A Comparative Study of Inhibition Function between High-Intensity Interval Training and Moderate-Intensity Continuous Training in Healthy People: A Systematic Review with Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Selection Criteria
2.3. Data Extraction
2.4. Quality Assessment
2.5. Statistical Methods
3. Results
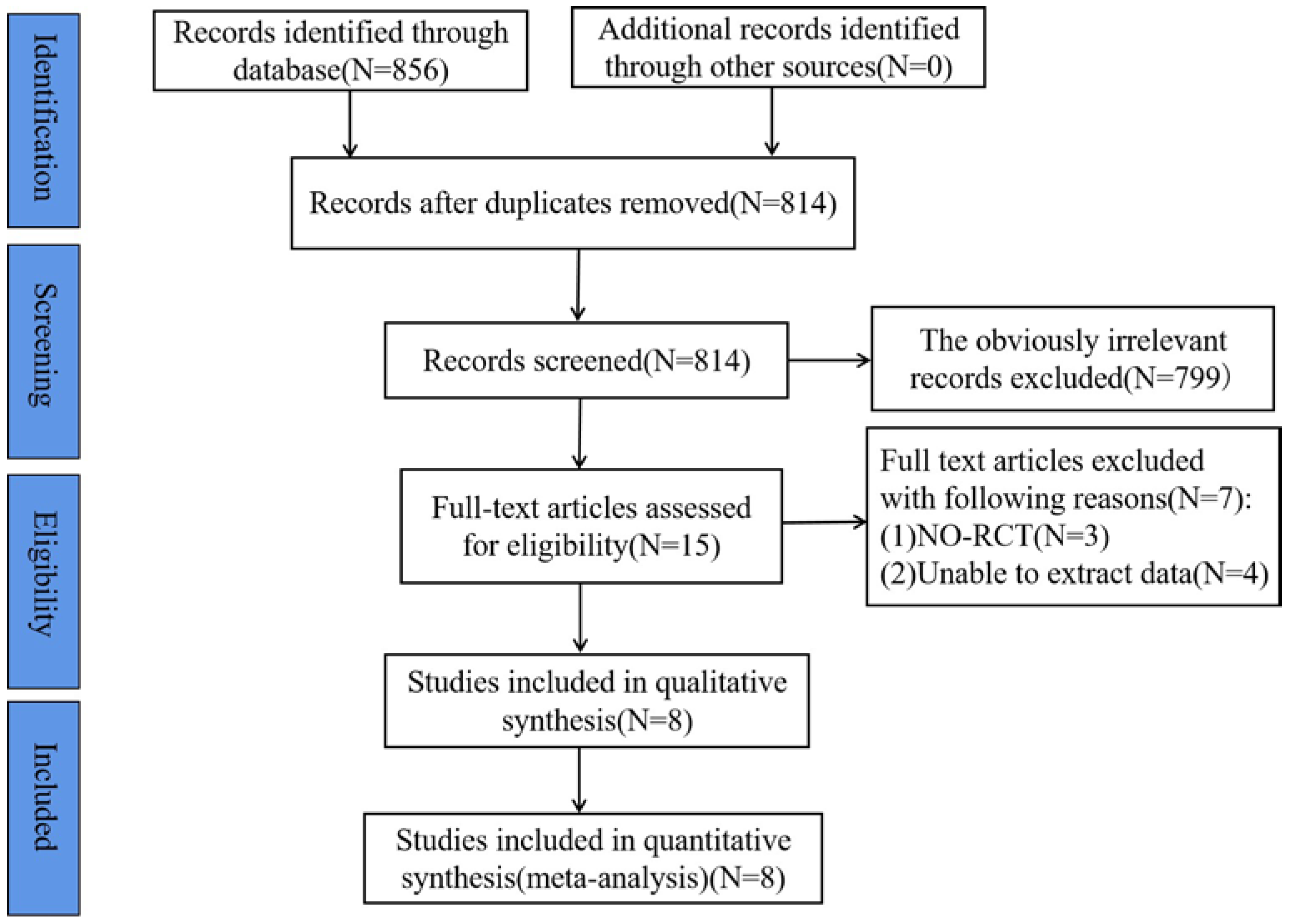
3.1. Results of the Literature Search
3.2. Risk of Bias Estimation
3.3. Research Characteristics
3.4. Results of Meta-Analysis
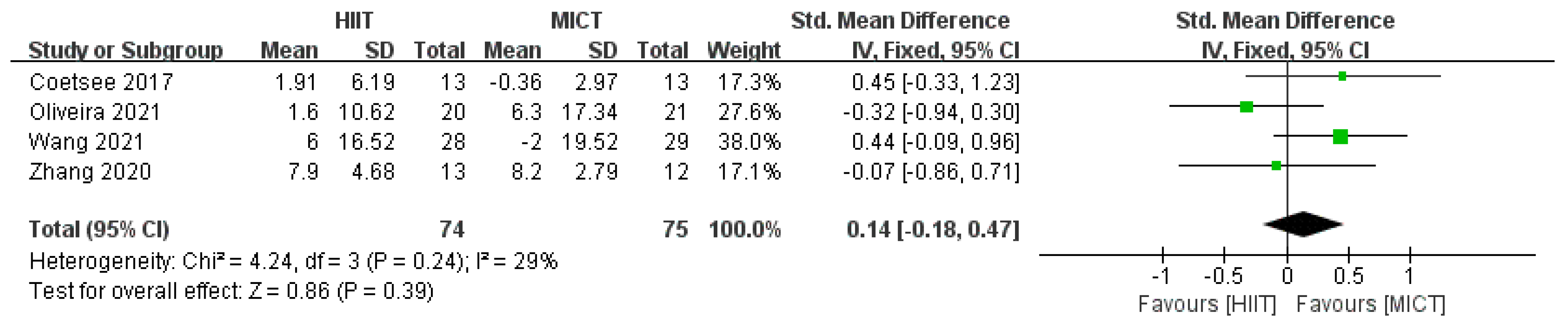
3.4.1. Correct Rate Results
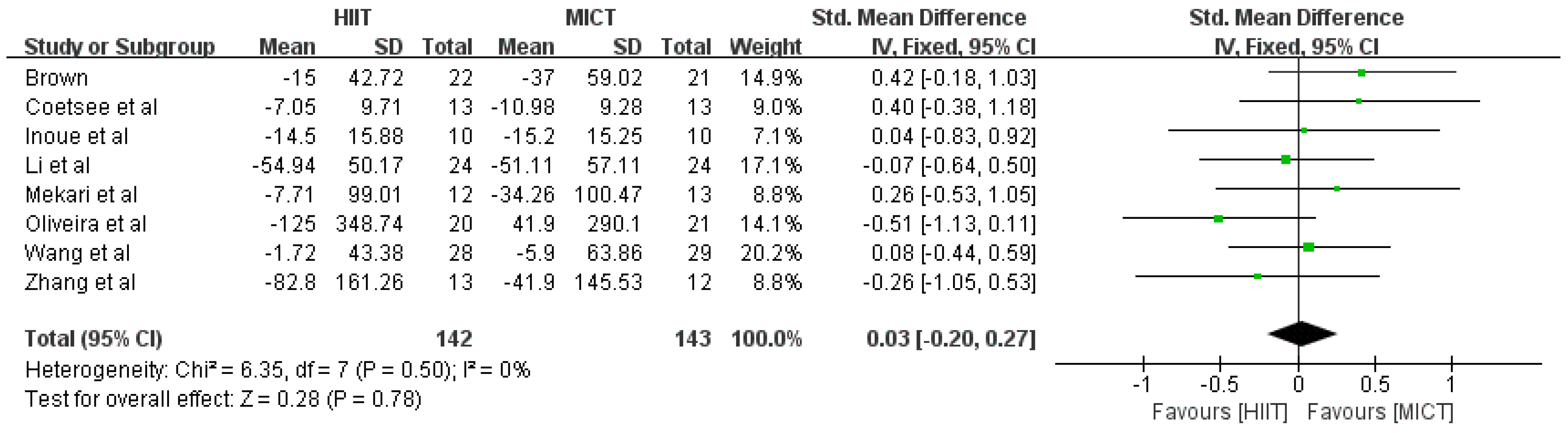
3.4.2. Response Time Results
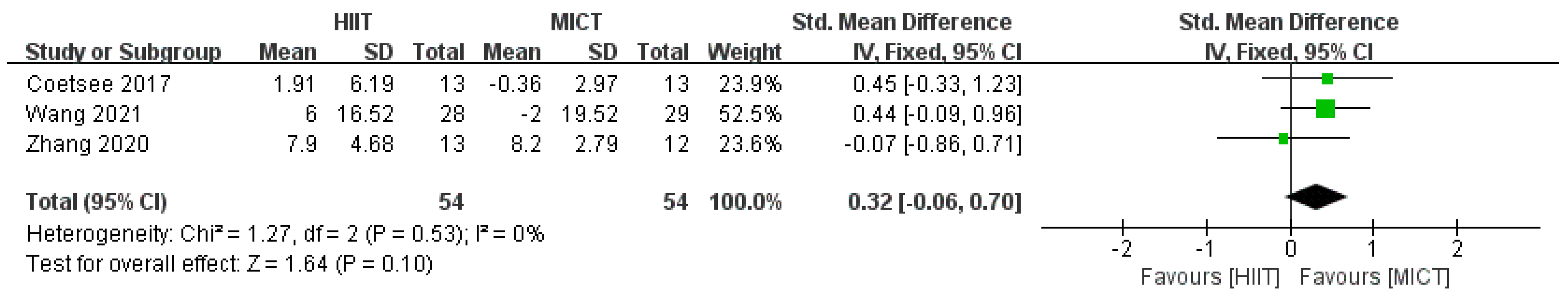
3.4.3. Results of Subgroup Analysis
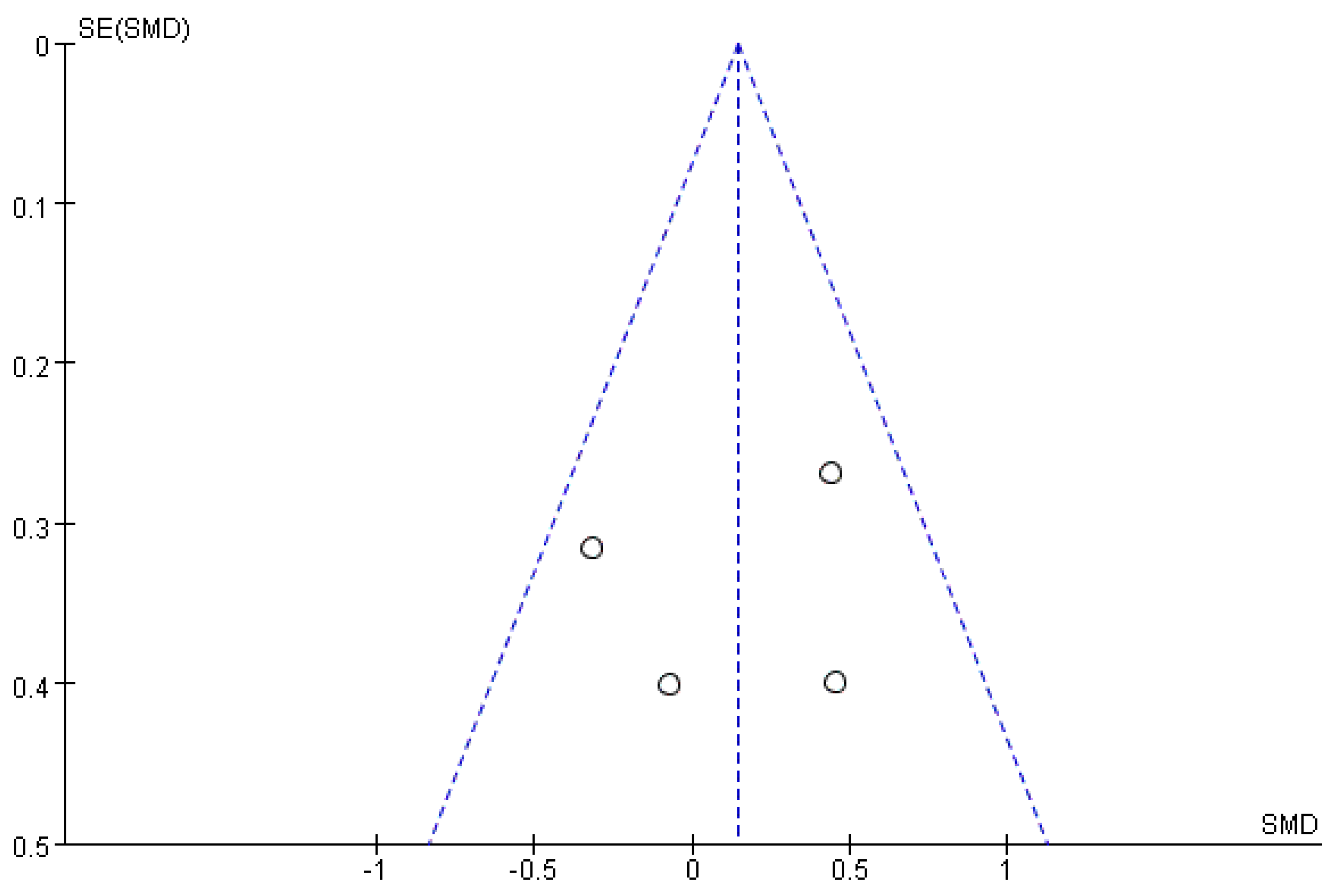
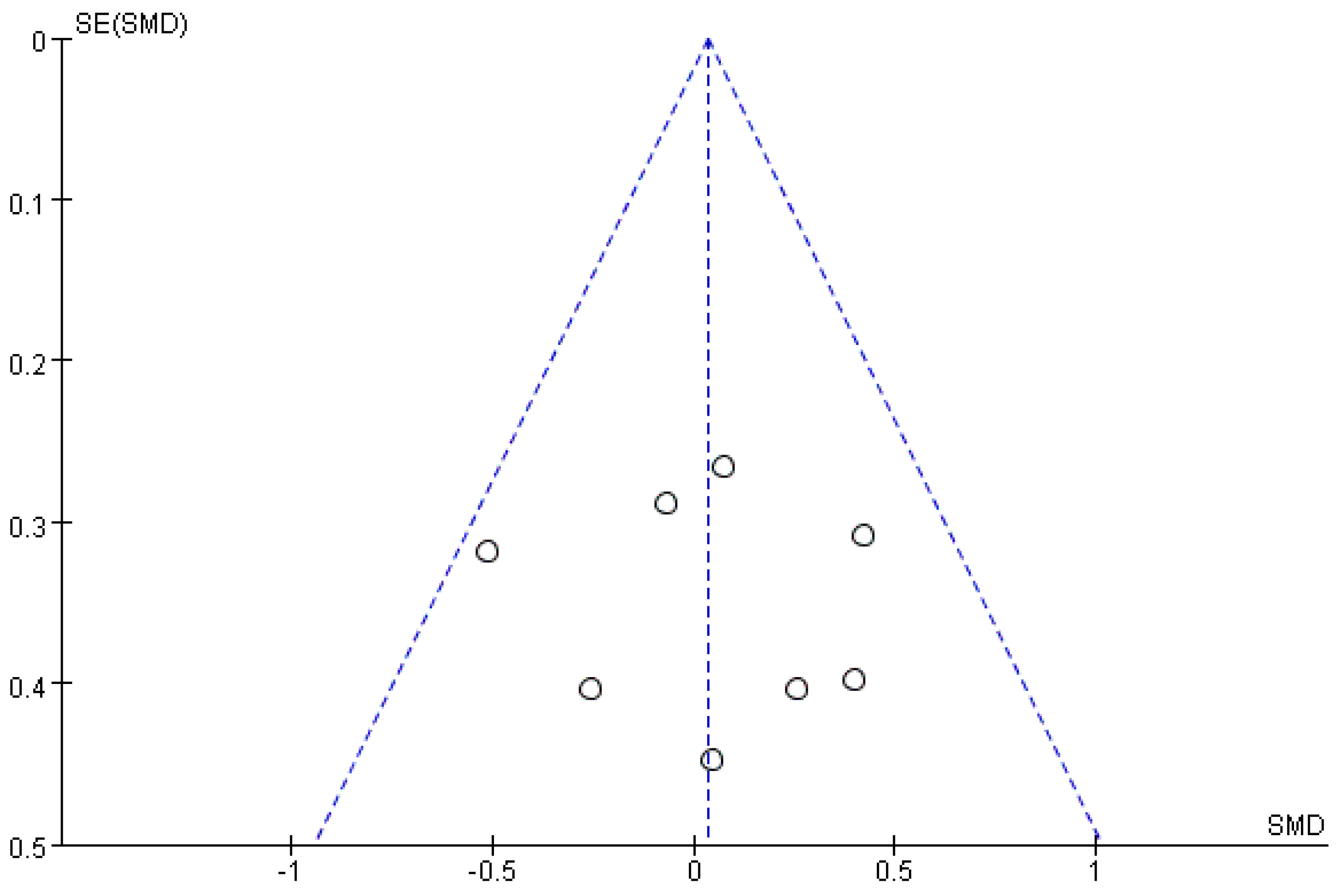
3.5. Publication Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hopfinger, J.B.; Slotnick, S.D. Attentional control and executive function. Cogn. Neurosci. 2020, 11, 1–4. [Google Scholar] [CrossRef]
- Penadés, R.; Franck, N.; González-Vallespí, L.; Dekerle, M. Neuroimaging studies of cognitive function in schizophrenia. Adv. Exp. Med. Biol. 2019, 1118, 117–134. [Google Scholar] [CrossRef]
- Christ, S.E.; Kester, L.E.; Bodner, K.E.; Miles, J.H. Evidence for selective inhibitory impairment in individuals with autism spectrum disorder. Neuropsychology 2011, 25, 690–701. [Google Scholar] [CrossRef]
- Bjorklund, D.F.; Harnishfegr, K.K. The evolution of inhibition mechanisms and their role in human cognition and behavior. In Interference and Inhibition in Cognition; Elsevier: Amsterdam, The Netherlands, 1995; pp. 141–173. [Google Scholar]
- Geronimi, E.M.C.; Arellano, B.; Woodruff-Borden, J. Relating mindfulness and executive function in children. Clin. Child Psychol. Psychiatry 2020, 25, 435–445. [Google Scholar] [CrossRef]
- Amodio, D.M.; Master, S.L.; Yee, C.M.; Taylor, S.E. Neurocognitive components of the behavioral inhibition and activation systems: Implications for theories of self-regulation. Psychophysiology 2008, 45, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Anna, B.; Thomsen, E.M. The acute effects of short bouts of exercise on inhibitory control in adolescents. Mental Health Phys. Act. 2018, 15, 17–21. [Google Scholar]
- Blakemore, S.J.; Robbins, T.W. Decision-making in the adolescent brain. Nat. Neurosci. 2012, 15, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.; Murphy, K.; Andrews, G. Cognitive and neural plasticity in old age: A systematic review of evidence from executive functions cognitive training. Ageing Res. Rev. 2019, 53, 100912. [Google Scholar] [CrossRef]
