors for denture stomatitis in complete denture wearers. Braz. J. Oral Sci. 2019, 18, e191414. [Google Scholar] [CrossRef]
- Muhvić-Urek, M.; Saltović, E.; Braut, A.; Kovačević Pavičić, D. Association between Vitamin D and Candida-Associated Denture Stomatitis. Dent. J. 2020, 8, 121. [Google Scholar] [CrossRef]
- Sardari, F.; Khalili, P.; Hakimi, H.; Mahmoudaghaei, S.; Abedi, P. The prevalence of denture stomatitis in cigarette and hookah smokers and opium addicts: Findings from Rafsanjan Cohort Study. BMC Oral Health 2021, 21, 455. [Google Scholar] [CrossRef]
- Contaldo, M.; Romano, A.; Mascitti, M.; Fiori, F.; Della Vella, F.; Serpico, R.; Santarelli, A. Association between denture stomatitis, Candida species and diabetic status. J. Biol. Regul. Homeost. Agents 2019, 33 (Suppl. S1), 35–41. [Google Scholar]
- Gual-Vaqués, P.; Jané-Salas, E.; Egido-Moreno, S.; Ayuso-Montero, R.; Marí-Roig, A.; López-López, J. Inflammatory papillary hyperplasia: A systematic review. Med. Oral Patol. Oral Cir. Bucal 2017, 22, e36–e42. [Google Scholar] [CrossRef]
- Gad, M.M.; Fouda, S.M. Current perspectives and the future of Candida albicans-associated denture stomatitis treatment. Dent. Med. Probl. 2020, 57, 95–102. [Google Scholar] [CrossRef]
- Galvan, R.; McBride, M.; Korioth, T.V.; Garcia-Godoy, F. Denture Hygiene as It Relates to Denture Stomatitis: A Review. Compend. Contin. Educ. Dent. 2021, 42, e1–e4. [Google Scholar]
- Alzayer, Y.M.; Gomez, G.F.; Eckert, G.J.; Levon, J.A.; Gregory, R.L. The Impact of Nicotine and Cigarette Smoke Condensate on Metabolic Activity and Biofilm Formation of Candida albicans on Acrylic Denture Material. J. Prosthodont. 2020, 29, 173–178. [Google Scholar] [CrossRef]
- Taebunpakul, P.; Jirawechwongsakul, P. Palatal Inflammation and the Presence of Candida in Denture-Wearing Patients. J. Int. Soc. Prev. Community Dent. 2021, 11, 272–280. [Google Scholar] [CrossRef]
- Alves, F.; Carmello, J.C.; Alonso, G.C.; Mima, E.G.O.; Bagnato, V.S.; Pavarina, A.C. A randomized clinical trial evaluating Photodithazine-mediated Antimicrobial Photodynamic Therapy as a treatment for Denture stomatitis. Photodiagnosis Photodyn. Ther. 2020, 32, 102041. [Google Scholar] [CrossRef]
- Dakka, A.; Nazir, Z.; Shamim, H.; Jean, M.; Umair, M.; Muddaloor, P.; Farinango, M.; Ansary, A.; Khan, S. Ill Effects and Complications Associated to Removable Dentures With Improper Use and Poor Oral Hygiene: A Systematic Review. Cureus 2022, 14, e28144. [Google Scholar] [CrossRef]
- Dodds, M.W.; Johnson, D.A.; Yeh, C.K. Health benefits of saliva: A review. J. Dent. 2005, 33, 223–233. [Google Scholar] [CrossRef]
- Sartawi, S.Y.; Abu-Hammad, S.; Salim, N.A.; Al-Omoush, S. Denture Stomatitis Revisited: A Summary of Systematic Reviews in the Past Decade and Two Case Reports of Papillary Hyperplasia of Unusual Locations. Int. J. Dent. 2021, 2021, 7338143. [Google Scholar] [CrossRef]
- Perić, M.; Živković, R.; Milić Lemić, A.; Radunović, M.; Miličić, B.; Arsić Arsenijević, V. The severity of denture stomatitis as related to risk factors and different Candida spp. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 126, 41–47. [Google Scholar] [CrossRef]
