Abstract
Several heterogeneous pathophysiology pathways have been hypothesized for being involved in the onset and course of Post-Traumatic Stress Disorder (PTSD). This systematic review aims to summarize the current evidence on the role of inflammation and immunological dysregulations in PTSD, investigating possible peripheral biomarkers linked to the neuroimmune response to stress. A total of 44 studies on the dysregulated inflammatory and metabolic response in subjects with PTSD with respect to controls were included. Eligibility criteria included full-text publications in the English language, human adult samples, studies involving both subjects with a clinical diagnosis of PTSD and a healthy control group. The research was focused on specific blood neuroimmune biomarkers, namely IL-1β, TNF-α, IL-6 and INF-γ, as well as on the potential harmful role of reduced antioxidant activity (involving catalase, superoxide dismutase and glutathione peroxidase). The possible role of the inflammatory-altered tryptophan metabolism was also explored. The results showed conflicting data on the role of pro-inflammatory cytokines in individuals with PTSD, and a lack of study regarding the other mediators investigated. The present research suggests the need for further studies in human samples to clarify the role of inflammation in the pathogenesis of PTSD, to define potential peripheral biomarkers.
1. Introduction
According to the fifth version-text revision of the Diagnostical and Statistical Manual for Mental Disorders (DSM-5-TR), Post-Traumatic Stress Disorder (PTSD) is a mental disorder characterized by the onset of typical symptoms following trauma exposure, such as natural disasters, war, rape or sexual abuse [1]. The lifetime prevalence of PTSD varies across studies upon methodological issues, such as the instruments adopted for the assessments, and it is estimated to be around 3.9% worldwide, with double the rates in female compared to male subjects (10–12% vs. 5–6%) [2].
The diagnostic symptoms of PTSD include intrusion symptoms, avoidance, negative alterations in cognition and mood and hyperarousal, lasting more than 1 month, leading to a global functioning impairment and other possible complications as an increased risk for suicide attempts [1,3]. Specifically, intrusion symptoms are characterized by re-experiencing the stressful event, such as dissociative flashbacks and intruding memories that play a key role in the development and persistence of the disorder. However, the clinical manifestations of PTSD are variable, and the majority of people exposed to trauma do not develop the disorder, suggesting that the development of the disease depends not only on the characteristics of the trauma, but also on individuals’ risk factors [4,5,6,7,8].
PTSD is a heterogeneous disorder and multiple pathophysiology pathways are hypothesized to contribute to its onset and endurance. In recent years, the scientific community has explored the possible biological pathways underlying the disorder, highlighting a probable linkage with the hypothalamic–pituitary–adrenal (HPA) axis, autonomic nervous system, monoaminergic transmission system, inflammation and immunological dysregulations, thus proposing a variety of so-called possible neuroimmune biomarkers of mental illnesses and PTSD [9,10,11].
Particularly, the HPA axis has been widely investigated in PTSD: stress exposure causes the release of corticotropin-releasing factor (CRF) and vasopressin from the paraventricular nucleus of the hypothalamus to stimulate the anterior pituitary gland, which in turn secretes the adrenocorticotropic hormone (ACTH) into the systemic circulation. The ACTH induces the release of glucocorticoids, especially cortisol, from the cortical part of adrenal glands; at the same time, stressful situations induce the release of catecholamines (epinephrine and norepinephrine) from the medulla of the adrenal glands. ACTH, cortisol and catecholamines responses depend on the type and intensity of the stressor [5,9,10].
Increasing evidence has been reported on the possible role of inflammation and immunological dysregulations in PTSD pathogenesis, so that pro-inflammatory cytokines are thought to relevantly contribute to the illness presentations, for instance, through the activation of the NF-κB and P38MAPK signal path. Particularly, alterations of interleukin-6 (IL-6), interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) as well as interferon-γ (INF- α) have been implicated in impaired processes of synaptic plasticity and neuroinflammation paths, underlying functional and cognitive anomalies related to PTSD [12]. Moreover, the increased levels of non-specific markers of inflammation such as Erythrocyte Sedimentation Rate (ESR) and C-Reactive Protein (CRP), together with the imbalance between reactive oxygen species (ROS) damages and the activity of antioxidant enzymes, such as catalase (CAT) and superoxide dismutase (SOD), have been highlighted [13]. Similarly, a link between PTSD and an increased risk of physical diseases, such as cardiovascular diseases and autoimmune diseases has been described, suggesting that a dysregulated inflammatory component can be a common subset of the condition [14].
Furthermore, proinflammatory cytokines induced by stress and traumatic event exposure have also been implicated in the upregulation of the indoleamine 2,3-dioxygenase (IDO), which is a crucial enzyme in the kynurenine shunt, a main pathway of tryptophan degradation. The activation of IDO results, at least acutely, in the decrease in tryptophan concentration and the increase in several metabolites, including kynurenic and quinoline acids, that have been involved in the NMDA neurotransmission and possible neurotoxicity [5,15,16].
Although the existing literature indicates possible changes in stress neuroimmune and inflammatory biomarkers in PTSD, the results in human models are still conflicting, while data have only focused mainly on IL-6, CRP and the HPA axis. As a consequence, there is no full consensus on their use as biomarkers in clinical practice. The aim of this systematic review was therefore to summarize evidence suggestive of the following:
- The presence of a dysregulated inflammatory response in individuals with PTSD versus controls, focusing on specific blood inflammatory biomarkers, as IL-1β, TNF-α, IL-6 and INF-γ, as well as on the potential harmful role on endothelial tissue integrity produced by the decreased clearance of ROS and the reduced antioxidant activity, involving CAT, glutathione peroxidase (GPX) and SOD activities;
- The role of tryptophan metabolism, or the serotonergic and kynurenine pathways, in the understanding of inflammation in PTSD;
- The usefulness of these molecular patterns as potential biomarkers of this disorder.