- Zhang, J. Experimental Research on Inhibitory Control among College Students with Different Types of Behavioral Inhibition; Liaoning Normal University: Dalian, China, 2012. [Google Scholar]
- Zeng, X.; Cai, L.; Wong, S.H.; Lai, L.; Lv, Y.; Tan, W.; Jing, J.; Chen, Y. Association of sedentary time and physical activity with executive function among children. Acad. Pediatr. 2021, 21, 63–69. [Google Scholar] [CrossRef]
- Runacres, A.; Mackintosh, K.A.; Knight, R.L.; Sheeran, L.; Thatcher, R.; Shelley, J.; McNarry, M.A. Impact of the COVID-19 pandemic on sedentary time and behaviour in children and adults: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2021, 18, 11286. [Google Scholar] [CrossRef]
- Cai, C.; Zhang, Y. Research progress on the mechanism of exercise improving brain executive function. J. Chengdu Sport Univ. 2019, 45, 120–126. [Google Scholar] [CrossRef]
- Jiang, D.; Zeng, C. The Effect of 8-weeks soccer exercise with medium intensity on executive function in preschool children. China Sport Sci. Technol. 2015, 51, 43–50. [Google Scholar] [CrossRef]
- Xiong, J.; Ye, M.; Wang, L.; Zheng, G. Effects of physical exercise on executive function in cognitively healthy older adults: A systematic review and meta-analysis of randomized controlled trials: Physical exercise for executive function. Int. J. Nurs. Stud. 2021, 114, 103810. [Google Scholar] [CrossRef] [PubMed]
- Verburgh, L.; Königs, M.; Scherder, E.J.; Oosterlaan, J. Physical exercise and executive functions in preadolescent children, adolescents and young adults: A meta-analysis. Br. J. Sports Med. 2014, 48, 973–979. [Google Scholar] [CrossRef]
- Li, Y. Effect of high-intensity interval training on different training populations. China Sport Sci. 2015, 35, 59–75+96. [Google Scholar] [CrossRef]
- Cao, M.; Quan, M.; Zhuang, J. Effect of high-intensity interval training versus moderate-intensity continuous training on cardiorespiratory fitness in children and adolescents: A meta-analysis. Int. J. Environ. Res. Public Health 2019, 16, 1533. [Google Scholar] [CrossRef]
- Alves, A.R.; Dias, R.; Neiva, H.P.; Marinho, D.A.; Marques, M.C.; Sousa, A.C.; Loureiro, V.; Loureiro, N. High-intensity interval training upon cognitive and psychological outcomes in youth: A systematic review. Int. J. Environ. Res. Public Health 2021, 18, 5344. [Google Scholar] [CrossRef]
- Smith, K.J.; Ainslie, P.N. Regulation of cerebral blood flow and metabolism during exercise. Exp. Physiol. 2017, 102, 1356–1371. [Google Scholar] [CrossRef]