- de Senna, A.M.; Vieira, M.M.F.; Machado-de-Sena, R.M.; Bertolin, A.O.; Núñez, S.C.; Ribeiro, M.S. Photodynamic inactivation of Candida ssp. on denture stomatitis. A clinical trial involving palatal mucosa and prosthesis disinfection. Photodiagnosis Photodyn. Ther. 2018, 22, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Le Bars, P.; Piloquet, P.; Daniel, A.; Giumelli, B. Immunohistochemical localization of type IV collagen and laminin (alpha1) in denture stomatitis. J. Oral Pathol. Med. 2001, 30, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Brantes, M.F.; Azevedo, R.S.; Rozza-de-Menezes, R.E.; Póvoa, H.C.; Tucci, R.; Gouvêa, A.F.; Takahama, A., Jr. Analysis of risk factors for maxillary denture-related oral mucosal lesions: A cross-sectional study. Med. Oral Patol. Oral Cir. Bucal 2019, 24, e305–e313. [Google Scholar] [CrossRef]
- Ansarifard, E.; Zareshahrabadi, Z. Evaluation of Antimicrobial and Antibiofilm Activities of Copper Oxide Nanoparticles within Soft Denture Liners against Oral Pathogens. Bioinorg. Chem. Appl. 2021, 2021, 9939275. [Google Scholar] [CrossRef]
- Cubera, K. [Denture stomatitis—Definition, etiology, classification and treatment]. Prz. Lek. 2013, 70, 947–949. [Google Scholar]
- de Souza, R.F.; Khiyani, M.F.; Chaves, C.A.L.; Feine, J.; Barbeau, J.; Fuentes, R.; Borie, E.; Crizostomo, L.C.; Silva-Lovato, C.H.; Rompre, P.; et al. Improving practice guidelines for the treatment of denture-related erythematous stomatitis: A study protocol for a randomized controlled trial. Trials 2017, 18, 211. [Google Scholar] [CrossRef]
- Newton, A.V. Denture sore mouth. A possible etiology. British Dental Journal 1962, 112, 357–360. [Google Scholar]
- Neppelenbroek, K.H.; Falcão Procópio, A.L.; Gurgel Gomes, A.C.; Campos Sugio, C.Y.; Maia Neves Garcia, A.A.; Porto, V.C.; Urban, V.M. A modified Newton classification for denture stomatitis. Prim. Dent. J. 2022, 11, 55–58. [Google Scholar] [CrossRef]
- Barbeau, J.; Séguin, J.; Goulet, J.P.; de Koninck, L.; Avon, S.L.; Lalonde, B.; Rompré, P.; Deslauriers, N. Reassessing the presence of Candida albicans in denture-related stomatitis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2003, 95, 51–59. [Google Scholar] [CrossRef]
- Schwartz, I.S.; Young, J.M.; Berrong, J.M. The effect of Listerine antiseptic on denture microbial flora and denture stomatitis. Int. J. Prosthodont. 1988, 1, 153–158. [Google Scholar] [PubMed]
- de Vasconcellos, A.A.; Gonçalves, L.M.; Del Bel Cury, A.A.; da Silva, W.J. Candida-Associated Denture Stomatitis: Clinical Relevant Aspects. In Oral Candidosis: Physiopathology, Decision Making, and Therapeutics; Ribeiro Rosa, E.A., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 53–57. [Google Scholar] [CrossRef]
- Adam, R.Z.; Kimmie-Dhansay, F. Prevalence of Denture-Related Stomatitis in Edentulous Patients at a Tertiary Dental Teaching Hospital. Front. Oral Health 2021, 2, 772679. [Google Scholar] [CrossRef] [PubMed]
- Davoudi, A.; Ebadian, B.; Nosouhian, S. Role of laser or photodynamic therapy in treatment of denture stomatitis: A systematic review. J. Prosthet. Dent. 2018, 120, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Meltem, K.; Tosun, T. Oral Mucosal Trauma and Injuries. In Trauma in Dentistry; Serdar, G., Ed.; IntechOpen: Rijeka, Croatia, 2019; Chapter 8. [Google Scholar] [CrossRef]
- da Costa, R.M.B.; Poluha, R.L.; De la Torre Canales, G.; Junior, J.F.S.; Conti, P.C.R.; Neppelenbroek, K.H.; Porto, V.C. The effectiveness of microwave disinfection in treating Candida-associated denture stomatitis: A systematic review and metaanalysis. Clin. Oral Investig. 2020, 24, 3821–3832. [Google Scholar] [CrossRef]
