Psychopathological Profile Associated with Food Addiction Symptoms in Adolescents with Eating Disorders
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. Psychopathological Measures
2.2.1. Youth Self-Report
2.2.2. Multidimensional Anxiety Scale for Children 2
2.2.3. Children Depression Inventory 2
2.2.4. Eating Disorder Inventory-3
2.2.5. Yale Food Addiction Scale 2.0
2.3. Statistical Analysis
3. Results
3.1. Patients
3.2. Food Addiction Profile and ED Subgroups
3.3. Association between Food Addiction and Psychopathological Symptoms
3.4. Multiple Correspondence Analysis (MCA)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Hoeken, D.; Hoek, H.W. Review of the Burden of Eating Disorders: Mortality, Disability, Costs, Quality of Life, and Family Burden. Curr. Opin. Psychiatry 2020, 33, 521–527. [Google Scholar] [CrossRef] [PubMed]
- CASA. Food for Thought: Substance Abuse and Eating Disorders; The National Center on Addiction and Substance Abuse (CASA) at Columbia University: New York, NY, USA, 2003. [Google Scholar]
- Pursey, K.; Stanwell, P.; Gearhardt, A.; Collins, C.; Burrows, T. The Prevalence of Food Addiction as Assessed by the Yale Food Addiction Scale: A Systematic Review. Nutrients 2014, 6, 4552–4590. [Google Scholar] [CrossRef]
- Granero, R.; Jiménez-Murcia, S.; Gearhardt, A.N.; Agüera, Z.; Aymamí, N.; Gómez-Peña, M.; Lozano-Madrid, M.; Mallorquí-Bagué, N.; Mestre-Bach, G.; Neto-Antao, M.I.; et al. Validation of the Spanish Version of the Yale Food Addiction Scale 2.0 (YFAS 2.0) and Clinical Correlates in a Sample of Eating Disorder, Gambling Disorder, and Healthy Control Participants. Front. Psychiatry 2018, 9, 208. [Google Scholar] [CrossRef] [PubMed]
- Cinelli, G.; Criscuolo, M.; Bifone, C.; Chianello, I.; Castiglioni, M.C.; De Lorenzo, A.; Di Renzo, L.; Tozzi, A.E.; Vicari, S.; Zanna, V. Food Addiction in a Group of Italian Adolescents Diagnosed for Eating Disorder. Nutrients 2020, 12, 1524. [Google Scholar] [CrossRef]
- Albayrak, Ö.; Föcker, M.; Kliewer, J.; Esber, S.; Peters, T.; de Zwaan, M.; Hebebrand, J. Eating-Related Psychopathology and Food Addiction in Adolescent Psychiatric Inpatients: Food Addiction in Youth Psychiatric Patients. Eur. Eat. Disord. Rev. 2017, 25, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Granero, R.; Hilker, I.; Agüera, Z.; Jiménez-Murcia, S.; Sauchelli, S.; Islam, M.A.; Fagundo, A.B.; Sánchez, I.; Riesco, N.; Dieguez, C.; et al. Food Addiction in a Spanish Sample of Eating Disorders: DSM-5 Diagnostic Subtype Differentiation and Validation Data: Food Addiction and ED Subtypes. Eur. Eat. Disord. Rev. 2014, 22, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Mann, A.P.; Accurso, E.C.; Stiles-Shields, C.; Capra, L.; Labuschagne, Z.; Karnik, N.S.; Le Grange, D. Factors Associated with Substance Use in Adolescents With Eating Disorders. J. Adolesc. Health 2014, 55, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Skinner, J.; Jebeile, H.; Burrows, T. Food Addiction and Mental Health in Adolescents: A Systematic Review. Lancet Child Adolesc. Health 2021, 5, 751–766. [Google Scholar] [CrossRef]