2. Materials and Methods
2.1. Literature Search
A systematic search was conducted in accordance with the PRISMA guidelines [17] and using the electronic databases PubMed, EMBASE and Web of Science. A combination of controlled vocabulary terms, free-text terms and keywords, without filters, restrictions or limitations, was used to identify all potentially eligible records. The basic search string used was (“IL-6” OR “interleukin-6” OR “IL-1β” OR “interleukin-1β” OR “IFN-γ” OR “interferon-γ” OR “kynurenine” OR “quinolinic acid” OR “ROS” OR “Reactive oxygen species” OR “superoxide dismutase” OR “SOD” OR “catalase” OR “CAT” OR “glutathione peroxidase” OR “GPX” OR “tumor necrosis factor-α” OR “TNF-α” OR “tryptophan” OR “melatonin”) AND (“PTSD” OR “Post-Traumatic Stress Disorder” OR “Posttraumatic stress disorder”). All studies from 1 January 1990, to 31 August 2022 were included in the database search.
2.2. Eligibility Criteria
The criteria for inclusion of studies in this review were as follows:
- Human studies;
- Studies involving subjects with a clinical diagnosis of PTSD;
- Studies involving a healthy control (HC) group;
- Studies that included only subjects aged > 17 years;
- Articles in English.
Because the aim of the study was to investigate possible in vivo biomarkers of PTSD patients, studies focusing on in vitro investigations (e.g., on cytokine gene expression patterns in cell cultures) and studies using animal models were excluded.
2.3. Screening and Selection Process
S.F., first evaluator, and D.G., second evaluator, conducted all phases of the literature selection. The initial database search produced a total of 2145 records. After that, 2000 articles were removed because of their title or abstract, as duplicates (n = 837) or as not relevant (n = 1163); the other 80 records were excluded because of different publication types (n = 78) or because their full text was not available or not in English (n = 2). Subsequently, 21 publications were excluded because they were studies that did not include a control group or a clinical PTSD diagnosis or other eligibility criteria. In addition, all references cited in the selected studies, including reviews and metanalyses, were manually screened. However, no suitable articles emerged from this further research. Finally, 44 articles were included in the present review. The first (S.F.) and second (D.G.) evaluator conducted this selection process independently. Any discrepancy that arose during the categorization phases was discussed and consensus was reached. The overall level of agreement between the two evaluators was good. Any disagreement concerning the inclusion or exclusion of a literature paper in the study was discussed and resolved by a third author (V.D.O.). Inclusion and exclusion decisions are summarized in a flowchart according to PRISMA recommendations [17]. The process of study selection is outlined in this flowchart (Figure 1).
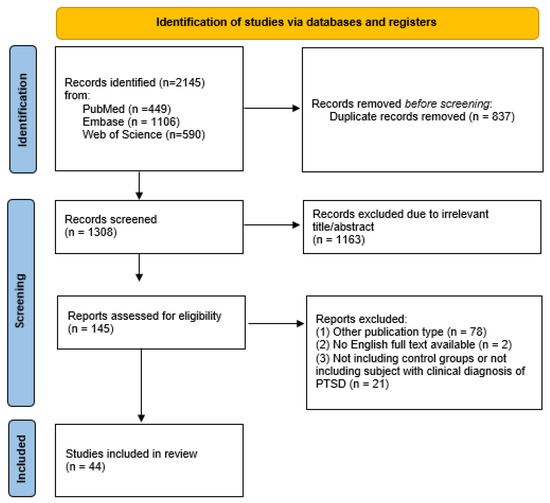
Figure 1.
PRISMA flow diagram of the study selection process. PRISMA, Preferred Reporting Items for Systematic reviews and Meta-Analyses; PTSD, Post-Traumatic Stress Disorder.
3. Results
The search provided the 44 studies included in the review, ranging from 1997 to 2022. Details of each study included in the review are reported in Table 1.

Table 1.
Characteristics of the included studies.
3.1. Characteristics of the Study Samples
3.1.1. Population
In the present search, most of the selected works (n = 19, 43.18%) examined samples from the general population, 15 of which included both genders, while four only evaluated females. Veterans were studied in 38.63% of all papers (n = 17), with 10 including only the male gender and seven including both genders. Of note, one of these studies included veterans with rheumatoid arthritis. Finally, 18.8% of the selected papers (n = eight) included other populations, such as earthquake survivors (n = two), war refugees (n = one), women with socioeconomic difficulties (n = one), abused women (n = one), drugs and alcohol abusers (n = one) or psychiatric inpatients (n = two). Regarding the medication status of the patients, 19 studies (43.18%) included a drug-free population. The indication “drug-free” means that patients were recruited if they did not take any drug, including psychotropic medication, or they were under a wash-out period of at least 2 weeks before the beginning of the investigation.
3.1.2. Type of Trauma
In most of the included articles (n = 25; 56.81%), the sample includes individuals who have been exposed to a specific type of traumatic event. Among these, exposure to war seems to be the most common (n = 18; 40.9% of all studies included). Other types of traumas were represented by interpersonal violence (n = two) and earthquakes (n = two); fire/multiple collision car crash (n = one), myocardial infarction (n = one) and urban violence (n = one). However, in 43.18% of the included studies (n = 19), the sample consists of subjects who have experienced various traumatic events.
3.1.3. Mean Ages
The mean age in the PTSD subsample groups was 40.52 years, while the mean age in the health control groups was 39.89 years. The mean ages of the populations were not available in four studies.
