Bone Health Status in Individuals with Amyotrophic Lateral Sclerosis: A Cross-Sectional Study on the Role of the Trabecular Bone Score and Its Implications in Neurorehabilitation
Abstract
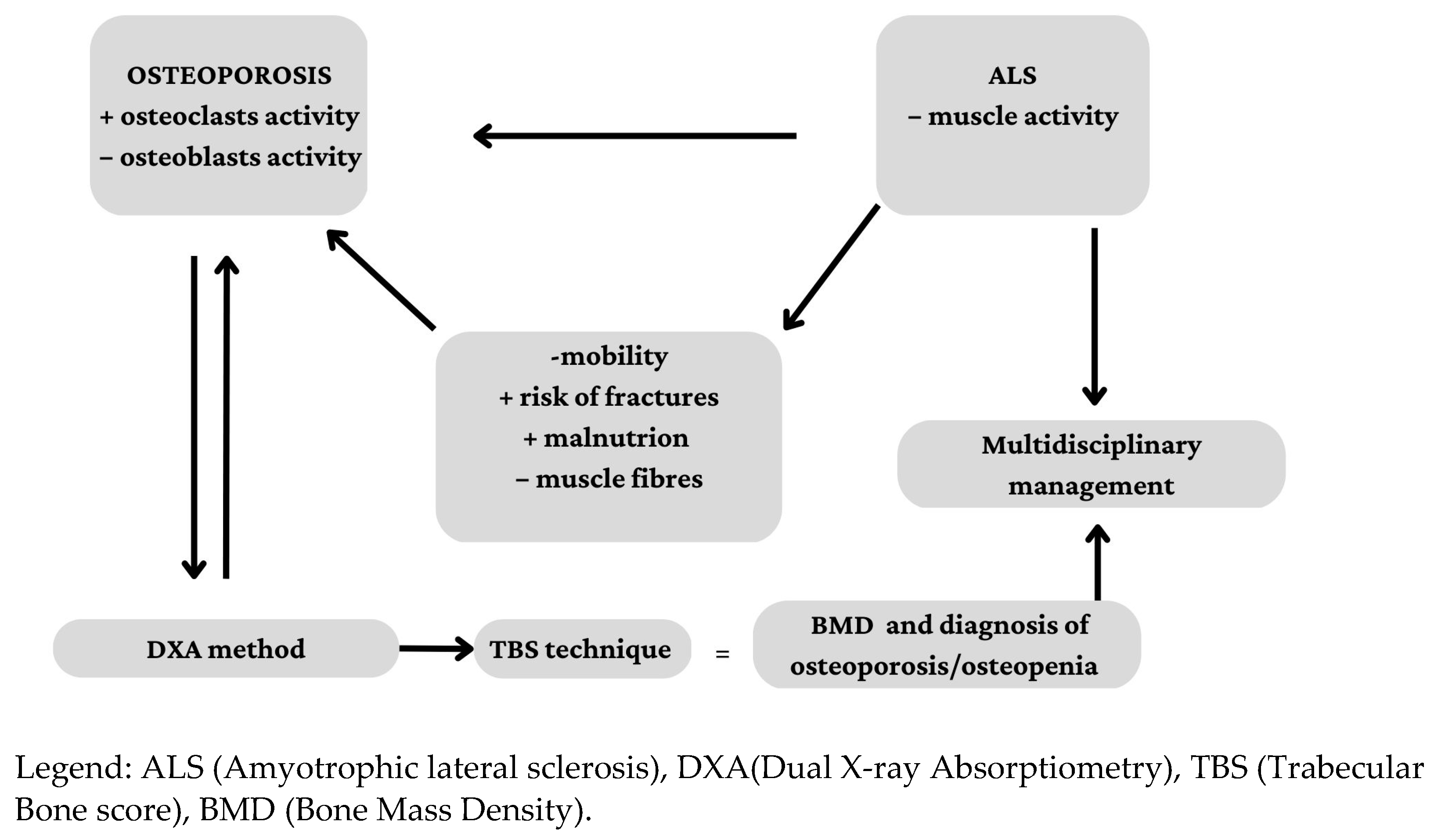
1. Introduction
2. Materials and Methods
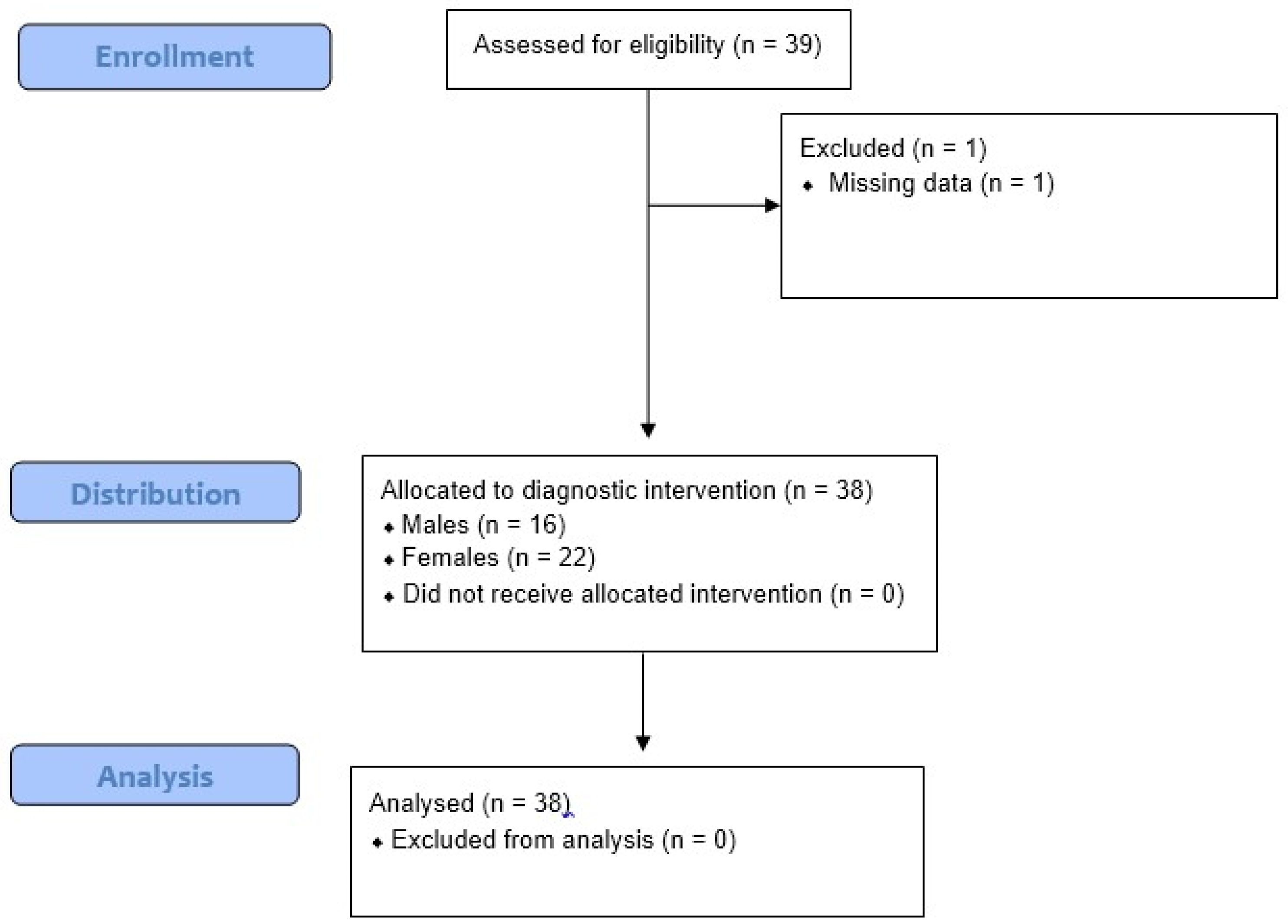
2.1. Study Population
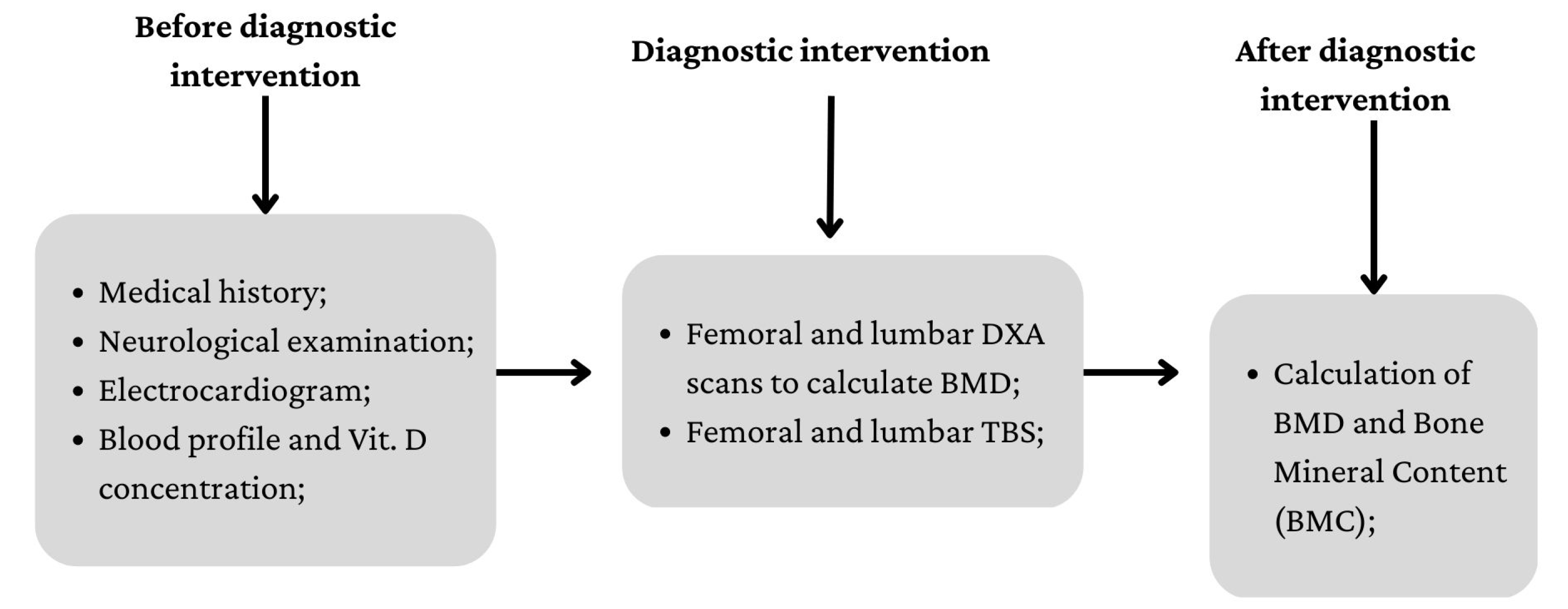
2.2. Procedures
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roos, P.M. Osteoporosis in neurodegeneration. J. Trace Elem. Med. Biol. 2014, 28, 418–421. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Burley, G.; Lin, S.; Shi, Y.C. Osteoporosis pathogenesis and treatment: Existing and emerging avenues. Cell Mol. Biol. Lett. 2022, 27, 72. [Google Scholar] [CrossRef] [PubMed]
- Salari, N.; Darvishi, N.; Bartina, Y.; Larti, M.; Kiaei, A.; Hemmati, M.; Shohaimi, S.; Mohammadi, M. Global prevalence of osteoporosis among the world older adults: A comprehensive systematic review and meta-analysis. J. Orthop. Surg. Res. 2021, 16, 669. [Google Scholar] [CrossRef] [PubMed]
- Karlamangla, A.S.; Burnett-Bowie, S.M.; Crandall, C.J. Bone Health During the Menopause Transition and Beyond. Obstet. Gynecol. Clin. 2018, 45, 695–708. [Google Scholar] [CrossRef]
- Kelly, R.R.; Sidles, S.J.; LaRue, A.C. Effects of Neurological Disorders on Bone Health. Front. Psychol. 2020, 11, 612366. [Google Scholar] [CrossRef]
- Siddiqui, J.A.; Partridge, N.C. Physiological Bone Remodelling: Systemic Regulation and Growth Factor Involvement. Physiology 2016, 31, 233–245. [Google Scholar] [CrossRef]
- Grad, L.I.; Rouleau, G.A.; Ravits, J.; Cashman, N.R. Clinical Spectrum of Amyotrophic Lateral Sclerosis (ALS). Cold Spring Harb. Perspect. Med. 2017, 7, a024117. [Google Scholar] [CrossRef]
- Sooragonda, B.G.; Agarwal, S.; Benjamin, R.N.; Prabhakar, A.T.; Sivadasan, A.; Kapoor, N.; Cherian, K.E.; Jebasingh, F.K.; Aaron, S.; Thomas, N.; et al. Bone Mineral Density and Body Composition in Males with Motor Neuron Disease: A Study from Teaching Hospital in Southern Part of India. Ann. Indian Acad. Neurol. 2021, 24, 211–216. [Google Scholar] [CrossRef]