- De Assis, G.G.; Gasanov, E.V.; de Sousa, M.B.C.; Kozacz, A.; Murawska-Cialowicz, E. Brain derived neutrophic factor, a link of aerobic metabolism to neuroplasticity. J. Physiol. Pharmacol. 2018, 69, 351–358. [Google Scholar] [CrossRef]
- Voelcker-Rehage, C.; Godde, B.; Staudinger, U.M. Cardiovascular and coordination training differentially improve cognitive performance and neural processing in older adults. Front. Hum. Neurosci. 2011, 5, 26. [Google Scholar] [CrossRef]
- Kao, S.C.D.E.; Ritondale, J.P. The acute effects of high-intensity interval training and moderate-intensity continuous exercise on declarative memory and inhibitory control. Psychol. Sport Exerc. 2018, 38, 90–99. [Google Scholar] [CrossRef]
- Coetsee, C.; Terblanche, E. The effect of three different exercise training modalities on cognitive and physical function in a healthy older population. Eur. Rev. Aging Phys. Act. 2017, 14, 13. [Google Scholar] [CrossRef]
- Zhang, Q.J. Effect of High-Intensity Interval Training on Brain Executive Function of Young Aged 12–14; Shandong Normal University: Jinan, China, 2020. [Google Scholar]
- Cumpston, M.S.; McKenzie, J.E.; Welch, V.A.; Brennan, S.E. Strengthening systematic reviews in public health: Guidance in the Cochrane Handbook for Systematic Reviews of Interventions, 2nd edition. J. Public Health 2022, 44, e588–e592. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Manchikanti, L.; Benyamin, R.M.; Helm, S.; Hirsch, J.A. Evidence-based medicine, systematic reviews, and guidelines in interventional pain management: Part 3: Systematic reviews and meta-analyses of randomized trials. Pain Physician 2009, 12, 35–72. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Leng, W.D. Theory and Practice of Systematic Review/Meta-Analysis; Military Medical Science Press: Beijing, China, 2013. [Google Scholar]
- Institute for Musculoskeletal Health, School of Public Health at the University of Sydney, Neuroscience Research Australia (NeuRA). Physiotherapy Evidence Database [EB/OL]. Available online: https://www.pedro.org.au/ (accessed on 22 May 2020).
- Tsai, C.L.; Chang, Y.C.; Pan, C.Y.; Wang, T.C.; Ukropec, J.; Ukropcová, B. Acute effects of different exercise intensities on executive function and oculomotor performance in middle-aged and older adults: Moderate-intensity continuous exercise vs. high-intensity interval exercise. Front. Aging Neurosci. 2021, 13, 743479. [Google Scholar] [CrossRef]