3.2. PTSD Diagnosis
To assess PTSD, 37 studies (84.09%) only used a scale. The most utilized scale was the Clinician-Administered PTSD Scale (CAPS) (n = 21; 47.72%): particularly, the CAPS was used for DSM-IV (CAPS-IV or CAPS) in 17 studies; the CAPS was used for DSM-5 (CAPS-5) in three studies; and the CAPS was used for DSM-III (CAPS-1) in one study. Eight studies (18.18%) used the DSM criteria to establish the PTSD diagnosis: the DSM-IV was used in four studies; the DSM-IV-R was in one study, the DSM-V was used in two studies and the DSM III-R was used in one case.
Further, seven studies (15.9%) utilized the Structured Clinical Interview for DSM (SCID) criteria: the SCID for DSM-III-R was used in two studies, and the SCID for DSM-IV was used in five studies. The remaining 15 studies (34.09%) assessed PTSD by means of other kinds of psychometric instruments, such as the Mini International Neuropsychiatric Interview (MINI, three studies), the 10th edition of the International Classification of Diseases (ICD-10, four studies), the 9th edition of International Classification of Diseases (ICD-9, one study), the Post-traumatic Stress Diagnostic Scale (PDS, two studies), the Perceived Stress Scale (PSS, one study), the Impact of Event Scale-Revised (IES-R, two studies), the Post-traumatic Checklist Scale (PCLS, one study) and the Composite International Diagnostic Interview (CIDI, one study).
3.3. Biomarkers
3.3.1. Biological Sample
In 24 studies (54.54%) the biochemical markers were analyzed in serum, while plasma was investigated in 14 studies (31.81%). In the remaining eight studies (18.18%) other types of biological matrices were collected, particularly whole blood (four studies) liquor (three studies) and saliva (two studies).
3.3.2. IL-6
IL-6 serum or plasma concentrations were investigated in 34 studies. In 20 studies (58.82%), no significant differences were found between IL-6 concentrations in PTSD and HC groups. In 13 studies (38.23%), a significantly higher plasma concentration of IL-6 was reported in PTSD patients compared with control subjects. Only one study (2.94%) detected significantly lower plasma levels of IL-6 in the PTSD group. In addition, one study measured the concentration of IL-6 in CSF; it showed a significantly higher IL-6 amount in the liquor of PTSD patients.
3.3.3. IL-1β
The measurements of IL-1β were obtained from 14 different studies. Seven of them (50%) showed higher serum and plasma concentrations in PTSD patients compared to the HC group, while only one (7.14%) study reported a significantly lower plasma concentration of IL-1β in PTSD than the HC group. Further, no significant difference was found in six studies (42.86%).
3.3.4. TNF-α
In 12 (52.17%) of the 23 studies that analyzed this outcome, higher TNF-α levels were found in the PTSD group compared to HC. There were no significant differences in the remaining 11 studies (47.83%).
3.3.5. IFN-γ
IFN-γ concentrations were investigated in 12 studies. Significantly higher concentrations of IFN-γ in blood and serum samples in PTSD patients than HC were described in three studies (25%). Conversely, no significant differences emerged in nine studies (75%).
3.3.6. Kynurenine and Tryptophan
There was no study investigating kynurenine and tryptophan levels independently. One included study analyzed the serum kynurenine/tryptophan ratio, but no statistically significant differences were highlighted between PTSD patients and HC groups.
3.3.7. Melatonin
Melatonin concentrations were investigated in one study which evidenced lower nocturne-melatonin salivary levels in PTSD patients with respect to HC, despite no statistical differences in the 24 h melatonin concentration were found in the two groups.
3.3.8. Superoxide Dismutase (SOD)
SOD levels were investigated in two studies: one study showed a lower serum SOD concentration in PTSD patients than HC, while no significant plasma SOD activity difference between the two groups was reported in the other study.
3.3.9. Catalase (CAT)
Measurements of CAT were extracted from two studies: one of them showed a significantly higher serum level of CAT in PTSD patients rather than HC, while no difference in the two groups was detected in the other one, where serum CAT levels were measured.
3.3.10. Glutathione Peroxidase (GPX)
The level of GPX was investigated in two studies, both showing a significantly lower serum concentration in PTSD patients with respect to the controls.
3.3.11. ROS/Quinolinic Acid
No studies comparing ROS or Quinolinic Acid levels in PTSD patients with respect to HC were found.
4. Discussion
In recent years, increasing interest has been devoted to the possible pathophysiological pathways underlying PTSD. The hypothalamic–pituitary–adrenal (HPA) axis, besides the autonomic nervous system, the monoaminergic transmission system, inflammation and immunological dysregulations, represent key systems hypothesized to be involved in the development of the aforementioned disorder; however, results in human models are still inconsistent [5,9,10,13,14]. As a result, there is no uniform consensus on the use of these mediators as possible biomarkers in clinical practice. Particularly, this review focused on the presence of evidence supporting a dysregulated inflammatory response in individuals with PTSD compared with controls. In this framework, it should be pointed out that, in accordance with the classification proposed by Davis et al., 2015 [62], the studies included in the present review were mostly examining biomarkers of PTSD status, while only a few assessed biomarkers of both trait and disease staging and/or biomarkers of drug response in PTSD (see Table 1).
Specifically, we have targeted some peripheral biomarkers, prevalently those that have been involved in the neuroendocrine response to stress and coping, commonly investigated in other mental illnesses and, therefore, defined as neuroimmune biomarkers [9,10]. These are the main cytokines belonging to the innate immune arsenal such as the aforementioned IL-1β, TNF-α, IL-6 and INF-γ. Furthermore, if considering the interrelationships existing between neuroinflammation, stress and metabolic adaptation to stress after the activation of the sympathetic system and HPA axis, the potential damaging role resulting from impaired ROS clearance and antioxidant activity was also investigated herein, as part of this same response. Thus, studies including the activities of CAT, glutathione peroxidase (GPX) and SOD, the first-line antioxidant enzymes, were comprised in the analysis. Finally, the possible role of inflammatory-altered tryptophan and serotonergic metabolism leading to the kynurenine signaling pathways and the accumulation of potentially neurotoxic metabolites in PTSD, was included.