- Bahji, A.; Mazhar, M.N.; Hudson, C.C.; Nadkarni, P.; MacNeil, B.A.; Hawken, E. Prevalence of Substance Use Disorder Comorbidity among Individuals with Eating Disorders: A Systematic Review and Meta-Analysis. Psychiatry Res. 2019, 273, 58–66. [Google Scholar] [CrossRef]
- Merlo, L.J.; Klingman, C.; Malasanos, T.H.; Silverstein, J.H. Exploration of Food Addiction in Pediatric Patients: A Preliminary Investigation. J. Addict. Med. 2009, 3, 26–32. [Google Scholar] [CrossRef]
- Blinder, B.J.; Cumella, E.J.; Sanathara, V.A. Psychiatric Comorbidities of Female Inpatients with Eating Disorders. Psychosom. Med. 2006, 68, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Braun, D.L.; Sunday, S.R.; Halmi, K.A. Psychiatric Comorbidity in Patients with Eating Disorders. Psychol. Med. 1994, 24, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, E.; Gray, K.; Miller, G.; Tyler, R.; Wiers, C.E.; Volkow, N.D. Food Addiction: A Common Neurobiological Mechanism with Drug Abuse. Front. Biosci.-Landmark 2018, 23, 811–836. [Google Scholar] [CrossRef]
- Serafine, K.M.; O’Dell, L.E.; Zorrilla, E.P. Converging Vulnerability Factors for Compulsive Food and Drug Use. Neuropharmacology 2021, 196, 108556. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, P.C.; Kenny, P.J. Food Addiction: A Valid Concept? Neuropsychopharmacology 2018, 43, 2506–2513. [Google Scholar] [CrossRef]
- Burrows, T.; Verdejo-Garcia, A.; Carter, A.; Brown, R.M.; Andrews, Z.B.; Dayas, C.V.; Hardman, C.A.; Loxton, N.; Sumithran, P.; Whatnall, M. Health Professionals’ and Health Professional Trainees’ Views on Addictive Eating Behaviours: A Cross-Sectional Survey. Nutrients 2020, 12, 2860. [Google Scholar] [CrossRef]
- Hebebrand, J.; Gearhardt, A.N. The Concept of “Food Addiction” Helps Inform the Understanding of Overeating and Obesity: NO. Am. J. Clin. Nutr. 2021, 113, 268–273. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5; American Psychiatric Association: Washington, DC, USA, 2013; Volume 10. [Google Scholar]
- Gearhardt, A.N.; Corbin, W.R.; Brownell, K.D. Preliminary Validation of the Yale Food Addiction Scale. Appetite 2009, 52, 430–436. [Google Scholar] [CrossRef]
- Gearhardt, A.N.; Corbin, W.R.; Brownell, K.D. Development of the Yale Food Addiction Scale Version 2.0. Psychol. Addict. Behav. 2016, 30, 113–121. [Google Scholar] [CrossRef]
- Whatnall, M.; Skinner, J.A.; Leary, M.; Burrows, T.L. Food Addiction: A Deep Dive into ‘Loss of Control’ and ‘Craving. ’ Curr. Addict. Rep. 2022, 9, 318–325. [Google Scholar] [CrossRef]
- Burrows, T.; Kay-Lambkin, F.; Pursey, K.; Skinner, J.; Dayas, C. Food Addiction and Associations with Mental Health Symptoms: A Systematic Review with Meta-Analysis. J. Hum. Nutr. Diet. Off. J. Br. Diet. Assoc. 2018, 31, 544–572. [Google Scholar] [CrossRef] [PubMed]
- Achenbach, T.M.; Rescorla, L. Manual for the ASEBA School-Age Forms & Profiles: An Integrated System of Multi-Informant Assessment; University of Vermont, Research Center for Children, Youth, & Families: Burlington, VT, USA, 2001. [Google Scholar]
- Frigerio, A.; Monterosso, R. La Valutazioen su Base Empirica dei Problemi Emotivo-Comportamentali su Base Emotiva; Infanzia e Adolescenza. 2002, pp. 38–48. Available online: https://www.infanziaeadolescenza.it/norme-editoriali/ (accessed on 30 December 2022).