- Mekari, S.; Earle, M.; Martins, R.; Drisdelle, S.; Killen, M.; Bouffard-Levasseur, V.; Dupuy, O. Effect of high intensity interval training compared to continuous training on cognitive performance in young healthy adults: A pilot study. Brain Sci. 2020, 10, 81. [Google Scholar] [CrossRef]
- Inoue, D.S.; Monteiro, P.A.; Gerosa-Neto, J.; Santana, P.R.; Peres, F.P.; Edwards, K.M.; Lira, F.S. Acute increases in brain-derived neurotrophic factor following high or moderate-intensity exercise is accompanied with better cognition performance in obese adults. Sci. Rep. 2020, 10, 13493. [Google Scholar] [CrossRef]
- Wang, J. A Comparative Study of the Effects of High-Intensity Interval Training and Moderate-Intensity Continuous Training on Executive Function and Cardiorespiratory Fitness of 11–12 Years; Shanghai University of Sport: Shanghai, China, 2021. [Google Scholar]
- Li, Y. Effect of High-Intensity Intermittent Exercise on Executive Function of College Students; Tianjin University of Sport: Tianjin, China, 2021. [Google Scholar]
- Amorim Oliveira, G.T.; Elsangedy, H.M.; Pereira, D.C.; de Melo Silva, R.; Campos Faro, H.K.; Bortolotti, H.; Costa, E.C.; Fontes, E.B. Effects of 12 weeks of high-intensity interval, moderate-intensity continuous and self-selected intensity exercise training protocols on cognitive inhibitory control in overweight/obese adults: A randomized trial. Eur. J. Sport Sci. 2022, 22, 1724–1733. [Google Scholar] [CrossRef]
- Hu, J.; Cai, M.; Shang, Q. Research advances on high-intensity interval training and cognitive function. Acta Physiol. Sinica 2021, 73, 126–136. [Google Scholar] [CrossRef]
- Hsieh, S.S.; Chueh, T.Y.; Huang, C.J.; Kao, S.C.; Hillman, C.H.; Chang, Y.K.; Hung, T.M. Systematic review of the acute and chronic effects of high-intensity interval training on executive function across the lifespan. J. Sports Sci. 2021, 39, 10–22. [Google Scholar] [CrossRef]
- Alves, C.R.; Tessaro, V.H.; Teixeira, L.A.; Murakava, K.; Roschel, H.; Gualano, B.; Takito, M.Y. Influence of acute high-intensity aerobic interval exercise bout on selective attention and short-term memory tasks. Percept. Mot. Skills. 2014, 118, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Kemppainen, J.; Aalto, S.; Fujimoto, T.; Kalliokoski, K.K.; Långsjö, J.; Oikonen, V.; Rinne, J.; Nuutila, P.; Knuuti, J. High intensity exercise decreases global brain glucose uptake in humans. J. Physiol. 2005, 568, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Tsukamoto, H.; Ando, S.; Ogoh, S. Effect of exercise on brain health: The potential role of lactate as a myokine. Metabolites 2021, 11, 813. [Google Scholar] [CrossRef]