In regards to IL-6 and INF-γ, most of the included studies [18,20,23,24,25,27,29,34,35,38,40,43,44,45,48,50,52,56,59,60] showed no significant differences between subjects with PTSD and HC. Likewise, differences in IL-β and TNF-α levels between subjects with PTSD and HC were found in slightly more than half of the studies examined. Although the “low-grade inflammation model” [33,35,63] is widely accepted in the scientific community, the results of the present review confirmed conflicting data about the role of pro-inflammatory cytokines in individuals with PTSD. Although some studies did not find significant statistical differences in IL -6 levels between the PTSD group and HC, important elements identified included an upward trend in IL6 [24], the isolated loss of the biphasic plasma peripheral IL-6 circadian pattern with attenuated plasma circadian variability in PTSD compared with HC [23], a positive correlation with symptom severity [34] and higher IL-6 salivary levels as a signal of anticipatory anxiety in the whole sample. This might suggest that the statistical result was influenced by the small number of the sample, resulting in type II error [64]. In addition, the heterogeneity of the studies (e.g., presence or absence of a polytrauma, isolated disorder, acute stress vs. spontaneous assessment) could explain the different results.
Further, scant data are available on the possible role of antioxidant and redox systems in PTSD. Only two studies [28,37] compared the serum levels of SOD in PTSD patients with those of HC, while two studies [37,41] explored the levels of CAT and two studies explored the GPX ones [28,53]. Although results on SOD and CAT were contradictory, both studies on GPX indicated its reduction in serum levels in subjects with PTSD compared to controls. It is noteworthy that, in the presence of oxidative stress, glutathione and other circulating proteins called thiols (P-SH) can be oxidized with a reversible process [65]. Thiol-based redox systems protect cells and organisms against ROS, maintain redox homeostasis and contribute to redox regulation [65,66,67,68], and data suggest that an imbalance in this system could be present in PTSD subjects.
The research yielded no study analyzing tryptophan and kynurenine levels independently, while only one study examined the ratio between kynurenine and tryptophan in serum, with no differences between PTSD patients and HC groups [44]. However, according to currently proposed biological models, proinflammatory cytokines seem to be involved in the upregulation of indoleamine 2,3-dioxygenase (IDO), which is a critical enzyme in the kynurenine shunt [5,15,16]. The activation of IDO leads, at least acutely, to a decrease in the tryptophan concentrations (and indole-conserving pathway metabolites), with an increase in several kynurenine metabolites (including kynurenic and quinolinic acid) which have been associated with NMDA neurotransmission and possible neurotoxicity [5,15,16]. The kynurenine shunt has acquired much interest in stress research and PTSD pathophysiology since it is the main metabolic route of free tryptophan and is a multi-branched pathway regulated by the HPA axis and cytokines [69,70]; it also contributes to balancing tryptophan, serotonin and melatonin amounts in the body, producing a variety of adaptogen derivatives, such as kynurenic acid, anthranilate, picolinic acid, quinolinic acid and the key energy metabolism coenzyme, NAD+ [69,70]. Among these compounds, kynurenine and quinolinic acid are particularly noteworthy because they are two main intermediates of the niacin/NAD+ branch, which links the glutamatergic neurotransmission with metabolism and redox reactions [70]. The essential amino acid tryptophan is also the precursor of the hormone melatonin by the indole-conserving pathway, which could be reduced when the kynurenine shunt is induced by inflammation [68]. It is relevant to investigate any changes in melatonin in patients with PTSD, since it regulates the sleep–wake circadian rhythm and sleep disturbances are part of the core symptoms of PTSD [1]. Nevertheless, only one study on this topic emerged [19], reporting the nocturnal melatonin levels in saliva of PTSD patients were lower than those of HC, although no differences in the 24- h melatonin concentration were found. Further studies seem to be necessary to explore this metabolic pathway in depth and its possible role in PTSD.
This review showed the current lack of data on the role of inflammation in subjects with PTSD and on possible biological biomarkers in the human population. Moreover, the most reported traumatic event was the war experience, as the studied sample consisted of veterans or war refugees. It is important to expand the data on different types of traumas to assess whether different events may elicit different pathophysiological responses. Finally, the use of psychotropic drugs could alter peripheral levels of the mediators examined, but less than half of the studies investigated a drug-free population.
In discussing our results, some limitations must be considered. First, we included only English language articles in our selection. Second, some of the included studies have small sample sizes, which may affect the statistical power of the study itself. Finally, the presence of other psychiatric or organic comorbidities as possible exclusion criteria was not always reported in the selected articles, nor were other variables (e.g., premorbid personality, family history, alcohol use), albeit their presence could have influenced the values of the mediators studied.
5. Conclusions
Scant and often conflicting data are currently available on the etiopathogenetic mechanisms and possible biomarkers in PTSD subjects. Despite the growing interest in this field, the present research suggests the need for further studies in human samples to deepen the knowledge on the role of inflammation in PTSD pathogenesis, to clearly define potential biomarkers that could be used in clinical practice.