- March, J.S. Multidimensional Anxiety Scale for Children (MASC 2): Technical Manual; Multi-Health Systems Incorporated: Toronto, ON, Canada, 2013. [Google Scholar]
- Paloscia, C.; Giangregorio, A.; Guerini, R.; Melchiori, F.M. MASC 2—Multidimensional Anxiety Scale for Children, 2nd ed.; Manuale Versione Italiana; Hogrefe: Firenze, Italy, 2017. [Google Scholar]
- Kovacs, M. Children’s Depression Inventory 2TM(CDI 2) North Tonawanda; Multi-Health Systems Incorporated: New York, NY, USA, 2010. [Google Scholar]
- Camuffo, M.; Cerutti, R.; Kovacs, M. CDI-2 Children’s Depression Inventory, 2nd ed.; Italian Adaptation; Hogrefe: Firenze, Italy, 2018. [Google Scholar]
- Garner, D. EDI-3, Eating Disorder Inventory-3: Professional Manual; Psychological Assessment Resources: Lutz, FL, USA, 2004; Volume 35, p. 479. [Google Scholar]
- Giannini, M.; Pannocchia, L.; Dalle Grave, R.; Muratori, F.; Viglione, V. EDI-3, Eating Disorders Inventory: Manuale. Adattamento Italiano; Giunti Organizzazioni Speciali (OS): Firenze, Italy, 2008. [Google Scholar]
- Aloi, M.; Rania, M.; Rodríguez Muñoz, R.C.; Jiménez Murcia, S.; Fernández-Aranda, F.; De Fazio, P.; Segura-Garcia, C. Validation of the Italian Version of the Yale Food Addiction Scale 2.0 (I-YFAS 2.0) in a Sample of Undergraduate Students. Eat. Weight Disord. EWD 2017, 22, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Aloi, M.; Verrastro, V.; Rania, M.; Sacco, R.; Fernández-Aranda, F.; Jiménez-Murcia, S.; De Fazio, P.; Segura-Garcia, C. The Potential Role of the Early Maladaptive Schema in Behavioral Addictions among Late Adolescents and Young Adults. Front. Psychol. 2019, 10, 3022. [Google Scholar] [CrossRef] [PubMed]