- Emami, E.; Kabawat, M.; Rompre, P.H.; Feine, J.S. Linking evidence to treatment for denture stomatitis: A meta-analysis of randomized controlled trials. J. Dent. 2014, 42, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Firoozi, P.; Farshidfar, N.; Fekrazad, R. Efficacy of antimicrobial photodynamic therapy compared to nystatin therapy in reducing Candida colony count in patients with Candida-associated denture stomatitis: A systematic review and meta-analysis. Evid.-Based Dent. 2021, 23, 47. [Google Scholar] [CrossRef]
- Hilgert, J.B.; Giordani, J.M.; de Souza, R.F.; Wendland, E.M.; D’Avila, O.P.; Hugo, F.N. Interventions for the Management of Denture Stomatitis: A Systematic Review and Meta-Analysis. J. Am. Geriatr. Soc. 2016, 64, 2539–2545. [Google Scholar] [CrossRef]
- Lyu, X.; Zhao, C.; Yan, Z.M.; Hua, H. Efficacy of nystatin for the treatment of oral candidiasis: A systematic review and meta-analysis. Drug Des. Dev. Ther. 2016, 10, 1161–1171. [Google Scholar] [CrossRef]
- Rai, A.; Misra, S.R. Nystatin Effectiveness in Oral Candidiasis Treatment: A Systematic Review & Meta-Analysis of Clinical Trials. Life 2022, 12, 1677. [Google Scholar] [CrossRef]
- Roomaney, I.A.; Holmes, H.K. Treatment of oral fungal infections using photodynamic therapy: Systematic review and meta-analysis. Clin. Exp. Dent. Res. 2021, 7, 354–364. [Google Scholar] [CrossRef]
- Shui, Y.; Li, J.; Lyu, X.; Wang, Y. Phytotherapy in the management of denture stomatitis: A systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2021, 35, 4111–4126. [Google Scholar] [CrossRef]
- Inácio Silveira, D.Q.; Lia, E.N.; Massignan, C.; Stefani, C.M. Natural products for the treatment of denture stomatitis: A systematic review. J. Prosthet. Dent. 2021. [Google Scholar] [CrossRef]
- Skupien, J.A.; Valentini, F.; Boscato, N.; Pereira-Cenci, T. Prevention and treatment of Candida colonization on denture liners: A systematic review. J. Prosthet. Dent. 2013, 110, 356–362. [Google Scholar] [CrossRef]
- Santos Sousa, T.M.; de Farias, O.R. Effectiveness of denture microwave disinfection for treatment of denture stomatitis: A systematic review and meta-analysis. Int. J. Dent. Hyg. 2021, 19, 62–77. [Google Scholar] [CrossRef]
- Verhaeghe, T.V.; Wyatt, C.C.; Mostafa, N.Z. The effect of overnight storage conditions on complete denture colonization by Candida albicans and dimensional stability: A systematic review. J. Prosthet. Dent. 2020, 124, 176–182. [Google Scholar] [CrossRef]
- Vila-Nova, T.E.L.; Leão, R.S.; Santiago Junior, J.F.; Pellizzer, E.P.; Vasconcelos, B.; Moraes, S.L.D. Photodynamic therapy in the treatment of denture stomatitis: A systematic review and meta-analysis. J. Prosthet. Dent. 2022. [Google Scholar] [CrossRef]
- Zhang, L.W.; Fu, J.Y.; Hua, H.; Yan, Z.M. Efficacy and safety of miconazole for oral candidiasis: A systematic review and meta-analysis. Oral Dis. 2016, 22, 185–195. [Google Scholar] [CrossRef]
- Aoun, G.; Cassia, A.; Berberi, A. Effectiveness of a Chlorhexidine Digluconate 0.12% and Cetylpyridinium Chloride 0.05% Solution in eliminating Candida albicans Colonizing Dentures: A Randomized Clinical in vivo Study. J. Contemp. Dent. Pract. 2015, 16, 433–436. [Google Scholar] [CrossRef]
- Badaró, M.M.; Bueno, F.L.; Arnez, R.M.; Oliveira, V.C.; Macedo, A.P.; de Souza, R.F.; Paranhos, H.F.O.; Silva-Lovato, C.H. The effects of three disinfection protocols on Candida spp., denture stomatitis, and biofilm: A parallel group randomized controlled trial. J. Prosthet. Dent. 2020, 124, 690–698. [Google Scholar] [CrossRef]