Author Contributions
Conceptualization, V.D., L.P., L.B., G.G. and C.C.; methodology, V.D., L.P., G.G. and C.C.; investigation, V.D., S.F. and D.G.; writing—original draft preparation, V.D., S.F., D.G., L.P. and C.C.; writing—review and editing, V.D., S.F., D.G., L.P. and C.C.; supervision, V.D., L.P., L.B., L.D., G.G. and C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Text-Revision (DSM-5 TR), 5th ed.; American Psychiatric Press: Washington, DC, USA, 2022. [Google Scholar]
- Passos, I.C.; Vasconcelos-Moreno, M.P.; Costa, L.G.; Kunz, M.; Brietzke, E.; Quevedo, J.; Salum, G.; Magalhães, P.V.; Kapczinski, F.; Kauer-Sant’Anna, M. Inflammatory markers in post-traumatic stress disorder: A systematic review, meta-analysis, and meta-regression. Lancet Psychiatry 2015, 2, 1002–1012. [Google Scholar] [CrossRef] [PubMed]
- Dell’Osso, L.; Carmassi, C.; Rucci, P.; Ciapparelli, A.; Paggini, R.; Ramacciotti, C.E.; Conversano, C.; Balestrieri, M.; Marazziti, D. Lifetime subthreshold mania is related to suicidality in posttraumatic stress disorder. CNS Spectr. 2009, 14, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Koenen, K.C.; Ratanatharathorn, A.; Ng, L.; McLaughlin, K.A.; Bromet, E.J.; Stein, D.J.; Karam, E.G.; Ruscio, A.M.; Benjet, C.; Scott, K.; et al. Posttraumatic stress disorder in the World Mental Health Surveys. Psychol. Med. 2017, 47, 2260–2274. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Amidfar, M.; Won, E. A review on inflammatory cytokine-induced alterations of the brain as potential neural biomarkers in post-traumatic stress disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 91, 103–112. [Google Scholar] [CrossRef]
- Carmassi, C.; Barberi, F.M.; Cordone, A.; Maglio, A.; Dell’Oste, V.; Dell’Osso, L. Trauma, PTSD and post-traumatic stress spectrum: 15 years’ experience on a multidimensional approach to trauma related psychopathology. J. Psychopathol. 2020, 26, 4–11. [Google Scholar] [CrossRef]
- Dell’Osso, L.; Carmassi, C.; Musetti, L.; Socci, C.; Shear, M.K.; Conversano, C.; Maremmani, I.; Perugi, G. Lifetime mood symptoms and adult separation anxiety in patients with complicated grief and/or post-traumatic stress disorder: A preliminary report. Psychiatry Res. 2012, 198, 436–440. [Google Scholar] [CrossRef]
- Stratta, P.; Capanna, C.; Dell’Osso, L.; Carmassi, C.; Patriarca, S.; Di Emidio, G.; Riccardi, I.; Collazzoni, A.; Rossi, A. Resilience and coping in trauma spectrum symptoms prediction: A structural equation modeling approach. Personal. Individ. Differ. 2015, 77, 55–61. [Google Scholar] [CrossRef]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr. Physiol. 2016, 6, 603–621. [Google Scholar] [CrossRef]
- Palego, L.; Giannaccini, G.; Betti, L. Neuroendocrine Response to Psychosocial Stressors, Inflammation Mediators and Brain-periphery Pathways of Adaptation. Cent. Nerv. Syst. Agents Med. Chem. 2021, 21, 2–19. [Google Scholar] [CrossRef]
- Miller, A.H. Mechanisms of cytokine-induced behavioral changes: Psychoneuroimmunology at the translational interface. Brain Behav. Immun. 2009, 23, 149–158. [Google Scholar] [CrossRef]
- Levin, S.G.; Godukhin, O.V. Modulating Effect of Cytokines on Mechanisms of Synaptic Plasticity in the Brain. Biochemistry 2017, 82, 264–274. [Google Scholar] [CrossRef]
- Karanikas, E. Psychologically Traumatic Oxidative Stress; A Comprehensive Review of Redox Mechanisms and Related Inflammatory Implications. Psychopharmacol. Bull. 2021, 51, 65–86. [Google Scholar]
- Levine, A.B.; Levine, L.M.; Levine, T.B. Posttraumatic stress disorder and cardiometabolic disease. Cardiology 2014, 127, 1–19. [Google Scholar] [CrossRef]
- Hori, H.; Kim, Y. Inflammation and post-traumatic stress disorder. Psychiatry Clin. Neurosci. 2019, 73, 143–153. [Google Scholar] [CrossRef]
- Katrinli, S.; Oliveira, N.C.S.; Felger, J.C.; Michopoulos, V.; Smith, A.K. The role of the immune system in posttraumatic stress disorder. Transl. Psychiatry 2022, 12, 313. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 105906. [Google Scholar]
- Mehta, N.D.; Stevens, J.S.; Li, Z.; Gillespie, C.F.; Fani, N.; Michopoulos, V.; Felger, J.C. Inflammation, reward circuitry and symptoms of anhedonia and PTSD in trauma-exposed women. Soc. Cogn. Affect. Neurosci. 2020, 15, 1046–1055. [Google Scholar] [CrossRef]
- Paul, M.A.; Love, R.J.; Jetly, R.; Richardson, J.D.; Lanius, R.A.; Miller, J.C.; MacDonald, M.; Rhind, S.G. Blunted Nocturnal Salivary Melatonin Secretion Profiles in Military-Related Posttraumatic Stress Disorder. Front. Psychiatry 2019, 10, 882. [Google Scholar] [CrossRef]