- Meule, A.; Gearhardt, A.N. Ten Years of the Yale Food Addiction Scale: A Review of Version 2.0. Curr. Addict. Rep. 2019, 6, 218–228. [Google Scholar] [CrossRef]
- Rodrigue, C.; Gearhardt, A.N.; Bégin, C. Food Addiction in Adolescents: Exploration of Psychological Symptoms and Executive Functioning Difficulties in a Non-Clinical Sample. Appetite 2019, 141, 104303. [Google Scholar] [CrossRef]
- Parnarouskis, L.; Schulte, E.M.; Lumeng, J.C.; Gearhardt, A.N. Development of the Highly Processed Food Withdrawal Scale for Children. Appetite 2020, 147, 104553. [Google Scholar] [CrossRef]
- Wiss, D.; Brewerton, T. Separating the Signal from the Noise: How Psychiatric Diagnoses Can Help Discern Food Addiction from Dietary Restraint. Nutrients 2020, 12, 2937. [Google Scholar] [CrossRef]
- Williamson, D.A.; Martin, C.K.; Stewart, T. Psychological Aspects of Eating Disorders. Best Pract. Res. Clin. Gastroenterol. 2004, 18, 1073–1088. [Google Scholar] [CrossRef]
- Jiménez-Murcia, S.; Granero, R.; Wolz, I.; Baño, M.; Mestre-Bach, G.; Steward, T.; Agüera, Z.; Hinney, A.; Diéguez, C.; Casanueva, F.F.; et al. Food Addiction in Gambling Disorder: Frequency and Clinical Outcomes. Front. Psychol. 2017, 8, 473. [Google Scholar] [CrossRef]
- Brooks, S.J.; Rask-Andersen, M.; Benedict, C.; Schiöth, H.B. A Debate on Current Eating Disorder Diagnoses in Light of Neurobiological Findings: Is It Time for a Spectrum Model? BMC Psychiatry 2012, 12, 76. [Google Scholar] [CrossRef]
