Soluble ST2 as a New Oxidative Stress and Inflammation Marker in Metabolic Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Echochardiography
2.3. Enzyme-Linked Immunosorbent Assay (ELISA)
2.4. Statistical Analysis
3. Results
3.1. Clinical Characteristics
3.2. sST2, Oxidative Stress and Inflammatory Markers in MS Patients
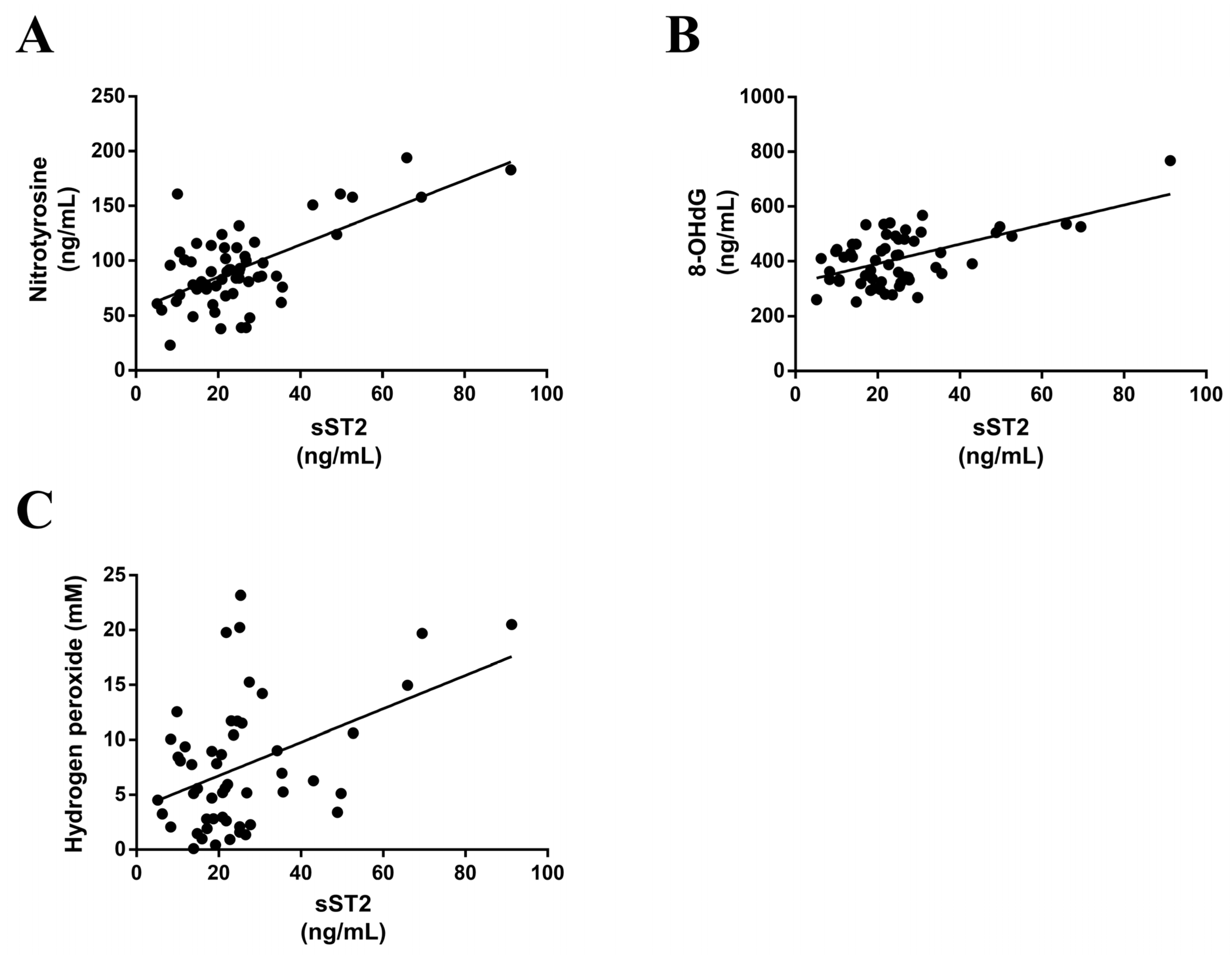
3.3. sST2 Positively Correlates with Oxidative Stress Markers in MS Patients
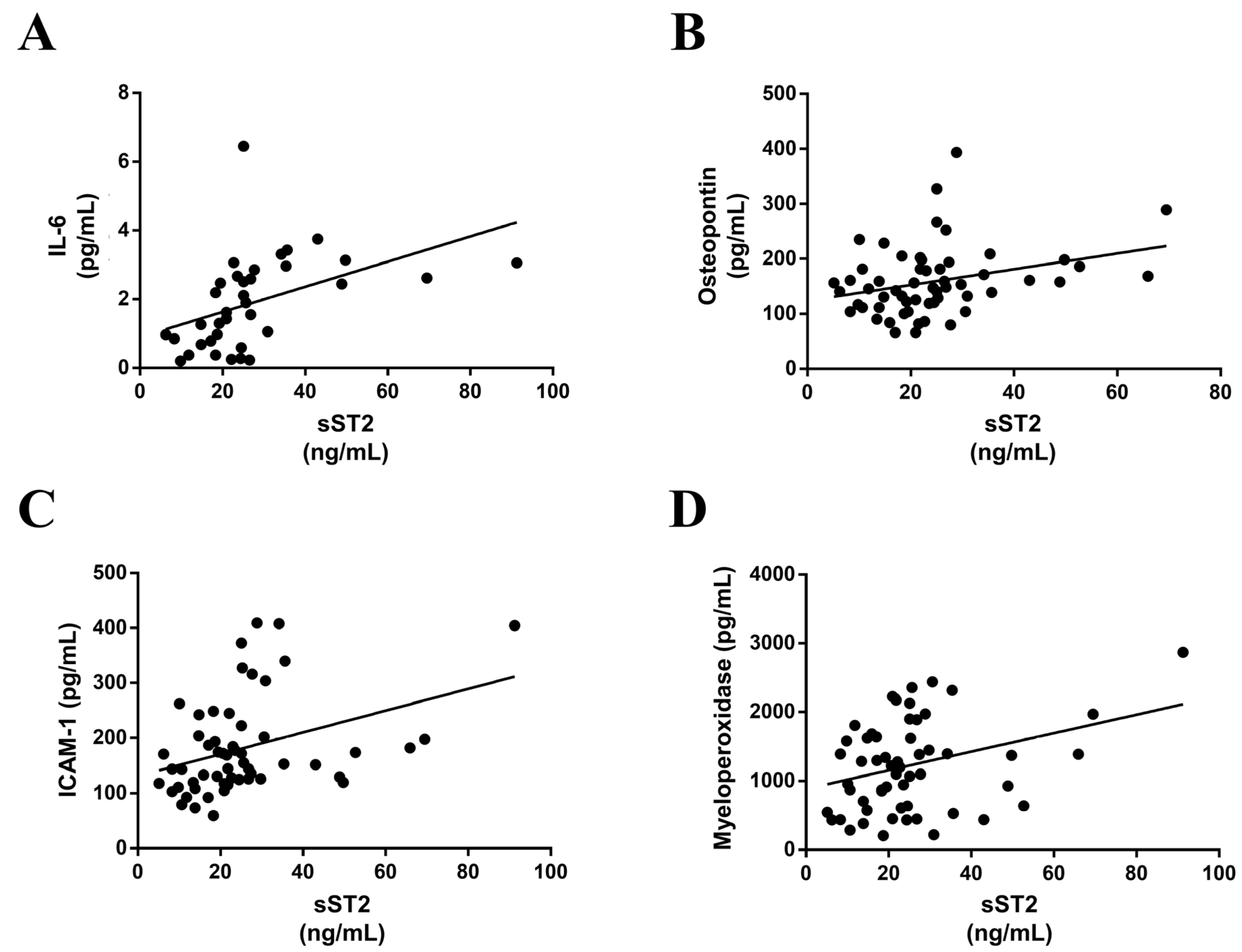
3.4. sST2 is Associated with Inflammatory Markers in MS Patients
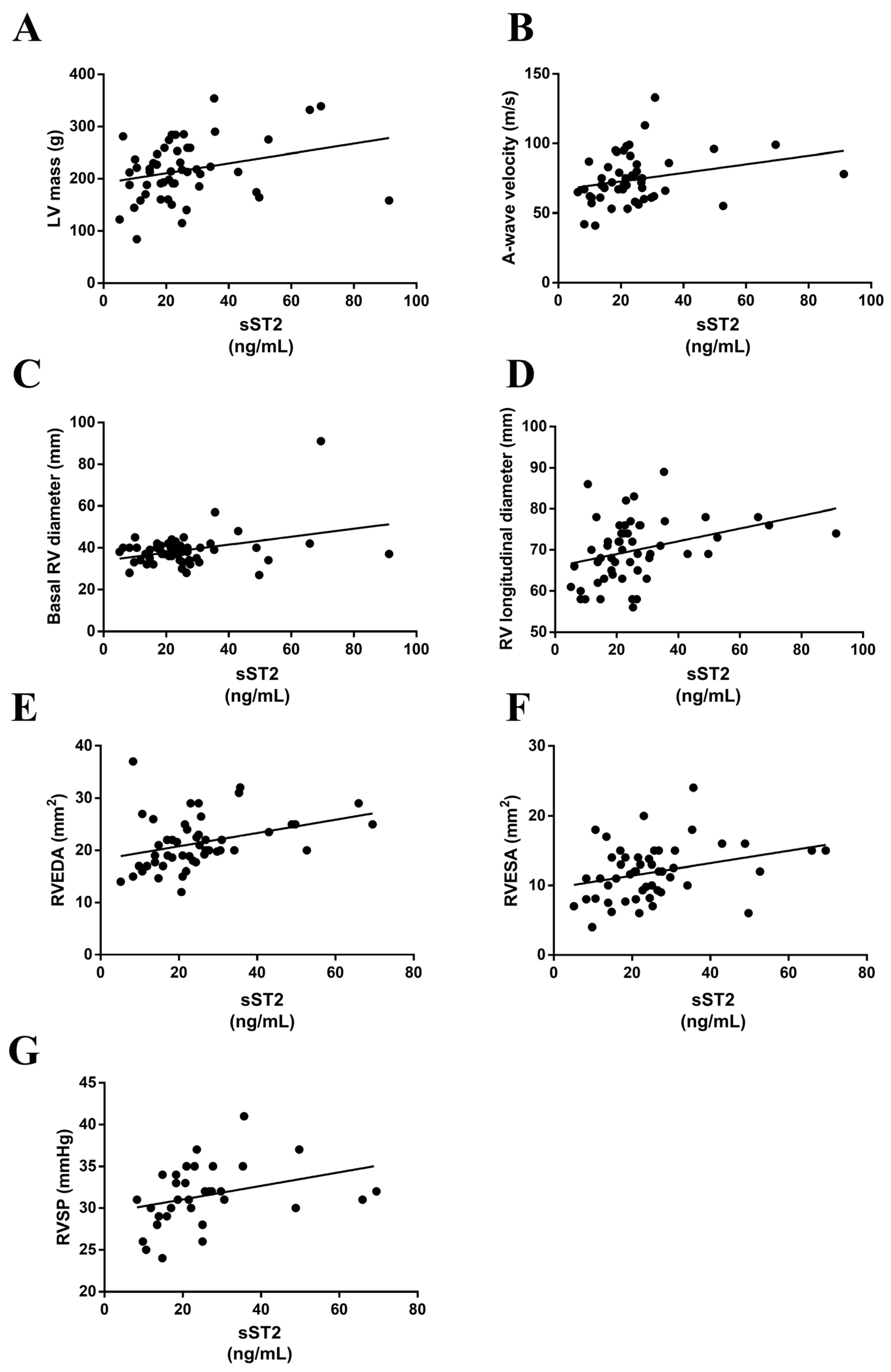
3.5. sST2 Positively Associated with Echocardiographic Parameters in MS Patients
4. Discussion
Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nolan, P.B.; Carrick-Ranson, G.; Stinear, J.W.; Reading, S.A.; Dalleck, L.C. Prevalence of metabolic syndrome and metabolic syndrome components in young adults: A pooled analysis. Prev. Med. Rep. 2017, 7, 211–215. [Google Scholar] [CrossRef]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- James, A.M.; Collins, Y.; Logan, A.; Murphy, M.P. Mitochondrial oxidative stress and the metabolic syndrome. Trends Endocrinol. Metab. 2012, 23, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.S.; Yi, Q.; Gerstein, H.; Lonn, E.; Jacobs, R.; Vuksan, V.; Teo, K.; Davis, B.; Montague, P.; Yusuf, S.; et al. Relationship of metabolic syndrome and fibrinolytic dysfunction to cardiovascular disease. Circulation 2003, 108, 420–425. [Google Scholar] [CrossRef]
- Esser, N.; Legrand-Poels, S.; Piette, J.; Scheen, A.J.; Paquot, N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 2014, 105, 141–150. [Google Scholar] [CrossRef]
- Spahis, S.; Borys, J.M.; Levy, E. Metabolic Syndrome as a Multifaceted Risk Factor for Oxidative Stress. Antioxid. Redox Signal. 2017, 26, 445–461. [Google Scholar] [CrossRef]
- Roberts, C.K.; Sindhu, K.K. Oxidative stress and metabolic syndrome. Life Sci. 2009, 84, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Marseglia, L.; Manti, S.; D’Angelo, G.; Nicotera, A.; Parisi, E.; Di Rosa, G.; Gitto, E.; Arrigo, T. Oxidative stress in obesity: A critical component in human diseases. Int. J. Mol. Sci. 2014, 16, 378–400. [Google Scholar] [CrossRef]
- Halliwell, B. The wanderings of a free radical. Free Radic. Biol. Med. 2009, 46, 531–542. [Google Scholar] [CrossRef]
- Tran, V.; De Silva, T.M.; Sobey, C.G.; Lim, K.; Drummond, G.R.; Vinh, A.; Jelinic, M. The Vascular Consequences of Metabolic Syndrome: Rodent Models, Endothelial Dysfunction, and Current Therapies. Front. Pharmacol. 2020, 11, 148. [Google Scholar] [CrossRef]