- Wang, W.; Wang, L.; Xu, H.; Cao, C.; Liu, P.; Luo, S.; Duan, Q.; Ellenbroek, B.; Zhang, X. Characteristics of pro- and anti-inflammatory cytokines alteration in PTSD patients exposed to a deadly earthquake. J. Affect. Disord. 2019, 248, 52–58. [Google Scholar] [CrossRef]
- Imai, R.; Hori, H.; Itoh, M.; Lin, M.; Niwa, M.; Ino, K.; Ogawa, S.; Ishida, M.; Sekiguchi, A.; Matsui, M.; et al. Inflammatory markers and their possible effects on cognitive function in women with posttraumatic stress disorder. J. Psychiatr. Res. 2018, 102, 192–200. [Google Scholar] [CrossRef]
- Imai, R.; Hori, H.; Itoh, M.; Lin, M.; Niwa, M.; Ino, K.; Ogawa, S.; Sekiguchi, A.; Kunugi, H.; Akechi, T.; et al. Relationships of blood proinflammatory markers with psychological resilience and quality of life in civilian women with posttraumatic stress disorder. Sci. Rep. 2019, 9, 17905. [Google Scholar] [CrossRef] [PubMed]
- Agorastos, A.; Hauger, R.L.; Barkauskas, D.A.; Lerman, I.R.; Moeller-Bertram, T.; Snijders, C.; Haji, U.; Patel, P.M.; Geracioti, T.D.; Chrousos, G.P.; et al. Relations of combat stress and posttraumatic stress disorder to 24-h plasma and cerebrospinal fluid interleukin-6 levels and circadian rhythmicity. Psychoneuroendocrinology 2019, 100, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.K.; Fonda, J.R.; Hauger, R.L.; Pinna, G.; Anderson, G.M.; Valovski, I.T.; Rasmusson, A.M. Composite contributions of cerebrospinal fluid GABAergic neurosteroids, neuropeptide Y and interleukin-6 to PTSD symptom severity in men with PTSD. Neurobiol. Stress 2020, 12, 100220. [Google Scholar] [CrossRef] [PubMed]
- Küffer, A.; Straus, L.D.; Prather, A.A.; Inslicht, S.S.; Richards, A.; Shigenaga, J.K.; Madden, E.; Metzler, T.J.; Neylan, T.C.; O’Donovan, A. Altered overnight levels of pro-inflammatory cytokines in men and women with posttraumatic stress disorder. Psychoneuroendocrinology 2019, 102, 114–120. [Google Scholar] [CrossRef]
- Brahmajothi, M.V.; Abou-Donia, M.B. PTSD Susceptibility and Challenges: Pathophysiological Consequences of Behavioral Symptoms. Mil. Med. 2020, 185 (Suppl. 1), 279–285. [Google Scholar] [CrossRef]
- Maloley, P.M.; England, B.R.; Sayles, H.; Thiele, G.M.; Michaud, K.; Sokolove, J.; Cannon, G.W.; Reimold, A.M.; Kerr, G.S.; Baker, J.F.; et al. Post-traumatic stress disorder and serum cytokine and chemokine concentrations in patients with rheumatoid arthritis. Semin. Arthritis Rheum. 2019, 49, 229–235. [Google Scholar] [CrossRef]
- Štefanović, L.B.; Kalinić, D.; Mimica, N.; Beer Ljubić, B.; Aladrović, J.; Mandelsamen Perica, M.; Curić, M.; Grošić, P.F.; Delaš, I. Oxidative status and the severity of clinical symptoms in patients with post-traumatic stress disorder. Ann. Clin. Biochem. 2015, 52 Pt 1, 95–104. [Google Scholar] [CrossRef]
- Gola, H.; Engler, H.; Sommershof, A.; Adenauer, H.; Kolassa, S.; Schedlowski, M.; Groettrup, M.; Elbert, T.; Kolassa, I.T. Posttraumatic stress disorder is associated with an enhanced spontaneous production of pro-inflammatory cytokines by peripheral blood mononuclear cells. BMC Psychiatry 2013, 13, 40. [Google Scholar] [CrossRef]
- Vidović, A.; Gotovac, K.; Vilibić, M.; Sabioncello, A.; Jovanović, T.; Rabatić, S.; Folnegović-Šmalć, V.; Dekaris, D. Repeated assessments of endocrine- and immune-related changes in posttraumatic stress disorder. Neuroimmunomodulation 2011, 18, 199–211. [Google Scholar] [CrossRef]
- Oganesyan, L.P.; Mkrtchyan, G.M.; Sukiasyan, S.H.; Boyajyan, A.S. Classic and alternative complement cascades in post-traumatic stress disorder. Bull. Exp. Biol. Med. 2009, 148, 859–861. [Google Scholar] [CrossRef]
- Von Känel, R.; Begré, S.; Abbas, C.C.; Saner, H.; Gander, M.L.; Schmid, J.P. Inflammatory biomarkers in patients with posttraumatic stress disorder caused by myocardial infarction and the role of depressive symptoms. Neuroimmunomodulation 2010, 17, 39–46. [Google Scholar] [CrossRef]
- Hoge, E.A.; Brandstetter, K.; Moshier, S.; Pollack, M.H.; Wong, K.K.; Simon, N.M. Broad spectrum of cytokine abnormalities in panic disorder and posttraumatic stress disorder. Depress. Anxiety 2009, 26, 447–455. [Google Scholar] [CrossRef]
- Song, Y.; Zhou, D.; Guan, Z.; Wang, X. Disturbance of serum interleukin-2 and interleukin-8 levels in posttraumatic and non-posttraumatic stress disorder earthquake survivors in northern China. Neuroimmunomodulation 2007, 14, 248–254. [Google Scholar] [CrossRef]
- Von Känel, R.; Hepp, U.; Kraemer, B.; Traber, R.; Keel, M.; Mica, L.; Schnyder, U. Evidence for low-grade systemic proinflammatory activity in patients with posttraumatic stress disorder. J. Psychiatr. Res. 2007, 41, 744–752. [Google Scholar] [CrossRef]