- Caplliure-Llopis, J.; Escrivá, D.; Benlloch, M.; de la Rubia Ortí, J.E.; Estrela, J.M.; Barrios, C. Poor Bone Quality in Patients With Amyotrophic Lateral Sclerosis. Front. Neurol. 2020, 11, 599216. [Google Scholar] [CrossRef]
- Peters, T.L.; Weibull, C.E.; Fang, F.; Sandler, D.P.; Lambert, P.C.; Ye, W.; Kamel, F. Association of fractures with the incidence of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Front. Degener. 2017, 18, 419–425. [Google Scholar] [CrossRef]
- Joyce, N.C.; Hache, L.P.; Clemens, P.R. Bone health and associated metabolic complications in neuromuscular diseases. Phys. Med. Rehabil. Clin. N. Am. 2012, 23, 773–799. [Google Scholar] [CrossRef]
- Patten, B.M.; Mallette, L.E. Motor neuron disease: Retrospective study of associated abnormalities. Dis. Nerv. Syst. 1976, 37, 318–321. [Google Scholar]
- Dupuis, L.; Pradat, P.F.; Ludolph, A.C.; Loeffler, J.P. Energy metabolism in amyotrophic lateral sclerosis. Lancet Neurol. 2011, 10, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Garach, A.; García-Fontana, B.; Muñoz-Torres, M. Nutrients and Dietary Patterns Related to Osteoporosis. Nutrients 2020, 12, 1986. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.; Maseeh, A. Vitamin D: The “sunshine” vitamin. J. Pharmacol. Pharmacother. 2012, 3, 118–126. [Google Scholar] [CrossRef]
- Zhou, J.; Yi, J.; Bonewald, L. Muscle-Bone Crosstalk in Amyotrophic Lateral Sclerosis. Curr. Osteoporos. Rep. 2015, 13, 274–279. [Google Scholar] [CrossRef]
- Iolascon, G.; Paoletta, M.; Liguori, S.; Curci, C.; Moretti, A. Neuromuscular Diseases and Bone. Front. Endocrinol. 2019, 10, 794. [Google Scholar] [CrossRef]
- Messina, C.; Bignotti, B.; Bazzocchi, A.; Phan, C.M.; Tagliafico, A.; Guglielmi, G.; Sardanelli, F.; Sconfienza, L.M. A critical appraisal of the quality of adult dual-energy X-ray absorptiometry guidelines in osteoporosis using the AGREE II tool: An EuroAIM initiative. Insights Imaging 2017, 8, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Marshall, D.; Johnell, O.; Wedel, H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ 1996, 312, 1254–1259. [Google Scholar] [CrossRef]