- Chen, S.J.; Yen, C.H.; Huang, Y.C.; Lee, B.J.; Hsia, S.; Lin, P.T. Relationships between inflammation, adiponectin, and oxidative stress in metabolic syndrome. PLoS ONE 2012, 7, e45693. [Google Scholar] [CrossRef] [PubMed]
- Matilla, L.; Ibarrola, J.; Arrieta, V.; Garcia-Pena, A.; Martinez-Martinez, E.; Sadaba, R.; Alvarez, V.; Navarro, A.; Fernandez-Celis, A.; Gainza, A.; et al. Soluble ST2 promotes oxidative stress and inflammation in cardiac fibroblasts: An in vitro and in vivo study in aortic stenosis. Clin. Sci. 2019, 133, 1537–1548. [Google Scholar] [CrossRef]
- Ojji, D.B.; Opie, L.H.; Lecour, S.; Lacerda, L.; Adeyemi, O.M.; Sliwa, K. The effect of left ventricular remodelling on soluble ST2 in a cohort of hypertensive subjects. J. Hum. Hypertens. 2014, 28, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Zeyda, M.; Wernly, B.; Demyanets, S.; Kaun, C.; Hammerle, M.; Hantusch, B.; Schranz, M.; Neuhofer, A.; Itariu, B.K.; Keck, M.; et al. Severe obesity increases adipose tissue expression of interleukin-33 and its receptor ST2, both predominantly detectable in endothelial cells of human adipose tissue. Int. J. Obes. 2013, 37, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Samuelsson, M.; Dereke, J.; Svensson, M.K.; Landin-Olsson, M.; Hillman, M.; on the behalf of the, D.S.g. Soluble plasma proteins ST2 and CD163 as early biomarkers of nephropathy in Swedish patients with diabetes, 15-34 years of age: A prospective cohort study. Diabetol. Metab. Syndr. 2017, 9, 41. [Google Scholar] [CrossRef]
- Daniels, L.B.; Bayes-Genis, A. Using ST2 in cardiovascular patients: A review. Future Cardiol. 2014, 10, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Celic, V.; Majstorovic, A.; Pencic-Popovic, B.; Sljivic, A.; Lopez-Andres, N.; Roy, I.; Escribano, E.; Beunza, M.; Melero, A.; Floridi, F.; et al. Soluble ST2 Levels and Left Ventricular Structure and Function in Patients With Metabolic Syndrome. Ann. Lab. Med. 2016, 36, 542–549. [Google Scholar] [CrossRef]
- Alberti, K.G.; Zimmet, P.; Shaw, J. Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef]
- Lang, R.M.; Bierig, M.; Devereux, R.B.; Flachskampf, F.A.; Foster, E.; Pellikka, P.A.; Picard, M.H.; Roman, M.J.; Seward, J.; Shanewise, J.; et al. Recommendations for chamber quantification. Eur. J. Echocardiogr. 2006, 7, 79–108. [Google Scholar] [CrossRef]