- Woods, A.B.; Page, G.G.; O’Campo, P.; Pugh, L.C.; Ford, D.; Campbell, J.C. The mediation effect of posttraumatic stress disorder symptoms on the relationship of intimate partner violence and IFN-gamma levels. Am. J. Community Psychol. 2005, 36, 159–175. [Google Scholar] [CrossRef]
- Tezcan, E.; Atmaca, M.; Kuloglu, M.; Ustundag, B. Free radicals in patients with post-traumatic stress disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2003, 253, 89–91. [Google Scholar] [CrossRef]
- Baker, D.G.; Ekhator, N.N.; Kasckow, J.W.; Hill, K.K.; Zoumakis, E.; Dashevsky, B.A.; Chrousos, G.P.; Geracioti, T.D., Jr. Plasma and cerebrospinal fluid interleukin-6 concentrations in posttraumatic stress disorder. Neuroimmunomodulation 2001, 9, 209–217. [Google Scholar] [CrossRef]
- Maes, M.; Lin, A.H.; Delmeire, L.; Van Gastel, A.; Kenis, G.; De Jongh, R.; Bosmans, E. Elevated serum interleukin-6 (IL-6) and IL-6 receptor concentrations in posttraumatic stress disorder following accidental man-made traumatic events. Biol. Psychiatry 1999, 45, 833–839. [Google Scholar] [CrossRef]
- O’Donovan, A.; Chao, L.L.; Paulson, J.; Samuelson, K.W.; Shigenaga, J.K.; Grunfeld, C.; Weiner, M.W.; Neylan, T.C. Altered inflammatory activity associated with reduced hippocampal volume and more severe posttraumatic stress symptoms in Gulf War veterans. Psychoneuroendocrinology 2015, 51, 557–566. [Google Scholar] [CrossRef]
- Ogłodek, E.A.; Just, M.J.; Szromek, A.R.; Araszkiewicz, A. Assessing the serum concentration levels of NT-4/5, GPX-1, TNF-α, and l-arginine as biomediators of depression severity in first depressive episode patients with and without posttraumatic stress disorder. Pharm. Rep. 2017, 69, 1049–1058. [Google Scholar] [CrossRef]
- Blessing, E.M.; Reus, V.; Mellon, S.H.; Wolkowitz, O.M.; Flory, J.D.; Bierer, L.; Lindqvist, D.; Dhabhar, F.; Li, M.; Qian, M.; et al. Biological predictors of insulin resistance associated with posttraumatic stress disorder in young military veterans. Psychoneuroendocrinology 2017, 82, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Jergović, M.; Bendelja, K.; Savić Mlakar, A.; Vojvoda, V.; Aberle, N.; Jovanovic, T.; Rabatić, S.; Sabioncello, A.; Vidović, A. Circulating levels of hormones, lipids, and immune mediators in post-traumatic stress disorder—A 3-month follow-up study. Front. Psychiatry 2015, 6, 49. [Google Scholar] [CrossRef] [PubMed]
- Neupane, S.P.; Bramness, J.G.; Lien, L. Comorbid post-traumatic stress disorder in alcohol use disorder: Relationships to demography, drinking and neuroimmune profile. BMC Psychiatry 2017, 17, 312. [Google Scholar] [CrossRef] [PubMed]
- Bruenig, D.; Mehta, D.; Morris, C.P.; Lawford, B.; Harvey, W.; McD Young, R.; Voisey, J. Correlation between interferon γ and interleukin 6 with PTSD and resilience. Psychiatry Res. 2018, 260, 193–198. [Google Scholar] [CrossRef]
- Bersani, F.S.; Wolkowitz, O.M.; Lindqvist, D.; Yehuda, R.; Flory, J.; Bierer, L.M.; Makotine, I.; Abu-Amara, D.; Coy, M.; Reus, V.I.; et al. Global arginine bioavailability, a marker of nitric oxide synthetic capacity, is decreased in PTSD and correlated with symptom severity and markers of inflammation. Brain Behav. Immun. 2016, 52, 153–160. [Google Scholar] [CrossRef]
- Lindqvist, D.; Dhabhar, F.S.; Mellon, S.H.; Yehuda, R.; Grenon, S.M.; Flory, J.D.; Bierer, L.M.; Abu-Amara, D.; Coy, M.; Makotkine, I.; et al. Increased pro-inflammatory milieu in combat related PTSD—A new cohort replication study. Brain Behav. Immun. 2017, 59, 260–264. [Google Scholar] [CrossRef]
- Lindqvist, D.; Wolkowitz, O.M.; Mellon, S.; Yehuda, R.; Flory, J.D.; Henn-Haase, C.; Bierer, L.M.; Abu-Amara, D.; Coy, M.; Neylan, T.C.; et al. Proinflammatory milieu in combat-related PTSD is independent of depression and early life stress. Brain Behav. Immun. 2014, 42, 81–88. [Google Scholar] [CrossRef]
- De Oliveira, J.F.; Wiener, C.D.; Jansen, K.; Portela, L.V.; Lara, D.R.; Souza, L.D.M.; da Silva, R.A.; Moreira, F.P.; Oses, J.P. Serum levels of interleukins IL-6 and IL-10 in individuals with posttraumatic stress disorder in a population-based sample. Psychiatry Res. 2018, 260, 111–115. [Google Scholar] [CrossRef]
- Teche, S.P.; Rovaris, D.L.; Aguiar, B.W.; Hauck, S.; Vitola, E.S.; Bau, C.H.D.; Freitas, L.H.; Grevet, E.H. Resilience to traumatic events related to urban violence and increased IL10 serum levels. Psychiatry Res. 2017, 250, 136–140. [Google Scholar] [CrossRef]
- Ogłodek, E.A.; Szota, A.M.; Moś, D.M.; Araszkiewicz, A.; Szromek, A.R. Serum concentrations of chemokines (CCL-5 and CXCL-12), chemokine receptors (CCR-5 and CXCR-4), and IL-6 in patients with posttraumatic stress disorder and avoidant personality disorder. Pharm. Rep. 2015, 67, 1251–1258. [Google Scholar] [CrossRef]