- Hwang, J.; Brothers, R.M.; Castelli, D.M.; Glowacki, E.M.; Chen, Y.T.; Salinas, M.M.; Kim, J.; Jung, Y.; Calvert, H.G. Acute high-intensity exercise-induced cognitive enhancement and brain-derived neurotrophic factor in young, healthy adults. Neurosci. Lett. 2016, 630, 247–253. [Google Scholar] [CrossRef]
- Haghighi, A.H.; Hajinia, M.; Askari, R.; Abbasian, S.; Goldfied, G. Effect of high-intensity interval training and high-intensity resistance training on irisin and fibroblast growth factor 21 in men with overweight and obesity. Can. J. Physiol. Pharmacol. 2022, 100, 937–944. [Google Scholar] [CrossRef]
- Herbert, P.; Hayes, L.D.; Sculthorpe, N.; Grace, F.M. High-intensity interval training (HIIT) increases insulin-like growth factor-I (IGF-I) in sedentary aging men but not masters’ athletes: An observational study. Aging Male 2017, 20, 54–59. [Google Scholar] [CrossRef]
- Pattij, T.; Janssen, M.C.; Vanderschuren, L.J.; Schoffelmeer, A.N.; van Gaalen, M.M. Involvement of dopamine D1 and D2 receptors in the nucleus accumbens core and shell in inhibitory response control. Psychopharmacology 2007, 191, 587–598. [Google Scholar] [CrossRef] [PubMed]
- McMorris, T.; Collard, K.; Corbett, J.; Dicks, M.; Swain, J.P. A test of the catecholamines hypothesis for an acute exercise-cognition interaction. Pharm. Biochem. Behav. 2008, 89, 106–115. [Google Scholar] [CrossRef]
- Yang, Y.; Wan, M.; Wan, X. Effect of high intensity interval exercise and moderate intensity continuous aerobic exercise on the executive function of college students. J. TUS 2021, 36, 733–738. [Google Scholar] [CrossRef]
- Cai, C.; Zhang, Y.; Zhu, Y.; Feng, S.; Liu, J. Study on the effects of intermittent exercise interventions on children’s executive function and its delayed benefits. J. Cap. Univ. Phys. Educ. Sports. 2021, 33, 541–548. [Google Scholar] [CrossRef]
- Tian, S.; Mou, H.; Qiu, F. Sustained effects of high-intensity interval exercise and moderate-intensity continuous exercise on inhibitory control. Int. J. Environ. Res. Public Health 2021, 18, 2687. [Google Scholar] [CrossRef] [PubMed]
- Lambrick, D.; Stoner, L.; Grigg, R.; Faulkner, J. Effects of continuous and intermittent exercise on executive function in children aged 8–10 years. Psychophysiology 2016, 53, 1335–1342. [Google Scholar] [CrossRef]