- Galderisi, M.; Henein, M.Y.; D’Hooge, J.; Sicari, R.; Badano, L.P.; Zamorano, J.L.; Roelandt, J.R.; European Association of, E. Recommendations of the European Association of Echocardiography: How to use echo-Doppler in clinical trials: Different modalities for different purposes. Eur. J. Echocardiogr. 2011, 12, 339–353. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Appleton, C.P.; Gillebert, T.C.; Marino, P.N.; Oh, J.K.; Smiseth, O.A.; Waggoner, A.D.; Flachskampf, F.A.; Pellikka, P.A.; Evangelisa, A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur. J. Echocardiogr. 2009, 10, 165–193. [Google Scholar] [CrossRef] [PubMed]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Tsang, W.; Adams, D.H.; Agricola, E.; Buck, T.; Faletra, F.F.; Franke, A.; Hung, J.; de Isla, L.P.; et al. EAE/ASE recommendations for image acquisition and display using three-dimensional echocardiography. J. Am. Soc. Echocardiogr. 2012, 25, 3–46. [Google Scholar] [CrossRef] [PubMed]
- Galderisi, M.; Nistri, S.; Mondillo, S.; Losi, M.A.; Innelli, P.; Mele, D.; Muraru, D.; D’Andrea, A.; Ballo, P.; Sgalambro, A.; et al. Methodological approach for the assessment of ultrasound reproducibility of cardiac structure and function: A proposal of the study group of Echocardiography of the Italian Society of Cardiology (Ultra Cardia SIC) part I. Cardiovasc. Ultrasound 2011, 9, 26. [Google Scholar] [CrossRef]
- Badano, L.P.; Boccalini, F.; Muraru, D.; Bianco, L.D.; Peluso, D.; Bellu, R.; Zoppellaro, G.; Iliceto, S. Current clinical applications of transthoracic three-dimensional echocardiography. J. Cardiovasc. Ultrasound 2012, 20, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Marsland, A.L.; McCaffery, J.M.; Muldoon, M.F.; Manuck, S.B. Systemic inflammation and the metabolic syndrome among middle-aged community volunteers. Metabolism 2010, 59, 1801–1808. [Google Scholar] [CrossRef]
- Marino, L.; Romano, G.P.; Santulli, M.; Bertazzoni, G.; Suppa, M. Soluble sST2 biomarker analysis for fibrosis development in atrial fibrillation. A case control study. Clin. Ter. 2021, 172, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Jialal, I.; Devaraj, S.; Adams-Huet, B.; Chen, X.; Kaur, H. Increased cellular and circulating biomarkers of oxidative stress in nascent metabolic syndrome. J. Clin. Endocrinol. Metab. 2012, 97, E1844–E1850. [Google Scholar] [CrossRef]
- Abd El Aziz, R.; Fawzy, M.W.; Khalil, N.; Abdel Atty, S.; Sabra, Z. Vascular affection in relation to oxidative DNA damage in metabolic syndrome. Ther. Adv. Endocrinol. Metab. 2018, 9, 43–51. [Google Scholar] [CrossRef]