- Hauck, C.; Cook, B.; Ellrott, T. Food Addiction, Eating Addiction and Eating Disorders. Proc. Nutr. Soc. 2020, 79, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.; Poinsot, P.; Guillaume, S.; Delaunay, D.; Bernetiere, M.; Bégin, C.; Fourneret, P.; Peretti, N.; Iceta, S. Food addiction as a proxy for anorexia nervosa severity: New data based on the yale food addiction scale 2.0. Psychiatry Res. 2020, 293, 113472. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, I.; Lucas, I.; Munguía, L.; Camacho-Barcia, L.; Giménez, M.; Sánchez-González, J.; Granero, R.; Solé-Morata, N.; Gearhardt, A.N.; Diéguez, C.; et al. Food Addiction in Anorexia Nervosa: Implications for the Understanding of Crossover Diagnosis. Eur. Eat. Disord. Rev. J. Eat. Disord. Assoc. 2022, 30, 278–288. [Google Scholar] [CrossRef] [PubMed]
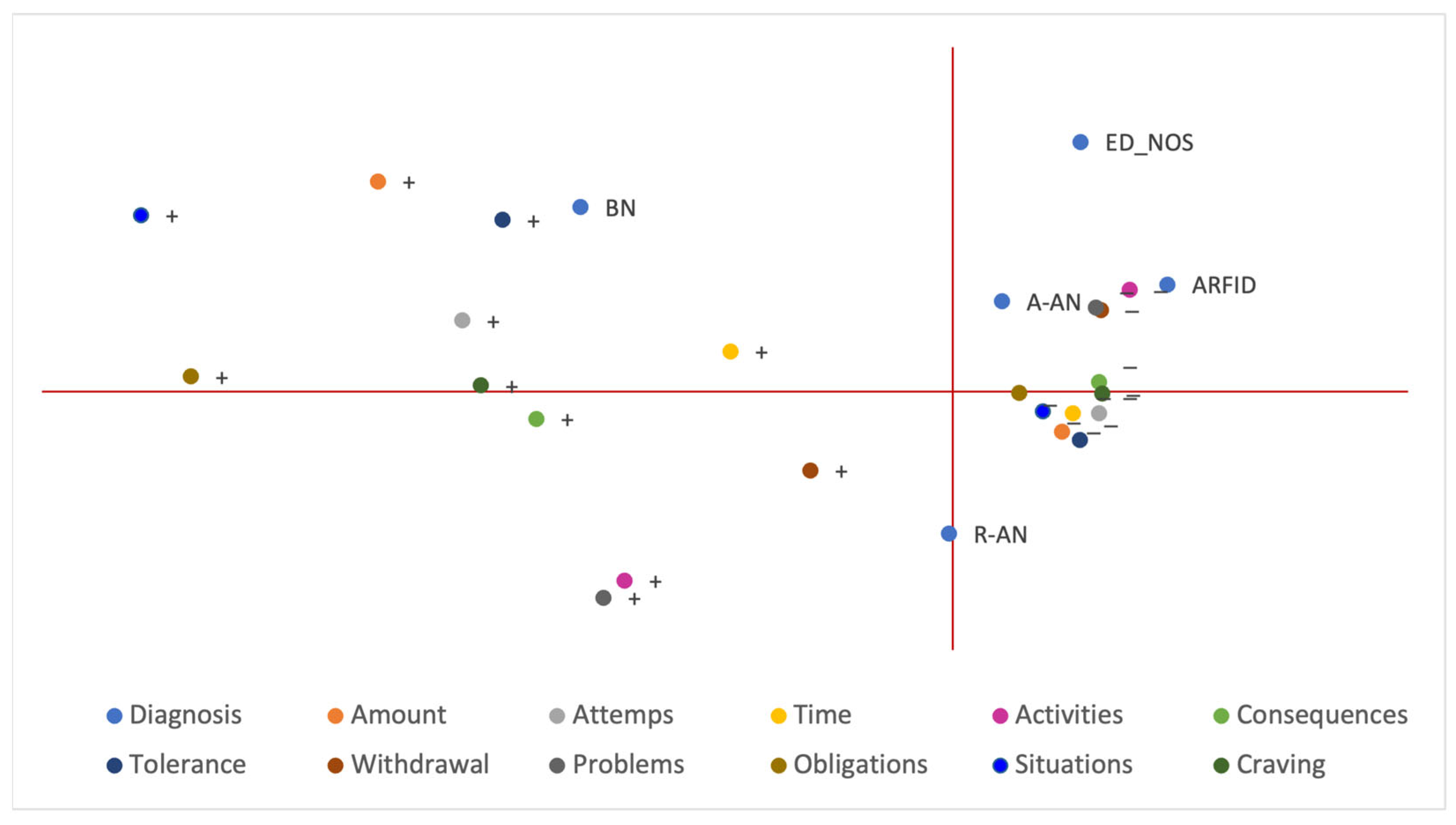
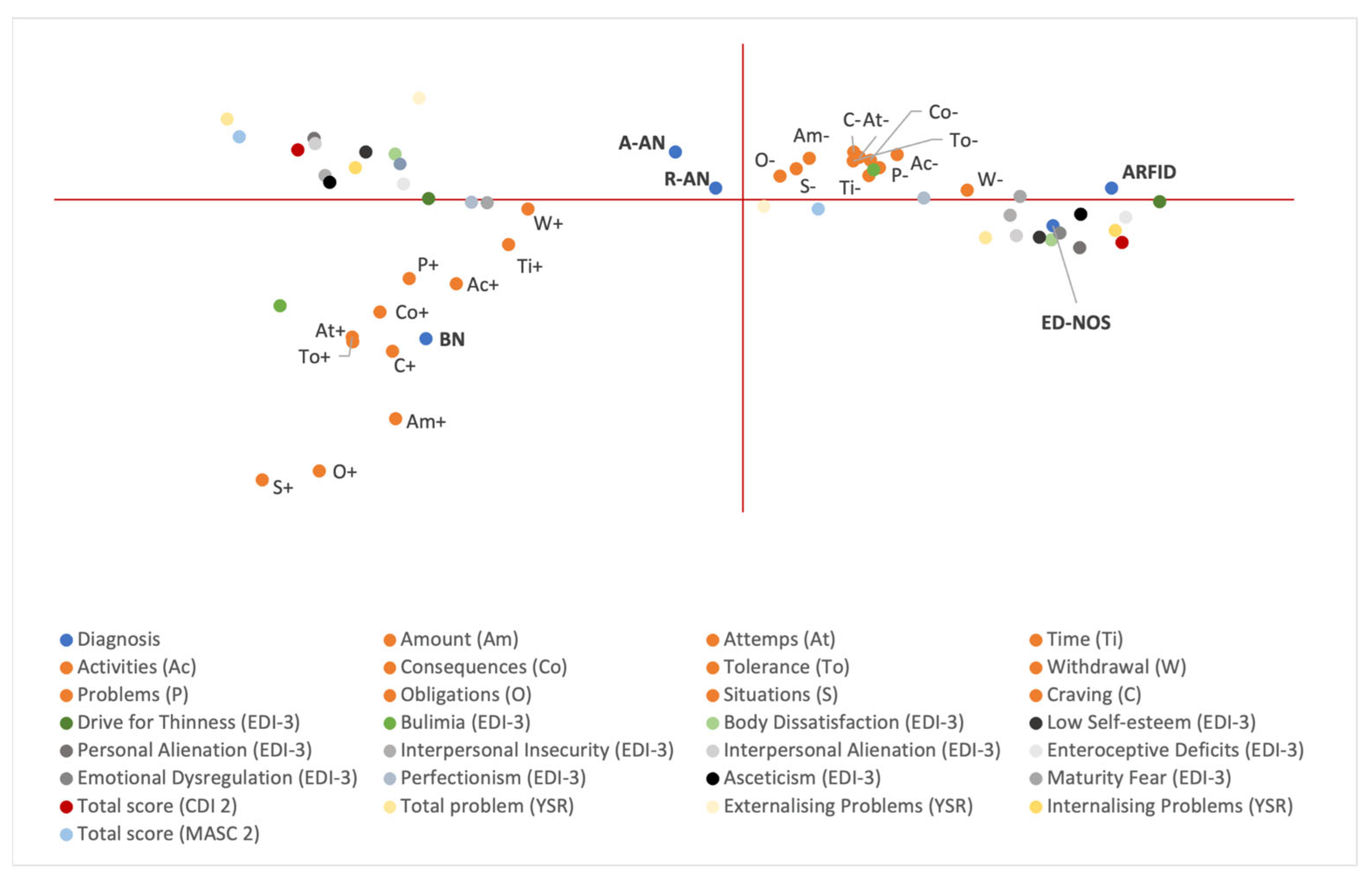
| Variable | n = 102 |
|---|---|
| Age | 15.6 (14.00–16.7) |
| Weight (kg) | 43.6 (38.6–51.0) |
| Height (cm) | 159.2 (154.5–164.0) |
| BMI | 17.3 (15.7–19.1) |
| BMI percentile | 10 (1.0–33.0) |
| Diagnosis | |
| ARFID | 7 (6.9) |
| R-AN | 51 (50.0) |
| A-AN | 21 (20.6) |
| BP-AN | 1 (1.0) |
| BN | 10 (9.8) |
| BED | 1 (1.0) |
| ED-NOS | 11 (10.8) |
| Whole Sample (n = 102) | ARFID (n = 7) | R-AN (n = 51) | A-AN (n = 21) | BP-AN (n = 1) | BN (n = 10) | BED (n = 1) | ED-NOS (n = 11) | |
|---|---|---|---|---|---|---|---|---|
| Symptom count | 2 (1–4) | 1 (0–4) | 2 (1–4) | 2 (1–4) | 2 (−) | 4.5 (1–8) | 9 (-) | 1 (0–2) |
| Amount | 17 (16.7) | 1 (14.3) | 4 (7.8) | 3 (14.3) | 0 (0.0) | 6 (60.0) | 1 (100.0) | 2 (18.2) |
| Attempts | 24 (19.6) | 0 (0.0) | 13 (25.5) | 5 (23.8) | 0 (0.0) | 3 (30.0) | 1 (100.0) | 2 (18.2) |
| Time | 37 (36.3) | 3 (42.7) | 17 (33.3) | 8 (38.1) | 1 (100.0) | 5 (50.0) | 1 (100.0) | 2 (18.2) |
| Activities | 35 (34.4) | 2 (28.6) | 20 (39.2) | 6 (28.6) | 0 (0.0) | 6 (60.0) | 0 (0.0) | 1 (9.1) |