- Shevroja, E.; Cafarelli, F.P.; Guglielmi, G.; Hans, D. DXA parameters, Trabecular Bone Score (TBS) and Bone Mineral Density (BMD), in fracture risk prediction in endocrine-mediated secondary osteoporosis. Endocrine 2021, 74, 20–28. [Google Scholar] [CrossRef]
- Pothuaud, L.; Carceller, P.; Hans, D. Correlations between grey-level variations in 2D projection images (TBS) and 3D microarchitecture: Applications in the study of human trabecular bone microarchitecture. Bone 2008, 42, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.C.; Leslie, W.D.; Resch, H.; Lamy, O.; Lesnyak, O.; Binkley, N.; McCloskey, E.V.; Kanis, J.A.; Bilezikian, J.P. Trabecular bone score: A noninvasive analytical method based upon the DXA image. J. Bone Miner. Res. 2014, 29, 518–530. [Google Scholar] [CrossRef]
- Palomo, T.; Muszkat, P.; Weiler, F.G.; Dreyer, P.; Brandão, C.M.A.; Silva, B.C. Update on trabecular bone score. Arch. Endocrinol. Metab. 2022, 66, 694–706. [Google Scholar] [CrossRef]
- Writing Group for the ISCD Position Development Conference. Position statement: Executive summary. The writing group for the International society for clinical densitometry (ISCD) position development conference. J. Clin. Densitom. 2004, 7, 7–12. [Google Scholar]
- Black, D.M.; Cauley, J.A.; Wagman, R.; Ensrud, K.; Fink, H.A.; Hillier, T.A.; Lui, L.Y.; Cummings, S.R.; Schousboe, J.T.; Napoli, N. The Ability of a Single BMD and Fracture History Assessment to Predict Fracture Over 25 Years in Postmenopausal Women: The Study of Osteoporotic Fractures. J. Bone Miner. Res. 2018, 33, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Rajaei, A.; Amiri, A.; Farsad, F.; Dehghan, P. The Correlation between Trabecular Bone Score and Lumbar Spine Bone Mineral Density in Patients with Normal and High Body Mass Index. Iran. J. Med. Sci. 2019, 44, 374–381. [Google Scholar] [CrossRef]
