Literature Review on Health Emigration in Rare Diseases—A Machine Learning Perspective
Abstract
1. Introduction
1.1. Presentation of Research Problems
- Settle or live there (as a permanent resident or naturalized citizen);
- Take up employment as a migrant worker;
- Live temporarily as a foreign worker.
- Re-emigration, or the return of emigrants to the country after a permanent stay abroad;
- Repatriation, i.e., the mass return to the country, organized by state authorities, of prisoners of war, internees, people who left the country, e.g., for political reasons;
- Deportation, i.e., forced expulsion from the national territory, most often having to do with illegal immigrants;
- Expatriation is the voluntary or forced departure from one’s national territory, signifying a break from the country.
1.2. Organization of the Paper
- Define the basic concepts related to the topics of the article: emigration and rare diseases.
- What socioeconomic and management issues dominate scientific analysis in the area of unequal access to the treatment of rare diseases?
- To what extent does machine learning help identify the state of knowledge and research on health emigration in rare diseases and its limitations?
- For what reasons is the phenomenon of health emigration relevant to the management strategy of the support system in rare diseases?
2. Materials and Methods
3. Results
- Cluster 1—red (149 items);
- Cluster 2—green (138 items);
- Cluster 3—blue (104 items);
- Cluster 4—yellow (92 items);
- Cluster 5—purple (82 items);
- Cluster 6—celeste (74 items);
- Cluster 7—orange (47 items);
- Cluster 8—brown (34 items);
- Cluster 9—pink (26 items).
- Cluster 1 (red) includes 10 latent variables: challenges, child, children, gene therapy, health, management, orphan disease, orphan drug, orphan medicines, and rare disease.
- In cluster 2 (green), there are nine latent variables: budget impact, cost-effectiveness, drug reimbursement, health policy, health technology assessment, market access, orphan drug, pricing, and reimbursement.
- Cluster 3 (blue) includes seven latent variables: access, clinical trials, drug development, enzyme replacement therapy, medicines, orphan diseases, and rare disease.
- Cluster 4 (yellow) includes four latent variables: drugs, legislation, orphan medicine product, and policy.
4. Discussion
5. Conclusions
- ML allowed us to objectively diagnose a research gap in the area of rare disease management—the issue of health emigration is not directly studied;
- Analysis of grey literature (including industry reports) confirmed the gap;
- The need to define the term “health emigration”;
- Health emigration as a derivative of health inequalities should be included on the social cost side of the rare disease management model at the national and supranational levels;
- The topic of health emigration in rare diseases needs further research at several levels.
- The selection of the base of the analyzed literature;
- Tthe supplementation of the analyzes with selected industry reports;
- The lack of references to domestic migration;
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The European Parliament and the Council. Regulation (EC) No 141/2000 of the European Parliament and of the Council of 16 December 1999 on Orphan Medicinal Products. Off. J. Eur. Communities 2000, L18/1. [Google Scholar]
- Libura, M.; Władusiuk, M.; Małowicka, M.; Grabowska, E.; Gałązka-Sobotka, M.; Gryglewicz, J. Choroby Rzadkie w Polsce: Stan Obecny i Perspektywy; Uczelnia Łazarskiego: Warszawa, Poland, 2016; ISBN 9788364054730. [Google Scholar]
- Wakap, N.S.; Lambert, D.M.; Olry, A.; Rodwell, C.; Gueydan, C.; Lanneau, V.; Murphy, D.; Le Cam, Y.; Rath, A. Estimating cumulative point prevalence of rare diseases: Analysis of the Orphanet database. Eur. J. Hum. Genet. 2020, 28, 165–173. [Google Scholar] [CrossRef]
- EURORDIS-Rare Diseases Europe. High-Level Ministerial Conference: Care and Innovation Pathways for a European Rare Diseases Policy; EURORDIS-Rare Diseases Europe: Paris, France, 2022. [Google Scholar]
- Juchniewicz, M.; Rzempała, J.; Skweres-Kuchta, M. Initiating and Defining a Sustainable Project on the Example of Rare Disease Therapy Initiating and Defining a Sustainable Project on the Example of Rare Disease Therapy. Eur. Res. Stud. J. 2021, XXIV, 663–681. [Google Scholar] [CrossRef]
- Emigration. Definition & Meaning. Available online: https://www.merriam-webster.com/dictionary/emigration (accessed on 17 September 2022).
- Kenton, W. What Is Emigration? Available online: https://www.investopedia.com/terms/e/emigration.asp (accessed on 17 September 2022).
- Emigration—Meaning, Definition. Available online: https://dictionary.cambridge.org/dictionary/english/emigration (accessed on 17 September 2022).
- Migration—Meaning, Definition. Available online: https://dictionary.cambridge.org/dictionary/english/migration (accessed on 17 September 2022).
- Ministerstwo Edukacji i Nauki. Zintegrowana Platforma Edukacyjna. Available online: https://zpe.gov.pl/a/przeczytaj/D10e2ylE1?fbclid=IwAR2lqsn4bhaOt_-7XG-fNh3lv_tPTwXn2qXGwqsenIVQQCkPEwZNt6xVpzM (accessed on 19 September 2022).
- Analiza Migracji Klientów (Churn)—StatSoft Polska. Available online: https://www.statsoft.pl/Rozwiazania/Zastosowania-biznesowe/Analiza-migracji-klientow-churn/ (accessed on 17 September 2022).
- Janicki, W. Migration Theories—a Review. Ann. Univ. Mariae Curie-Skłodowska Sect. B Geogr. Geol. Mineral. Et Petrogr. 2007, 62, 285–304. [Google Scholar]
- Imigracja—Definicja, Synonimy, Przykłady Użycia. Available online: https://sjp.pwn.pl/slowniki/imigracja.html (accessed on 19 September 2022).