- da Fonseca, L.J.; Nunes-Souza, V.; Guedes Gda, S.; Schettino-Silva, G.; Mota-Gomes, M.A.; Rabelo, L.A. Oxidative status imbalance in patients with metabolic syndrome: Role of the myeloperoxidase/hydrogen peroxide axis. Oxid. Med. Cell. Longev. 2014, 2014, 898501. [Google Scholar] [CrossRef]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Litvinova, L.; Atochin, D.N.; Fattakhov, N.; Vasilenko, M.; Zatolokin, P.; Kirienkova, E. Nitric oxide and mitochondria in metabolic syndrome. Front. Physiol. 2015, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Hopps, E.; Caimi, G. Matrix metalloproteinases in metabolic syndrome. Eur. J. Intern. Med. 2012, 23, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.F.; Xie, S.L.; Chen, Y.X.; Mai, J.T.; Wang, J.F.; Zhu, W.L.; Zhu, L.G. Altered serum levels of IL-33 in patients with advanced systolic chronic heart failure: Correlation with oxidative stress. J. Transl. Med. 2012, 10, 120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J. Biomarkers of endothelial activation and dysfunction in cardiovascular diseases. Rev. Cardiovasc. Med. 2022, 23, 73. [Google Scholar] [CrossRef] [PubMed]
- Gimbrone, M.A., Jr.; Garcia-Cardena, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef]
- Ridker, P.M.; Kastelein, J.J.; Genest, J.; Koenig, W. C-reactive protein and cholesterol are equally strong predictors of cardiovascular risk and both are important for quality clinical care. Eur. Heart J. 2013, 34, 1258–1261. [Google Scholar] [CrossRef]
- Sarbijani, H.M.; Khoshnia, M.; Marjani, A. The association between Metabolic Syndrome and serum levels of lipid peroxidation and interleukin-6 in Gorgan. Diabetes Metab. Syndr. 2016, 10, S86–S89. [Google Scholar] [CrossRef]
- van de Stolpe, A.; van der Saag, P.T. Intercellular adhesion molecule-1. J. Mol. Med. 1996, 74, 13–33. [Google Scholar] [CrossRef]
- Esposito, K.; Giugliano, D. The metabolic syndrome and inflammation: Association or causation? Nutr. Metab. Cardiovasc. Dis. 2004, 14, 228–232. [Google Scholar] [CrossRef]
- Pascual-Figal, D.A.; Januzzi, J.L. The biology of ST2: The International ST2 Consensus Panel. Am. J. Cardiol. 2015, 115, 3B–7B. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Martinez, E.; Lopez-Andres, N.; Jurado-Lopez, R.; Rousseau, E.; Bartolome, M.V.; Fernandez-Celis, A.; Rossignol, P.; Islas, F.; Antequera, A.; Prieto, S.; et al. Galectin-3 Participates in Cardiovascular Remodeling Associated With Obesity. Hypertension 2015, 66, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Chang, B.; Huang, A.; Hao, S.; Gao, M.; Sun, Y.; Shi, M.; Jin, L.; Zhang, W.; Zhao, J.; et al. Plasma levels of soluble ST2, but not IL-33, correlate with the severity of alcoholic liver disease. J. Cell. Mol. Med. 2019, 23, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Lin, A.; Yu, Y.; Zhang, L.; Yang, G.; Hu, H.; Luo, Y. Serum Soluble ST2 as a Novel Inflammatory Marker in Acute Ischemic Stroke. Clin. Lab. 2018, 64, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Walzl, G.; Matthews, S.; Kendall, S.; Gutierrez-Ramos, J.C.; Coyle, A.J.; Openshaw, P.J.; Hussell, T. Inhibition of T1/ST2 during respiratory syncytial virus infection prevents T helper cell type 2 (Th2)- but not Th1-driven immunopathology. J. Exp. Med. 2001, 193, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Coyle, A.J.; Lloyd, C.; Tian, J.; Nguyen, T.; Erikkson, C.; Wang, L.; Ottoson, P.; Persson, P.; Delaney, T.; Lehar, S.; et al. Crucial role of the interleukin 1 receptor family member T1/ST2 in T helper cell type 2-mediated lung mucosal immune responses. J. Exp. Med. 1999, 190, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Bartosinska, J.; Przepiorka-Kosinska, J.; Sarecka-Hujar, B.; Raczkiewicz, D.; Kowal, M.; Chyl-Surdacka, K.; Bartosinski, J.; Kosinski, J.; Krasowska, D.; Chodorowska, G. Osteopontin Serum Concentration and Metabolic Syndrome in Male Psoriatic Patients. J. Clin. Med. 2021, 10, 755. [Google Scholar] [CrossRef]
- Kiefer, F.W.; Zeyda, M.; Gollinger, K.; Pfau, B.; Neuhofer, A.; Weichhart, T.; Saemann, M.D.; Geyeregger, R.; Schlederer, M.; Kenner, L.; et al. Neutralization of osteopontin inhibits obesity-induced inflammation and insulin resistance. Diabetes 2010, 59, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Galijasevic, S.; Saed, G.M.; Diamond, M.P.; Abu-Soud, H.M. Myeloperoxidase up-regulates the catalytic activity of inducible nitric oxide synthase by preventing nitric oxide feedback inhibition. Proc. Natl. Acad. Sci. USA 2003, 100, 14766–14771. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Hazen, S.L. Myeloperoxidase and cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1102–1111. [Google Scholar] [CrossRef]
- Tang, Y.; Xu, Q.; Peng, H.; Liu, Z.; Yang, T.; Yu, Z.; Cheng, G.; Li, X.; Zhang, G.; Shi, R. The role of vascular peroxidase 1 in ox-LDL-induced vascular smooth muscle cell calcification. Atherosclerosis 2015, 243, 357–363. [Google Scholar] [CrossRef]