- Shevroja, E.; Mo Costabella, F.; Gonzalez Rodriguez, E.; Lamy, O.; Hans, D. The fracture predictive ability of lumbar spine BMD and TBS as calculated based on different combinations of the lumbar spine vertebrae. Arch. Osteoporos. 2022, 17, 83. [Google Scholar] [CrossRef]
- Park, D.; Kwak, S.G.; Park, J.S.; Choo, Y.J.; Chang, M.C. Can Therapeutic Exercise Slow Down Progressive Functional Decline in Patients With Amyotrophic Lateral Sclerosis? A Meta-Analysis. Front. Neurol. 2020, 11, 853. [Google Scholar] [CrossRef]
- Manzano, R.; Toivonen, J.M.; Moreno-Martínez, L.; de la Torre, M.; Moreno-García, L.; López-Royo, T.; Molina, N.; Zaragoza, P.; Calvo, A.C.; Osta, R. What skeletal muscle has to say in amyotrophic lateral sclerosis: Implications for therapy. Br. J. Pharmacol. 2021, 178, 1279–1297. [Google Scholar] [CrossRef]
- Zhao, R. Irisin at the crossroads of inter-organ communications: Challenge and implications. Front. Endocrinol. 2022, 13, 989135. [Google Scholar] [CrossRef] [PubMed]
- Lunetta, C.; Lizio, A.; Tremolizzo, L.; Ruscica, M.; Macchi, C.; Riva, N.; Weydt, P.; Corradi, E.; Magni, P.; Sansone, V. Serum irisin is upregulated in patients affected by amyotrophic lateral sclerosis and correlates with functional and metabolic status. J. Neurol. 2018, 265, 3001–3008. [Google Scholar] [CrossRef]
- Roddam, A.W.; Neale, R.; Appleby, P.; Allen, N.E.; Tipper, S.; Key, T.J. Association between Plasma 25-Hydroxyvitamin D Levels and Fracture Risk The EPIC-Oxford Study. Am. J. Epidemiol. 2007, 166, 1327–1336. [Google Scholar] [CrossRef]
- Karam, C.; Barrett, M.J.; Imperato, T.; MacGowan, D.J.; Scelsa, S. Vitamin D deficiency and its supplementation in patients with amyotrophic lateral sclerosis. J. Clin. Neurosci. 2013, 20, 1550–1553. [Google Scholar] [CrossRef]
- Farghali, M.; Ruga, S.; Morsanuto, V.; Uberti, F. Can Brain Health Be Supported by Vitamin D-Based Supplements? A Critical Review. Brain. Sci. 2020, 10, 660. [Google Scholar] [CrossRef]