- Slusher, A.L.; Patterson, V.T.; Schwartz, C.S.; Acevedo, E.O. Impact of high intensity interval exercise on executive function and brain derived neurotrophic factor in healthy college aged males. Physiol. Behav. 2018, 191, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Telenius, E.W.; Engedal, K.; Bergland, A. Long-term effects of a 12 weeks high-intensity functional exercise program on physical function and mental health in nursing home residents with dementia: A single blinded randomized controlled trial. BMC Geriatr. 2015, 15, 158. [Google Scholar] [CrossRef]
- Ramos, J.S.; Dalleck, L.C.; Tjonna, A.E.; Beetham, K.S.; Coombes, J.S. The impact of high-intensity interval training versus moderate-intensity continuous training on vascular function: A systematic review and meta-analysis. Sports Med. 2015, 45, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G. Effect of High Intensity Interval Training on Physical Fitness and Executive Control Ability of Children; Shandong Normal University: Jinan, China, 2019. [Google Scholar]
- Fang, G.; Zhang, L.; Han, T.; Zou, X.; Zhang, H.; Li, X.; Wang, H.; Shen, Y. Effects of high intensity interval training on congnitive function in older adults. China Sport Sci. Technol. 2020, 56, 32–37. [Google Scholar] [CrossRef]
- Jung, M.E.; Bourne, J.E.; Little, J.P. Where does HIT fit? An examination of the affective response to high-intensity intervals in comparison to continuous moderate- and continuous vigorous-intensity exercise in the exercise intensity-affect continuum. PLoS ONE 2014, 9, e114541. [Google Scholar] [CrossRef]
- Luo, L.; Li, C.; Deng, Y.; Wang, Y.; Meng, P.; Wang, Q. High-intensity interval training on neuroplasticity, balance between brain-derived neurotrophic factor and precursor brain-derived neurotrophic factor in poststroke depression rats. J. Stroke Cereb. Dis. 2019, 28, 672–682. [Google Scholar] [CrossRef]
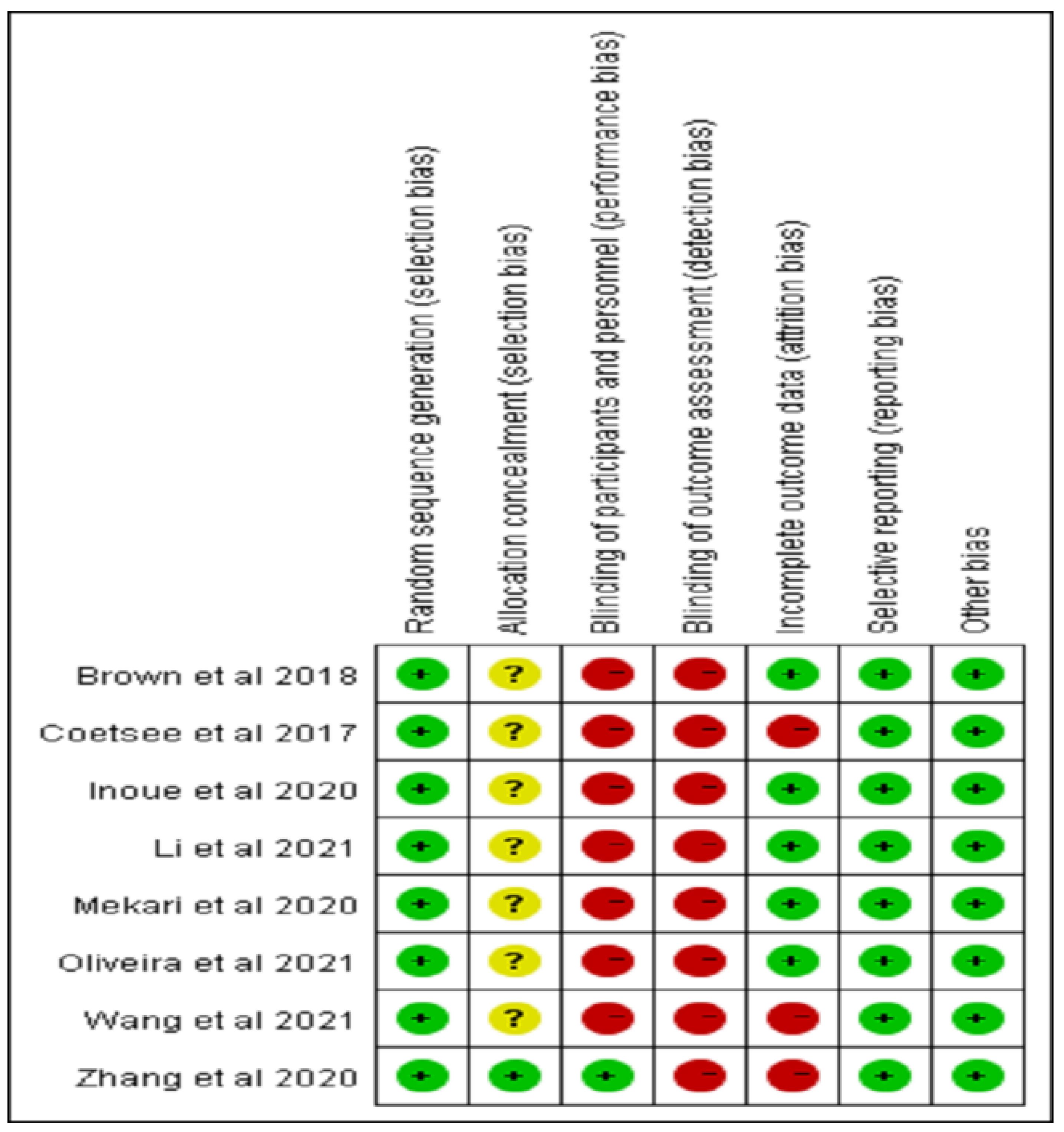
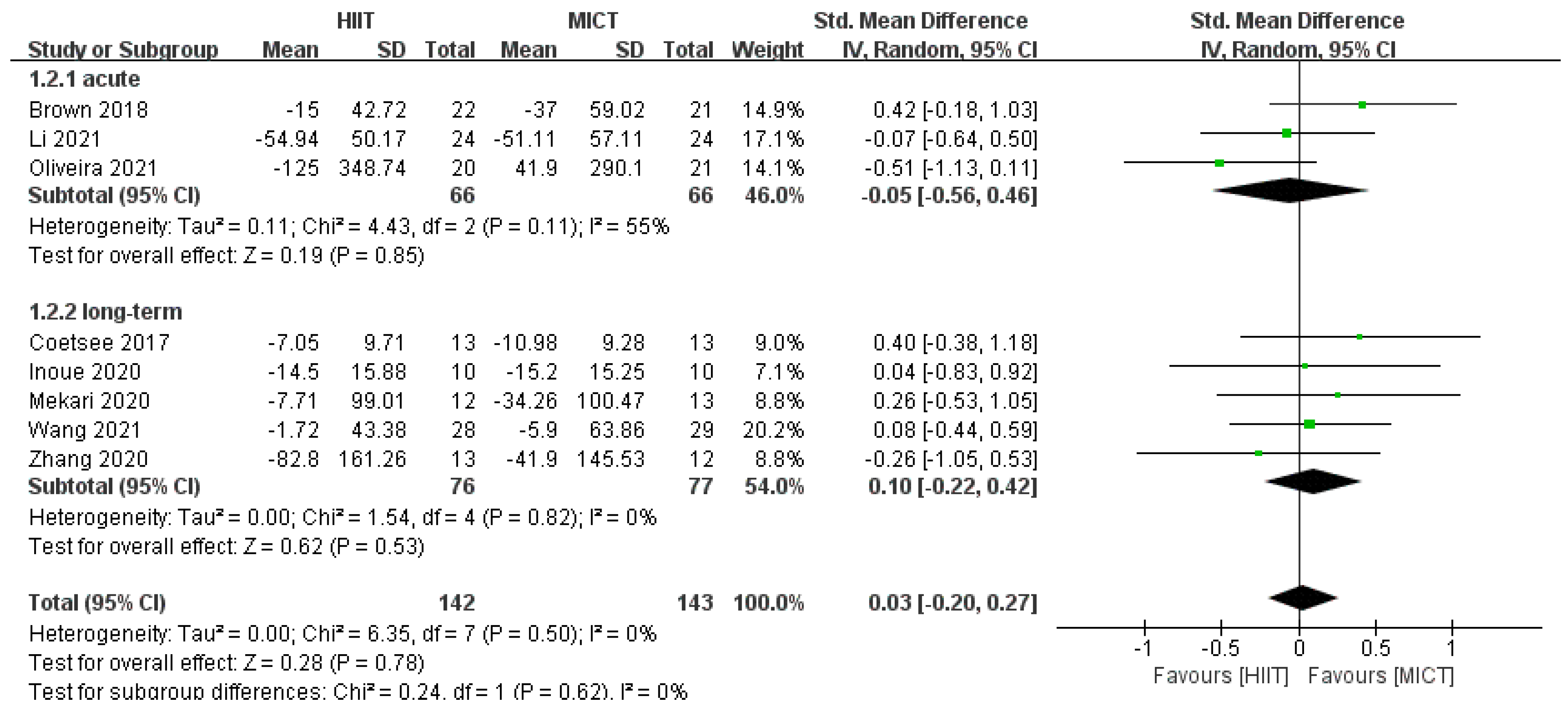
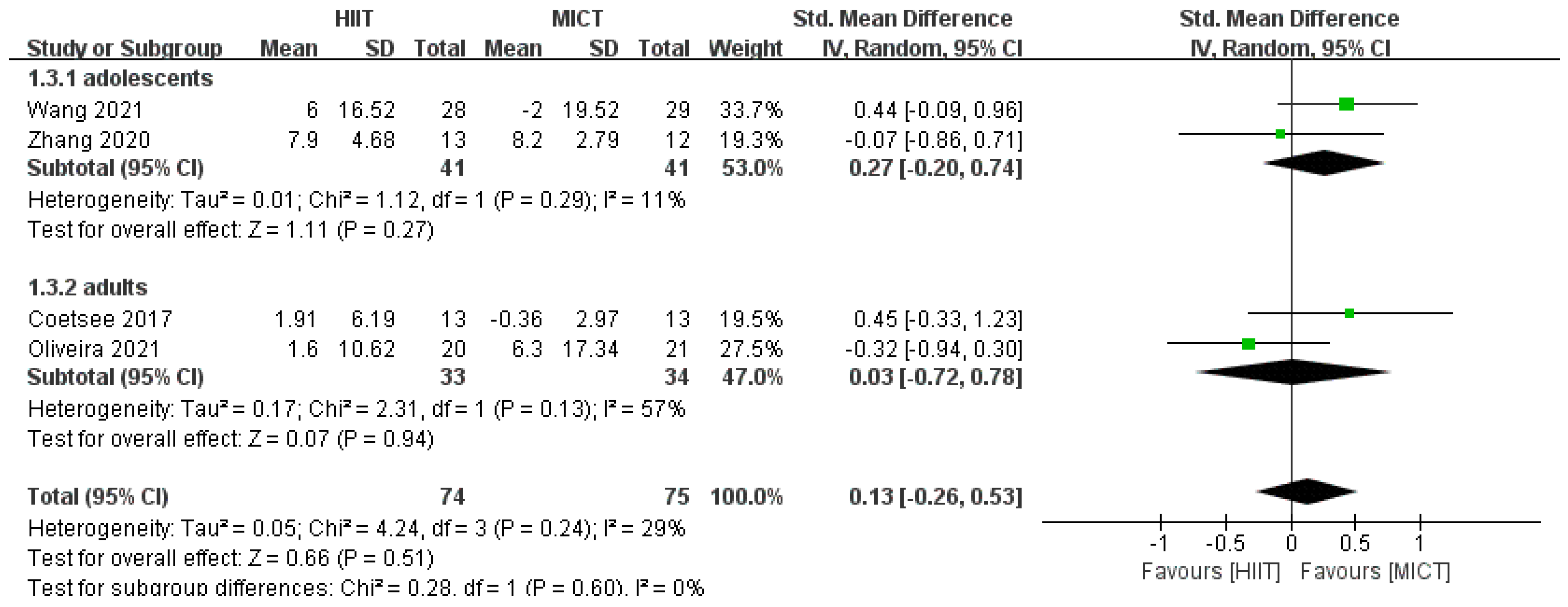
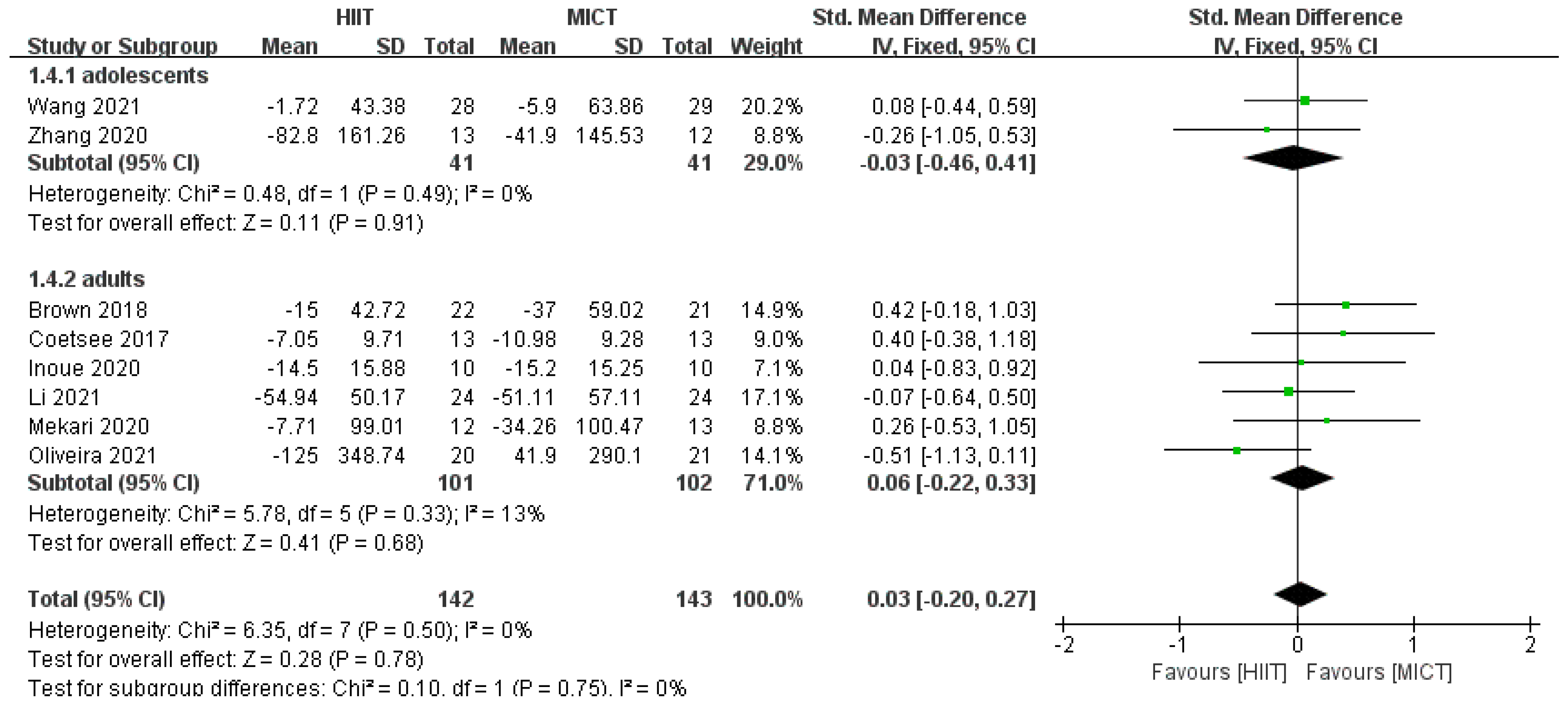
| Study | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Total Score | Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coetsee 2017 [24] | Yes | Yes | No | Yes | No | No | No | No | No | Yes | Yes | 4 | Medium |
| Brown 2018 [31] | Yes | Yes | No | Yes | No | No | No | Yes | No | Yes | Yes | 5 | Medium |
| Zhang 2020 [25] | Yes | Yes | Yes | Yes | Yes | No | No | No | No | Yes | Yes | 6 | High |
| Mekari 2020 [32] | Yes | Yes | No | Yes | No | No | No | Yes | No | Yes | Yes | 5 | Medium |
| Inoue 2020 [33] | Yes | Yes | No | Yes | No | No | No | Yes | No | Yes | Yes | 5 | Medium |