- Procópio, A.L.F.; Lara, V.S.; Porto, V.C.; Soares, S.; Fernandes, M.H.; Urban, V.M.; Neppelenbroek, K.H. Resilient liner modified by antimicrobials for denture stomatitis treatment: A randomized controlled trial. J. Dent. 2022, 126, 104297. [Google Scholar] [CrossRef]
- Raghavendra Swamy, K.N.; Alla, R.K.; Mohammed, S.; Konakanchi, A. The role of antifungal agents in treating denture stomatitis. Res. J. Pharm. Technol. 2018, 11, 1365–1369. [Google Scholar] [CrossRef]
- Bukhari, M.A.; Algahtani, M.A.; Alsuwailem, F.A.; Alogaiel, R.M.; Almubarak, S.H.; Alqahtani, S.S.; Alabdullatif, R.A.; Alghimlas, R.Y.; Alotaibi, N.F.; Qahtani, A.R.A.; et al. Epidemiology, etiology, and treatment of denture stomatitis. Int. J. Community Med. Public Health 2022, 9, 981–986. [Google Scholar] [CrossRef]
- Alfouzan, A.; Alotiabi, H.; Labban, N.; Al-Otaibi, H.; Al Taweel, S.; AlShehri, H. Color stability of 3D-printed denture resins: Effect of aging, mechanical brushing and immersion in staining medium. J. Adv. Prosthodont. 2021, 13, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Alfouzan, A.F.; Alotiabi, H.M.; Labban, N.; Al-Otaibi, H.N.; Al Taweel, S.M.; AlShehri, H.A. Effect of aging and mechanical brushing on surface roughness of 3D printed denture resins: A profilometer and scanning electron microscopy analysis. Technol. Health Care 2022, 30, 161–173. [Google Scholar] [CrossRef]
- Valentini-Mioso, F.; Maske, T.T.; Cenci, M.S.; Boscato, N.; Pereira-Cenci, T. Chemical hygiene protocols for complete dentures: A crossover randomized clinical trial. J. Prosthet. Dent. 2019, 121, 83–89. [Google Scholar] [CrossRef]
- Alfouzan, A.F.; AlNouwaisar, A.N.; AlAzzam, N.F.; Al-Otaibi, H.N.; Labban, N.; Alswaidan, M.H.; Al Taweel, S.M.; Alshehri, H.A. Power brushing and chemical denture cleansers induced color changes of pre-polymerized CAD/CAM denture acrylic resins. Mater. Res. Express 2021, 8, 085402. [Google Scholar] [CrossRef]
- Raszewski, Z.; Nowakowska, D.; Więckiewicz, W.; Nowakowska-Toporowska, A. The Effect of Chlorhexidine Disinfectant Gels with Anti-Discoloration Systems on Color and Mechanical Properties of PMMA Resin for Dental Applications. Polymers 2021, 13, 1800. [Google Scholar] [CrossRef]
- Contaldo, M.; Di Stasio, D.; Romano, A.; Fiori, F.; Della Vella, F.; Rupe, C.; Lajolo, C.; Petruzzi, M.; Serpico, R.; Lucchese, A. Oral candidiasis and novel therapeutic strategies: Antifungals, phytotherapy, probiotics, and photodynamic therapy. Curr. Drug Deliv. 2022. [Google Scholar] [CrossRef]
- Vidya, S.; Varsha, P.; Anurag, A.; Sanath, S. Efficacy of Anti-Fungal Agents Incorporated in Tissue Conditioners in Inhibiting the Growth of Candida albicans. J. Evolution. Med. Dent. Sci. 2020, 9, 3904–3908. [Google Scholar]
- Shaikh, M.S.; Alnazzawi, A.; Habib, S.R.; Lone, M.A.; Zafar, M.S. Therapeutic Role of Nystatin Added to Tissue Conditioners for Treating Denture-Induced Stomatitis: A Systematic Review. Prosthesis 2021, 3, 61–74. [Google Scholar] [CrossRef]
- Carolina, Y.; Maria, G.; Anna, C.; Amanda, A.; Thaís, M.; Karin, H. Use of Natural Products in the Prevention and Treatment of Denture Stomatitis. Open Access J. Biomed. Sci. 2020, 2, e146. [Google Scholar]