- Wang, M.; Liu, Z.; Sun, W.; Yuan, Y.; Jiao, B.; Zhang, X.; Shen, L.; Jiang, H.; Xia, K.; Tang, B.; et al. Association Between Vitamins and Amyotrophic Lateral Sclerosis: A Center-Based Survey in Mainland China. Front. Neurol. 2020, 11, 488. [Google Scholar] [CrossRef]
- Suzuki, M.; Yoshioka, M.; Hashimoto, M.; Murakami, M.; Noya, M.; Takahashi, D.; Urashima, M. Randomised, double-blind, placebo-controlled trial of vitamin D supplementation in Parkinson disease. Am. J. Clin. Nutr. 2013, 97, 1004–1013. [Google Scholar] [CrossRef]
- Libonati, L.; Onesti, E.; Gori, M.C.; Ceccanti, M.; Cambieri, C.; Fabbri, A.; Inghilleri, M. Vitamin D in amyotrophic lateral sclerosis. Funct. Neurol. 2017, 32, 35–40. [Google Scholar] [CrossRef]
- Harvey, N.C.; Glüer, C.C.; Binkley, N.; McCloskey, E.V.; Brandi, M.L.; Cooper, C.; Kendler, D.; Lamy, O.; Laslop, A.; Camargos, B.M.; et al. Trabecular bone score (TBS) as a new complementary approach for osteoporosis evaluation in clinical practice. Bone 2015, 78, 216–224. [Google Scholar] [CrossRef]
- Hans, D.; Šteňová, E.; Lamy, O. The Trabecular Bone Score (TBS) Complements DXA and the FRAX as a Fracture Risk Assessment Tool in Routine Clinical Practice. Curr. Osteoporos. Rep. 2017, 15, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Iandolo, R.; Marini, F.; Semprini, M.; Laffranchi, M.; Mugnosso, M.; Cherif, A.; De Michieli, L.; Chiappalone, M.; Zenzeri, J. Perspectives and Challenges in Robotic Neurorehabilitation. Appl. Sci. 2019, 9, 3183. [Google Scholar] [CrossRef]
- Calabrò, R.S.; Cacciola, A.; Bertè, F.; Manuli, A.; Leo, A.; Bramanti, A.; Naro, A.; Milardi, D.; Bramanti, P. Robotic gait rehabilitation and substitution devices in neurological disorders: Where are we now? Neurol. Sci. 2016, 37, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Morioka, H.; Hirayama, T.; Sugisawa, T.; Murata, K.; Shibukawa, M.; Ebina, J.; Sawada, M.; Hanashiro, S.; Nagasawa, J.; Yanagihashi, M.; et al. Robot-assisted training using hybrid assistive limb ameliorates gait ability in patients with amyotrophic lateral sclerosis. J. Clin. Neurosci. 2022, 99, 158–163. [Google Scholar] [CrossRef]