| Wang 2021 [34] | Yes | Yes | No | Yes | No | No | No | Yes | No | Yes | Yes | 5 | Medium |
| Li 2021 [35] | Yes | Yes | No | Yes | No | No | No | Yes | No | Yes | Yes | 5 | Medium |
| Oliveira 2021 [36] | Yes | Yes | No | Yes | No | No | No | Yes | No | Yes | Yes | 5 | Medium |
| Author | Year | Intervention Methods | Sample Size | F/M | Age | Intervention Programs | Intervention Frequency | Intervention Period | Ending Indicators |
|---|---|---|---|---|---|---|---|---|---|
| Coetsee [24] | 2017 | HIIT | 13 | 3/10 | 64.5 ± 6.3 | Warm-up; then 4 min intervals on the treadmill at an exercise intensity of 90–95% HRmax, with 3 min active recovery periods at an exercise intensity of 70% HRmax, lasting about 30 min; recovery. | 3 times/week | 16 weeks | Stroop b, a |
| MICT | 13 | 3/10 | 61.6 ± 5.8 | Warm up; then run continuously on the treadmill for 47 min at an exercise intensity of 70–75% HRmax; recovery. | |||||
| Brown [31] | 2018 | HIIT | 22 | - | 19.95 ± 1.96 | 1 min exercise intensity of 70% PPO power cycling (90–120 rpm), followed by 1 min exercise intensity of 12.5% PPO power cycling (60–90 rpm), for a total of 10 sets. | 1 time | Stroop b | |
| MICT | 21 | - | 20.14 ± 1.90 | Continuous power cycling exercise at a fixed exercise intensity of 41.25% PPO (60–90 rpm). | |||||
| Zhang [25] | 2020 | HIIT | 13 | 6/7 | 12.77 ± 0.44 | Warm-up 5 min; 1 min running (exercise intensity about 90% HRmax), 2 min rest between each group, interval to 50–60% HRmax exercise intensity slow walking, a total of 10 groups; relaxation for 5 min. | 3 times/week | 8 weeks | Stroop b, a |
| MICT | 12 | 5/7 | 12.73 ± 0.65 | Warm-up 5 min; 30 min of continuous aerobic running at 65% HRmax; relaxation for 5 min. | |||||
| Mekari [32] | 2020 | HIIT | 12 | 9/3 | 29 ± 10.3 | Warm-up 5 min; power bike exercise at 100% PPO for 20 min, then rest for five minutes and perform two sets; 55 min total. | 3 times/week | 6 weeks | Stroop b |
| MICT | 13 | 9/4 | 35 ± 7.4 | Warm-up 5 min; power cycling at 60% PPO for 35 min with a final rest of five minutes; total 45 min. | |||||
| Inoue [33] | 2020 | HIIT | 10 | - | 30 ± 5.4 | Warm-up 5 min; 10 interval runs on the treadmill (10 × 1:1-1 min at 100% MAV, interspersed with 1 min of passive recovery). | 3 times/week | 6 weeks | Stroop b |
| MICT | 10 | - | 30 ± 5.4 | Warm-up 5 min; continuous running on the treadmill at 65% MAV for about 35 min. | |||||