- Jergović, M.; Tomičević, M.; Vidović, A.; Bendelja, K.; Savić, A.; Vojvoda, V.; Rac, D.; Lovrić-Čavar, D.; Rabatić, S.; Jovanovic, T.; et al. Telomere shortening and immune activity in war veterans with posttraumatic stress disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 54, 275–283. [Google Scholar] [CrossRef]
- Ogłodek, E.A. Evaluation of ADMA, carbonyl groups, CAT and NKA in depressed patients with and without posttraumatic stress disorder. Pharm. Rep. 2017, 69, 730–737. [Google Scholar] [CrossRef]
- Tucker, P.; Ruwe, W.D.; Masters, B.; Parker, D.E.; Hossain, A.; Trautman, R.P.; Wyatt, D.B. Neuroimmune and cortisol changes in selective serotonin reuptake inhibitor and placebo treatment of chronic posttraumatic stress disorder. Biol. Psychiatry 2004, 56, 121–128. [Google Scholar] [CrossRef]
- Spivak, B.; Shohat, B.; Mester, R.; Avraham, S.; Gil-Ad, I.; Bleich, A.; Valevski, A.; Weizman, A. Elevated levels of serum interleukin-1 beta in combat-related posttraumatic stress disorder. Biol. Psychiatry 1997, 42, 345–348. [Google Scholar] [CrossRef]
- Park, J.; Marvar, P.J.; Liao, P.; Kankam, M.L.; Norrholm, S.D.; Downey, R.M.; McCullough, S.A.; Le, N.A.; Rothbaum, B.O. Baroreflex dysfunction and augmented sympathetic nerve responses during mental stress in veterans with post-traumatic stress disorder. J. Physiol. 2017, 595, 4893–4908. [Google Scholar] [CrossRef]
- Guo, M.; Liu, T.; Guo, J.C.; Jiang, X.L.; Chen, F.; Gao, Y.S. Study on serum cytokine levels in posttraumatic stress disorder patients. Asian Pac. J. Trop. Med. 2012, 5, 323–325. [Google Scholar] [CrossRef]
- Dalgard, C.; Eidelman, O.; Jozwik, C.; Olsen, C.H.; Srivastava, M.; Biswas, R.; Eudy, Y.; Rothwell, S.W.; Mueller, G.P.; Yuan, P.; et al. The MCP-4/MCP-1 ratio in plasma is a candidate circadian biomarker for chronic post-traumatic stress disorder. Transl. Psychiatry 2017, 7, e1025. [Google Scholar] [CrossRef]
- Newton, T.L.; Fernandez-Botran, R.; Miller, J.J.; Burns, V.E. Interleukin-6 and soluble interleukin-6 receptor levels in posttraumatic stress disorder: Associations with lifetime diagnostic status and psychological context. Biol. Psychol. 2014, 99, 150–159. [Google Scholar] [CrossRef]
- Renner, V.; Schellong, J.; Bornstein, S.; Petrowski, K. Stress-induced pro- and anti-inflammatory cytokine concentrations in female PTSD and depressive patients. Transl. Psychiatry 2022, 12, 158. [Google Scholar] [CrossRef]
- Toft, H.; Bramness, J.; Tilden, T.; Bolstad, I.; Lien, L. Persistent level of mental distress in PTSD patients is not reflected in cytokine levels 1 year after the treatment. Acta Neuropsychiatr. 2021, 33, 254–260. [Google Scholar] [CrossRef]
- Davis, J.; Maes, M.; Andreazza, A.; McGrath, J.J.; Tye, S.J.; Berk, M. Towards a classification of biomarkers of neuropsychiatric disease: From encompass to compass. Mol. Psychiatry 2015, 20, 152–153. [Google Scholar] [CrossRef] [PubMed]
- Speer, K.; Upton, D.; Semple, S.; McKune, A. Systemic low-grade inflammation in posttraumatic stress disorder: A systematic review. J. Inflamm. Res. 2018, 11, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Streiner, D.L. Sample size and power in psychiatric research. Can. J. Psychiatry 1991, 36, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Bachi, A.; Dalle-Donne, I.; Scaloni, A. Redox proteomics: Chemical principles, methodological approaches and biological/biomedical promises. Chem. Rev. 2013, 113, 596–698. [Google Scholar] [CrossRef]
- Ulrich, K.; Jakob, U. The role of thiols in antioxidant systems. Free Radic. Biol. Med. 2019, 140, 14–27. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Milzani, A.; Gagliano, N.; Colombo, R.; Giustarini, D.; Rossi, R. Molecular mechanisms and potential clinical significance of S-glutathionylation. Antioxid. Redox Signal. 2008, 10, 445–473. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Rossi, R.; Colombo, G.; Giustarini, D.; Milzani, A. Protein S-glutathionylation: A regulatory device from bacteria to humans. Trends Biochem. Sci. 2009, 34, 85–96. [Google Scholar] [CrossRef]
- Palego, L.; Betti, L.; Rossi, A.; Giannaccini, G. Tryptophan Biochemistry: Structural, Nutritional, Metabolic, and Medical Aspects in Humans. J. Amino Acids 2016, 2016, 8952520. [Google Scholar] [CrossRef]
- Badawy, A.A. Kynurenine Pathway of Tryptophan Metabolism: Regulatory and Functional Aspects. Int. J. Tryptophan Res. 2017, 10, 1178646917691938. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).