- Portaro, S.; Cimino, V.; Accorinti, M.; Pidalà, A.; Naro, A.; Calabrò, R.S. A promising tool for flail arm in amyotrophic lateral sclerosis rehabilitation: A case report. Eur. J. Phys. Rehabil. Med. 2019, 55, 515–518. [Google Scholar] [CrossRef]
- Calabrò, R.S.; Portaro, S.; Manuli, A.; Leo, A.; Naro, A.; Bramanti, A. Rethinking the robotic rehabilitation pathway for people with amyotrophic lateral sclerosis: A need for clinical trials. Innov. Clin. Neurosci. 2019, 16, 11–12. [Google Scholar]
| Total (n = 38) | Male (n = 16) | Female (n = 22) | p-Value | |
|---|---|---|---|---|
| Age (years) | 61.82 ± 11.25 | 64.31 ± 12.91 | 60.00 ± 9.77 | 0.293 |
| Over 50 | 31 (81.58) | 14 (87.50) | 17 (77.27) | 0.705 |
| Menopausal | 19 (50.0) | - | 19 (86.4) | - |
| TBS | 1283.7 ± 151.3 | 1313.4 ± 176.6 | 1262.1 ± 130.0 | 0.487 |
| LS-BMD | 1.06 ± 0.21 | 1.09 ± 0.23 | 1.03 ± 0.19 | 0.544 |
| FN-BMD | 0.76 ± 0.18 | 0.80 ±0.18 | 0.73 ± 0.17 | 0.214 |
| FDM-TBS (n = 13) | PDM-TBS (n = 12) | NM-TBS (n = 13) | p-Value | |
|---|---|---|---|---|
| Age (years) | 59.46 ± 11.63 | 65.17 ± 10.05 | 61.08 ± 12.01 | 0.307 |
| Over 50 | 10 (76.92) | 10 (83.33) | 11 (84.61) | 0.864 |
| Female | 8 (61.54) | 8 (66.67) | 6 (46.15) | 0.553 |
| LS-BMD | 1.13 ± 0.22 | 1.03 ± 0.23 | 1.01 ± 0.82 | 0.241 |
| FN-BMD | 0.82 ± 0.21 | 0.74 ±0.16 | 0.71 ± 0.15 | 0.333 |
| Osteoporosis (n = 11) | Osteopenia (n = 11) | Normal (n = 16) | p-Value | |
|---|---|---|---|---|
| Age (years) | 62.54 ± 12.18 | 62.09 ± 13.49 | 61.12 ± 9.52 | 0.694 |
| Over 50 | 9 (81.82) | 8 (72.73) | 14 (87.50) | 0.623 |
| Female | 8 (72.73) | 6 (54.54) | 8 (50.00) | 0.484 |
| TBS | 1270.3 ± 160.7 | 1298.5 ± 166.7 | 1282.7 ± 142.9 | 0.163 |
| Osteoporosis (n = 18) | Osteopenia (n = 12) | Normal (n = 8) | p-Value | |
|---|---|---|---|---|