| Wang [34] | 2021 | HIIT | 28 | - | 11–12 | Warm-up 3 min; basic stage 10 m fast folding run, open and closed jump, upgrade stage 10 m fast folding run, Bobbi jump, exercise intensity 85–90% HRmax; recovery 2 min. | 3 times/week | 8 weeks | Flanker b, a |
| MICT | 29 | - | 11–12 | Warm-up 3 min; basic stage before and after the swing arm alternating jump, unarmed deep squat, upgrade stage prone knee lift, prone open jump, exercise intensity 60–69% HRmax; recovery 2 min. | |||||
| Li [35] | 2021 | HIIT | 24 | 10/14 | 23.79 ± 1.82 | Warm-up 5 min; 20 min of exercise in 1:1 interval mode (85% HRmax or higher intensity running exercise for 1 min with 1 min interval). | 1 time | Stroop b | |
| MICT | 24 | 12/12 | 23.13 ± 1.96 | Warm-up 5 min; run for 20 min at 60–70% HRmax exercise intensity. | |||||
| Oliveira [36] | 2021 | HIIT | 20 | 13/7 | 29.7 ± 8.3 | Warm-up 5 min; then RPE was kept at 15–17 with 10 intervals on the treadmill (10 × 1, 1 min intervals with slow walking); recovery 5 min. | 1 time | Stroop b, a | |
| MICT | 21 | 10/11 | 33.2 ± 6.6 | Warm-up 5 min; then RPE held at 13, 30 min on treadmill; recovery 5 min. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Q.; Niu, X.; Zhang, Y.; Song, J.; Chi, A. A Comparative Study of Inhibition Function between High-Intensity Interval Training and Moderate-Intensity Continuous Training in Healthy People: A Systematic Review with Meta-Analysis. Int. J. Environ. Res. Public Health 2023, 20, 2859. https://doi.org/10.3390/ijerph20042859
Wu Q, Niu X, Zhang Y, Song J, Chi A. A Comparative Study of Inhibition Function between High-Intensity Interval Training and Moderate-Intensity Continuous Training in Healthy People: A Systematic Review with Meta-Analysis. International Journal of Environmental Research and Public Health. 2023; 20(4):2859. https://doi.org/10.3390/ijerph20042859
Chicago/Turabian StyleWu, Qianqian, Xiaodan Niu, Yan Zhang, Jing Song, and Aiping Chi. 2023. "A Comparative Study of Inhibition Function between High-Intensity Interval Training and Moderate-Intensity Continuous Training in Healthy People: A Systematic Review with Meta-Analysis" International Journal of Environmental Research and Public Health 20, no. 4: 2859. https://doi.org/10.3390/ijerph20042859
APA StyleWu, Q., Niu, X., Zhang, Y., Song, J., & Chi, A. (2023). A Comparative Study of Inhibition Function between High-Intensity Interval Training and Moderate-Intensity Continuous Training in Healthy People: A Systematic Review with Meta-Analysis. International Journal of Environmental Research and Public Health, 20(4), 2859. https://doi.org/10.3390/ijerph20042859