- Abu-Soud, H.M.; Hazen, S.L. Nitric oxide modulates the catalytic activity of myeloperoxidase. J. Biol. Chem. 2000, 275, 5425–5430. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.L. Unraveling the links between diabetes, obesity, and cardiovascular disease. Circ. Res. 2005, 96, 1129–1131. [Google Scholar] [CrossRef] [PubMed]
- Giblin, F.J. Glutathione: A vital lens antioxidant. J. Ocul. Pharmacol. Ther. 2000, 16, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Gunawardena, H.P.; Silva, R.; Sivakanesan, R.; Ranasinghe, P.; Katulanda, P. Poor Glycaemic Control Is Associated with Increased Lipid Peroxidation and Glutathione Peroxidase Activity in Type 2 Diabetes Patients. Oxid. Med. Cell. Longev. 2019, 2019, 9471697. [Google Scholar] [CrossRef]
- da Silva, F.C.; de Araujo, B.J.; Cordeiro, C.S.; Arruda, V.M.; Faria, B.Q.; Guerra, J.; Araujo, T.G.; Furstenau, C.R. Endothelial dysfunction due to the inhibition of the synthesis of nitric oxide: Proposal and characterization of an in vitro cellular model. Front. Physiol. 2022, 13, 978378. [Google Scholar] [CrossRef]
- Gaxiola-Robles, R.; Bitzer-Quintero, O.K.; Mendez-Rodriguez, L.C.; Labrada-Martagon, V.; Garcia-Gonzalez, A.; Ramirez-Jirano, L.J.; Velez-Alavez, M.; Zenteno-Savin, T. Lipid peroxidation and the response of the antioxidant defense system in the obese type 2 diabetic compared with the non-obese type 2 diabetic. Nutr. Hosp. 2013, 28, 1905–1911. [Google Scholar] [PubMed]
- Frijhoff, J.; Winyard, P.G.; Zarkovic, N.; Davies, S.S.; Stocker, R.; Cheng, D.; Knight, A.R.; Taylor, E.L.; Oettrich, J.; Ruskovska, T.; et al. Clinical Relevance of Biomarkers of Oxidative Stress. Antioxid. Redox Signal. 2015, 23, 1144–1170. [Google Scholar] [CrossRef]
- Ueba, T.; Nomura, S.; Inami, N.; Yokoi, T.; Inoue, T. Elevated RANTES level is associated with metabolic syndrome and correlated with activated platelets associated markers in healthy younger men. Clin. Appl. Thromb. 2014, 20, 813–818. [Google Scholar] [CrossRef]
- Mombouli, J.V.; Vanhoutte, P.M. Endothelial dysfunction: From physiology to therapy. J. Mol. Cell. Cardiol. 1999, 31, 61–74. [Google Scholar] [CrossRef]
- Bayes-Genis, A.; Januzzi, J.L.; Gaggin, H.K.; de Antonio, M.; Motiwala, S.R.; Zamora, E.; Galan, A.; Domingo, M.; Urrutia, A.; Lupon, J. ST2 pathogenetic profile in ambulatory heart failure patients. J. Card. Fail. 2015, 21, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.G.; Yang, T.; He, J.G.; Chen, G.; Liu, Z.H.; Xiong, C.M.; Gu, Q.; Ni, X.H.; Zhao, Z.H. Plasma soluble ST2 levels correlate with disease severity and predict clinical worsening in patients with pulmonary arterial hypertension. Clin. Cardiol. 