- Lu, H.; Shrivastava, M.; Whiteway, M.; Jiang, Y. Candida albicans targets that potentially synergize with fluconazole. Crit. Rev. Microbiol. 2021, 47, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ji, Z.; Feng, Y.; Yan, T.; Cao, Y.; Lu, H.; Jiang, Y. Myriocin enhances the antifungal activity of fluconazole by blocking the membrane localization of the efflux pump Cdr1. Front. Pharmacol. 2022, 13, 1101553. [Google Scholar] [CrossRef] [PubMed]
- De Matteis, V.; Cascione, M.; Toma, C.C.; Albanese, G.; De Giorgi, M.L. Silver Nanoparticles Addition in Poly(Methyl Methacrylate) Dental Matrix: Topographic and Antimycotic Studies. Int. J. Mol. Sci. 2019, 20, 4691. [Google Scholar] [CrossRef]
- Cascione, M.; De Matteis, V. Improvement of PMMA Dental Matrix Performance by Addition of Titanium Dioxide Nanoparticles and Clay Nanotubes. Nanomaterials 2021, 11, 2027. [Google Scholar] [CrossRef]
- Bajunaid, S.O. How Effective Are Antimicrobial Agents on Preventing the Adhesion of Candida albicans to Denture Base Acrylic Resin Materials? A Systematic Review. Polymers 2022, 14, 908. [Google Scholar] [PubMed]
- An, S.; Evans, J.L.; Hamlet, S.; Love, R.M. Incorporation of antimicrobial agents in denture base resin: A systematic review. J. Prosthet. Dent. 2021, 126, 188–195. [Google Scholar] [CrossRef] [PubMed]
- An, S.; Evans, J.L.; Hamlet, S.; Love, R.M. Overview of incorporation of inorganic antimicrobial materials in denture base resin: A scoping review. J. Prosthet. Dent. 2021. [Google Scholar] [CrossRef]
- Yousef, M.; Abdelaziz, A.; Essa, M.; Fahmi, M. Assessment of Photodynamic Therapy and Miconazole in the Management of Denture Stomatitis. Int. J. Dent. Sci. Res. 2018, 6, 83–87. [Google Scholar] [CrossRef]
- Ahmad Khan, M.S.; Ahmad, I. Chapter 1—Herbal Medicine: Current Trends and Future Prospects. In New Look to Phytomedicine; Ahmad Khan, M.S., Ahmad, I., Chattopadhyay, D., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 3–13. [Google Scholar] [CrossRef]
- Iyer, M.S.; Gujjari, A.K.; Paranthaman, S.; Abu Lila, A.S.; Almansour, K.; Alshammari, F.; Khafagy, E.-S.; Arab, H.H.; Gowda, D.V. Development and Evaluation of Clove and Cinnamon Supercritical Fluid Extracts-Loaded Emulgel for Antifungal Activity in Denture Stomatitis. Gels 2022, 8, 33. [Google Scholar] [CrossRef]


| Author/Year | Intervention(s) | Number of RCTs Included | Main Findings | Risk of Bias |
|---|---|---|---|---|
| da Costa et al., 2020 [37] | Microwave | 5 | Microwave disinfection is equally effective as 0.2% chlorhexidine, 0.02% sodium hypochlorite, and topical nystatin (100,000 IU/mL), and superior to topical miconazole | Moderate |
| Davoudi 2018 [35] | Low-level laser therapy (LLLT) or photodynamic therapy (PDT | 6 | LLLT has a significant role in the clinical treatment of DS PDT and showed similar results to conventional antifungal therapies | Low–moderate |
| Emami E 2014 [38] | Efficacy of antifungal therapy with alternative methods | 14 | Disinfection methods could be considered as an adjunct to antifungal medications; no statistically significant difference between antifungals and disinfection for clinical and microbiological outcomes | Moderate–high |
| Firoozi et al., 2021 [39] | Antimicrobial Photodynamic therapy (aPDT) compared to Nystatin | 3 | aPDT may be effective in reducing Candida colony count and treating DS but is not superior to nystatin | Moderate |
| Hilgert et al., 2016 [40] | Any agent or procedure to treat DS | 35 | Nystatin and disinfecting agents can be an effective treatment for DS | High |