| Consequences | 27 (26.5) | 1 (14.3) | 15 (29.4) | 3 (14.3) | 0 (0.0) | 5 (50.0) | 1 (100.0) | 2 (18.2) |
| Tolerance | 23 (22.5) | 1 (14.3) | 10 (19.6) | 5 (23.8) | 0 (0.0) | 4 (40.0) | 1 (100.0) | 2 (18.2) |
| Withdrawal | 52 (51.0) | 2 (28.6) | 24 (47.1) | 13 (61.9) | 0 (0.0) | 7 (70.0) | 1 (100.0) | 5 (45.4) |
| Problems | 31 (30.4) | 1 (14.3) | 19 (37.2) | 4 (19.0) | 1 (100.0) | 3 (30.0) | 1 (100.0) | 2 (18.2) |
| Obligations | 8 (7.8) | 0 (0.0) | 3 (5.9) | 3 (14.3) | 0 (0.0) | 1 (10.0) | 0 (0.0) | 1 (9.1) |
| Situations | 11 (10.8) | 0 (0.0) | 5 (9.8) | 2 (9.5) | 0 (0.0) | 2 (20.0) | 1 (100.0) | 1 (9.1) |
| Craving | 25 (24.5) | 1 (14.3) | 14 (27.4) | 2 (9.5) | 0 (0.0) | 5 (50.0) | 1 (100.0) | 2 (18.2) |
| Amount | Attempts | Time | Activities | Consequences | Tolerance | Withdrawal | Problems | Obligations | Situations | Craving | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Internalising Problems (YSR) | 0.010 | 0.010 | 0.002 | 0.001 | 0.008 | ||||||
| Externalising Problems (YSR) | |||||||||||
| Total Problems (YSR) | 0.017 | 0.001 | 0.014 | ||||||||
| Total score (MASC 2) | 0.043 | 0.017 | |||||||||
| Total score (CDI 2) | 0.029 | 0.009 | 0.009 | 0.030 | |||||||
| Drive for Thinness (EDI-3) | 0.020 | 0.040 | 0.001 | 0.035 | 0.019 | 0.010 | <0.001 | ||||
| Bulimia (EDI-3) | <0.001 | 0.010 | 0.011 | 0.008 | <0.001 | 0.043 | 0.005 | 0.007 | <0.001 | ||
| Body dissatisfaction (EDI-3) | |||||||||||
| Low Self-esteem (EDI-3) | |||||||||||
| Personal Alienation (EDI-3) | 0.044 | ||||||||||
| Interpersonal Insecurity (EDI-3) | 0.001 | 0.011 | 0.037 | 0.023 | 0.013 | ||||||
| Interpersonal Alienation (EDI-3) | 0.037 | ||||||||||