2014, 37, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Ojji, D.B.; Lecour, S.; Adeyemi, O.M.; Sliwa, K. Soluble ST2 correlates with some indicators of right ventricular function in hypertensive heart failure. Vasc. Health Risk Manag. 2017, 13, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Ky, B.; French, B.; McCloskey, K.; Rame, J.E.; McIntosh, E.; Shahi, P.; Dries, D.L.; Tang, W.H.; Wu, A.H.; Fang, J.C.; et al. High-sensitivity ST2 for prediction of adverse outcomes in chronic heart failure. Circ. Heart Fail. 2011, 4, 180–187. [Google Scholar] [CrossRef] [PubMed]
| Variables [Normal Reference Value] | Value |
|---|---|
| Age (years) | 54.9 ± 11.6 |
| Male (%) | 70.7 |
| SBP/DBP (mmHg) [<120/80] | 143.3 ± 16/93.1 ± 15.2 |
| BMI (Kg/m2) [18.5–24.9] | 33.1 ± 4.5 |
| BSA (m2) [men: 1.9; women: 1.6] | 2 ± 0.2 |
| Smoking (%) | 33.3 |
| Diabetes mellitus (%) | 34.5 |
| Diabetes mellitus duration (years) | 6.8 ± 4.9 |
| Hypertension (%) | 89.7 |
| Hypertension duration (years) | 9.9 ± 6.9 |
| Antihypertensive drugs: | |
| 25.9 50 27.6 39.7 32.8 |
| ASA (%) | 20.7 |
| Creatinine (mg/dL), [men: 0.7–1.3; women: 0.6–1.1] | 0.9 ± 0.3 |
| Urea (mg/dL) [6–24] | 36.6 ± 9.8 |
| Total cholesterol (mg/dL) [<200] | 194.4 ± 49.9 |
| HDL cholesterol (mg/dL) [>40] | 44.7 ± 14.7 |
| LDL cholesterol (mg/dL) [≤129] | 122.2 ± 36.2 |
| Triglycerides (mg/dL) | 184.9 ± 197.2 |
| Glucose (mg/dL) [<100] | 120.8 ± 36.2 |
| HbA1c (%) [<5.7] | 7.1 ± 1.3 |
| 2DE Variables | Value |
|---|---|
| LV end-diastolic diameter (mm) | 57.8 ± 62.9 |
| LV end-systolic diameter (mm) | 31.3 ± 5.2 |
| LV end-diastolic volume (mL) | 108.3 ± 25.9 |
| LV end-systolic volume (mL) | 40.9 ± 16.7 |
| Ejection fraction biplane (%) | 62.7 ± 10.2 |
| Global longitudinal LV strain (%) | −17.1 ± 3.9 |
| Interventricular septum thickness (mm) | 10.4 ± 1.4 |
| Posterior wall thickness (mm) | 10.4 ± 1.3 |
| LV mass (g) | 215.9 ± 56.7 |
| LV mass index (g/m2) | 103 ± 23.2 |
| LA diameter in parasternal long axis view (mm) | 40.3 ± 4.8 |
| LA volume (ml) | 62.4 ± 26.8 |
| Mitral E-wave velocity (m/s) | 72.3 ± 21.9 |
| Mitral A-wave velocity (m/s) | 69.9 ± 23.9 |
| Mitral E/A ratio | 1.05 ± 0.3 |
| DT (s) | 226.6 ± 52.2 |
| IVRT (ms) | 106.1 ± 22.9 |
| Mitral E/e’ average | 7.4 ± 3.8 |
| 2DE Variables | Value |
|---|---|
| RV basal diameter (mm) | 38.8 ± 8.9 |
| RV longitudinal diameter (mm) | 69.9 ± 7.5 |