| Lyu et al., 2016 [41] | Comparison of nystatin with other antifungal agents | 11 | Nystatin pastille alone or pastille with suspension is more effective than suspension alone; prolonged treatment duration (4 weeks) can increase the efficacy of nystatin; Fluconazole superior in infants, children, or HIV patients | Moderate–high |
| Rai et al., 2022 [42] | Compared topical nystatin to other antifungal agents or placebo | 24 | Equal efficacy of 100,000 IU of nystatin suspension and six sessions of PDT for the treatment of DS | Moderate–high |
| Roomaney 2021 [43] | Photodynamic therapy | 5 | PDT comparable to systemic and topical antifungals | Low–moderate |
| Shui et al., 2021 [44] | Phytotherapy | 19 | Phytomedicines had fewer side effects and more patient satisfaction than antifungals or disinfectants; no statistical difference between propolis and miconazole for clinical and microbiological parameters | High |
| Silveira et al., 2021 [45] | Natural products (Propolis, Green tea, Ginger, Zataria multiflora, chitosan, garlic, Artemisia, Schinus terebinthifolius Raddi, Uncaria tomentosa, Punica granatum, and Ricinus communis) | 14 | Natural products showed similar efficacy and safety when compared with nystatin or miconazole | High |
| Skupien JA 2013 [46] | Nystatin (500,000 units) and Sodium hypochlorite 0.5% | 7 | Sodium hypochlorite 0.5% can disinfect denture liners & tissue conditioners; incorporation of nystatin is also beneficial | High |
| Sousa et al., 2021 [47] | Microwave disinfection | 4 | Efficient as an antifungal therapy | High |
| Verhaeghe et al., 2020 [48] | Overnight storage conditions | 3 | Cleaning dentures before overnight storage reduces C. albicans; overnight dry storage could reduce C. albicans colonization | Low |
| Vila-Nova TEL et al., 2022 [49] | Photodynamic therapy (PDT) | 4 | Photodynamic therapy is effective in improving the efficacy of antifungals | Low to moderate |
| Zhang et al. 2016 [50] | Miconazole | 17 | Miconazole oral gel may be more effective than other formulations with regard to long-term results | Moderate to high |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abuhajar, E.; Ali, K.; Zulfiqar, G.; Al Ansari, K.; Raja, H.Z.; Bishti, S.; Anweigi, L. Management of Chronic Atrophic Candidiasis (Denture Stomatitis)—A Narrative Review. Int. J. Environ. Res. Public Health 2023, 20, 3029. https://doi.org/10.3390/ijerph20043029
Abuhajar E, Ali K, Zulfiqar G, Al Ansari K, Raja HZ, Bishti S, Anweigi L. Management of Chronic Atrophic Candidiasis (Denture Stomatitis)—A Narrative Review. International Journal of Environmental Research and Public Health. 2023; 20(4):3029. https://doi.org/10.3390/ijerph20043029
Chicago/Turabian StyleAbuhajar, Eman, Kamran Ali, Gulraiz Zulfiqar, Khalifa Al Ansari, Hina Zafar Raja, Shaza Bishti, and Lamyia Anweigi. 2023. "Management of Chronic Atrophic Candidiasis (Denture Stomatitis)—A Narrative Review" International Journal of Environmental Research and Public Health 20, no. 4: 3029. https://doi.org/10.3390/ijerph20043029
APA StyleAbuhajar, E., Ali, K., Zulfiqar, G., Al Ansari, K., Raja, H. Z., Bishti, S., & Anweigi, L. (2023). Management of Chronic Atrophic Candidiasis (Denture Stomatitis)—A Narrative Review. International Journal of Environmental Research and Public Health, 20(4), 3029. https://doi.org/10.3390/ijerph20043029