| Interoceptive Deficits (EDI-3) | 0.013 | 0.022 | 0.001 | 0.003 | 0.048 | ||||||
| Emotional Dysregulation (EDI-3) | 0.017 | 0.002 | |||||||||
| Perfectionism (EDI-3) | 0028 | 0.028 | |||||||||
| Asceticism (EDI-3) | 0.029 | 0.012 | 0.027 | 0.009 | 0.002 | ||||||
| Maturity Fear (EDI-3) | 0.026 | 0.034 | 0.010 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Criscuolo, M.; Cinelli, G.; Croci, I.; Chianello, I.; Caramadre, A.M.; Tozzi, A.E.; Zanna, V. Psychopathological Profile Associated with Food Addiction Symptoms in Adolescents with Eating Disorders. Int. J. Environ. Res. Public Health 2023, 20, 3014. https://doi.org/10.3390/ijerph20043014
Criscuolo M, Cinelli G, Croci I, Chianello I, Caramadre AM, Tozzi AE, Zanna V. Psychopathological Profile Associated with Food Addiction Symptoms in Adolescents with Eating Disorders. International Journal of Environmental Research and Public Health. 2023; 20(4):3014. https://doi.org/10.3390/ijerph20043014
Chicago/Turabian StyleCriscuolo, Michela, Giulia Cinelli, Ileana Croci, Ilenia Chianello, Anna Maria Caramadre, Alberto Eugenio Tozzi, and Valeria Zanna. 2023. "Psychopathological Profile Associated with Food Addiction Symptoms in Adolescents with Eating Disorders" International Journal of Environmental Research and Public Health 20, no. 4: 3014. https://doi.org/10.3390/ijerph20043014
APA StyleCriscuolo, M., Cinelli, G., Croci, I., Chianello, I., Caramadre, A. M., Tozzi, A. E., & Zanna, V. (2023). Psychopathological Profile Associated with Food Addiction Symptoms in Adolescents with Eating Disorders. International Journal of Environmental Research and Public Health, 20(4), 3014. https://doi.org/10.3390/ijerph20043014