| RV end-diastolic area (mm2) | 25.5 ± 30.3 |
| RV end-systolic area (mm2) | 11.8 ± 3.9 |
| RV free wall thickness from subcostal view (mm) | 4.3 ± 1.1 |
| TAPSE (mm) | 22 ± 3.4 |
| FAC (%) | 42.9 ± 13.9 |
| RV systolic pressure (mmHg) | 32.4 ± 8 |
| S tricuspid annulus peak velocity (cm/s) | 11.8 ± 2.9 |
| Right atrial volume (mm3) | 54.8 ± 32.4 |
| IVC diameter (mm) | 16.9 ± 3.9 |
| IVC collapse with sniff (%) | 58.7 ± 13.2 |
| Variables | Value |
|---|---|
| sST2 (ng/mL) | 25.4 ± 18 |
| Nitrotyrosine (ng/mL) | 93.0 ± 36 |
| 8-OHdG (ng/mL) | 411 ± 98 |
| Hydrogen peroxide (mM) | 7.6 ± 6 |
| TBARs (µM) | 0.80 ± 0.4 |
| Total nitric oxide (µM) | 50 ± 26 |
| CRP (mg/mL) | 1.30 ± 1.1 |
| IL-6 (pg/mL) | 1.89 ± 1.3 |
| Osteopontin(pg/mL) | 159 ± 62 |
| Myeloperoxidase (pg/mL) | 1227 ± 661 |
| PGE2α (pg/mL) | 7.9 ± 0.6 |
| PGF2α (pg/mL) | 84 ± 27 |
| RANTES (ng/mL) | 71 ± 31 |
| ICAM-1 (pg/mL) | 181 ± 87 |
| CD14 (pg/mL) | 1409 ± 254 |
| MMP-1/TIMP-1 (pg/mL) | 167 ± 136.6 |
| MMP-2/TIMP-2 ratio (ng/mL) | 343.4 ± 169 |
| MMP-9/TIMP-2 ratio (pg/mL) | 3 ± 2.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy, I.; Jover, E.; Matilla, L.; Alvarez, V.; Fernández-Celis, A.; Beunza, M.; Escribano, E.; Gainza, A.; Sádaba, R.; López-Andrés, N. Soluble ST2 as a New Oxidative Stress and Inflammation Marker in Metabolic Syndrome. Int. J. Environ. Res. Public Health 2023, 20, 2579. https://doi.org/10.3390/ijerph20032579
Roy I, Jover E, Matilla L, Alvarez V, Fernández-Celis A, Beunza M, Escribano E, Gainza A, Sádaba R, López-Andrés N. Soluble ST2 as a New Oxidative Stress and Inflammation Marker in Metabolic Syndrome. International Journal of Environmental Research and Public Health. 2023; 20(3):2579. https://doi.org/10.3390/ijerph20032579
Chicago/Turabian StyleRoy, Ignacio, Eva Jover, Lara Matilla, Virginia Alvarez, Amaya Fernández-Celis, Maite Beunza, Elena Escribano, Alicia Gainza, Rafael Sádaba, and Natalia López-Andrés. 2023. "Soluble ST2 as a New Oxidative Stress and Inflammation Marker in Metabolic Syndrome" International Journal of Environmental Research and Public Health 20, no. 3: 2579. https://doi.org/10.3390/ijerph20032579
APA StyleRoy, I., Jover, E., Matilla, L., Alvarez, V., Fernández-Celis, A., Beunza, M., Escribano, E., Gainza, A., Sádaba, R., & López-Andrés, N. (2023). Soluble ST2 as a New Oxidative Stress and Inflammation Marker in Metabolic Syndrome. International Journal of Environmental Research and Public Health, 20(3), 2579. https://doi.org/10.3390/ijerph20032579