| Age (years) | 61.44 ± 11.73 | 61.67 ± 13.15 | 62.87 ± 7.83 | 0.536 |
| Over 50 | 14 (77.78) | 9 (75.00) | 8 (100.00) | 0.313 |
| Female | 10 (55.56) | 7 (58.33) | 5 (62.50) | 0.946 |
| TBS | 1268.8 ± 151.3 | 1353.7 ± 171.7 | 1329.2 ± 107.8 | 0.242 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morini, E.; Portaro, S.; Leonetti, D.; De Cola, M.C.; De Luca, R.; Bonanno, M.; Quartarone, A.; Calabrò, R.S. Bone Health Status in Individuals with Amyotrophic Lateral Sclerosis: A Cross-Sectional Study on the Role of the Trabecular Bone Score and Its Implications in Neurorehabilitation. Int. J. Environ. Res. Public Health 2023, 20, 2923. https://doi.org/10.3390/ijerph20042923
Morini E, Portaro S, Leonetti D, De Cola MC, De Luca R, Bonanno M, Quartarone A, Calabrò RS. Bone Health Status in Individuals with Amyotrophic Lateral Sclerosis: A Cross-Sectional Study on the Role of the Trabecular Bone Score and Its Implications in Neurorehabilitation. International Journal of Environmental Research and Public Health. 2023; 20(4):2923. https://doi.org/10.3390/ijerph20042923
Chicago/Turabian StyleMorini, Elisabetta, Simona Portaro, Danilo Leonetti, Maria Cristina De Cola, Rosaria De Luca, Mirjam Bonanno, Angelo Quartarone, and Rocco Salvatore Calabrò. 2023. "Bone Health Status in Individuals with Amyotrophic Lateral Sclerosis: A Cross-Sectional Study on the Role of the Trabecular Bone Score and Its Implications in Neurorehabilitation" International Journal of Environmental Research and Public Health 20, no. 4: 2923. https://doi.org/10.3390/ijerph20042923
APA StyleMorini, E., Portaro, S., Leonetti, D., De Cola, M. C., De Luca, R., Bonanno, M., Quartarone, A., & Calabrò, R. S. (2023). Bone Health Status in Individuals with Amyotrophic Lateral Sclerosis: A Cross-Sectional Study on the Role of the Trabecular Bone Score and Its Implications in Neurorehabilitation. International Journal of Environmental Research and Public Health, 20(4), 2923. https://doi.org/10.3390/ijerph20042923