- Immigration Definition—US Immigration Glossary. Available online: https://www.usimmigration.org/glossary/immigration (accessed on 17 September 2022).
- Zakład Demografii i Gerontologii Społecznej UŁ Migracje Ludności. Materiały Dydaktyczne. Available online: http://www.demografia.uni.lodz.pl/dlastud/migracje.pdf?fbclid=IwAR21hmPCRMo_IS1iERNB8Wmb-v37EyPagtYOpsSQYM6xa8T_psZcCg5Ox_k (accessed on 19 September 2022).
- Ringold, S.; Burke, A.; Glass, R.M. Refugee Mental Health. JAMA 2005, 294, 646. [Google Scholar] [CrossRef]
- Yorimitsu, M. A Review on the Determinants of Migration. Hitotsubashi J. Soc. Stud. 1985, 17, 17–27. [Google Scholar]
- Patnaik, B.C.M.; Satpathy, I.; Mandal, A. Determinants of Migration—A Review of Literature. Online Int. Interdiscip. Res. J. 2014, 4, 349–357. [Google Scholar]
- De Haas, H. The Determinants of International Migration. Conceptualising Policy, Origin and Destination Effects. IMI Work. Pap. Ser. 2011, 32. [Google Scholar]
- Simpson, N.B. Demographic and Economic Determinants of Migration. Push and Pull Factors Drive the Decision to Stay or Move. IZA World Labor 2022, 373. [Google Scholar] [CrossRef]
- De Haas, H.; Czaika, M.; Flahaux, M.-L.; Mahendra, E.; Natter, K.; Vezzoli, S.; Villares-Varela, M. Notes and Commentary International Migration: Trends, Determinants, and Policy Effects. Popul. Dev. Rev. 2019, 45, 885–922. [Google Scholar] [CrossRef]
- Massimo, L.M.; Wiley, T.J.; Caprino, D. Health Emigration: A Challenge in Paediatric Oncology. J. Child Health Care 2008, 12, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Skweres-Kuchta, M. Choroby Rzadkie Wśród Dzieci—Zarządzanie Systemem z Perspektywy Rodziny Pacjenta. In Zdrowie i style życia. Wyzwania Ekonomiczne i Społeczne; Nowak, W., Szalonka, K., Eds.; E-Wydawnictwo; Prawnicza i Ekonomiczna Biblioteka Cyfrowa. Wydział Prawa, Administracji i Ekonomii Uniwersytetu Wrocławskiego: Wrocław, Poland, 2019; pp. 299–310. [Google Scholar]
- Czy Emigracja Zdrowotna to Jedyny Lek Na CLN2? Available online: https://wpolityce.pl/spoleczenstwo/467935-czy-emigracja-zdrowotna-to-jedyny-lek-na-cln2 (accessed on 29 September 2022).
- Krawitz, P.M. Artificial Intelligence in the Diagnosis of Rare Disorders: The Development of Phenotype Analysis. Bundesgesundheitsblatt Gesundh. Gesundh. 2022, 65, 1159–1163. [Google Scholar] [CrossRef]
- Mahesh, B. Machine Learning Algorithms—A Review. Int. J. Sci. Res. 2018, 9, 381–386. [Google Scholar] [CrossRef]
- el Naqa, I.; Murphy, M.J. What Is Machine Learning? In Machine Learning in Radiation Oncology; el Naqa, I., Li, R.M.M., Eds.; Springer: Cham, Switzerland, 2015; pp. 3–11. [Google Scholar]
- Kamila, N.K.; Fonda, J.; Pani, S.K.; Das, R.; Islam, S.M.N.; Bharti, P.K.; Muduli, K. Machine Learning Model Design for High Performance Cloud Computing & Load Balancing Resiliency: An Innovative Approach. J. King Saud Univ. —Comput. Inf. Sci. 2022, 34, 9991–10009. [Google Scholar] [CrossRef]
- Ren, Y.-S.; Ma, C.-Q.; Kong, X.-L.; Baltas, K.; Zureigat, Q. Past, Present, and Future of the Application of Machine Learning in Cryptocurrency Research. Res. Int. Bus. Finance 2022, 63, 101799. [Google Scholar] [CrossRef]
- Lundberg, I.; Brand, J.E.; Jeon, N. Researcher Reasoning Meets Computational Capacity: Machine Learning for Social Science. Soc. Sci. Res. 2022, 108, 102807. [Google Scholar] [CrossRef]
- Document Search—Web of Science Core Collection. Available online: https://www.webofscience.com/wos/woscc/basic-search (accessed on 1 August 2022).
- Scopus—Document Search. Available online: https://www.scopus.com/search/form.uri?display=basic#basic (accessed on 1 August 2022).
- PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov (accessed on 1 August 2022).
- Ning, W.; Lin, S.; Zhou, J.; Guo, Y.; Zhang, Y.; Peng, D.; Deng, W.; Xue, Y. WocEA: The Visualization of Functional Enrichment Results in Word Clouds. J. Genet. Genom. 2018, 45, 415–417. [Google Scholar] [CrossRef]
- Jin, Y. Development of Word Cloud Generator Software Based on Python. Procedia Eng. 2017, 174, 788–792. [Google Scholar] [CrossRef]
- van Eck, N.J.L.; Waltman, L. Visualizing Bibliometric Networks. In Measuring Scholarly Impact; Ding, Y., Rousseau, R., Wolfram, D., Eds.; Springer International Publishing: Cham, Switzerland, 2014; pp. 285–320. ISBN 19103778_13. [Google Scholar]
- Szaruga, E.; Załoga, E. Qualitative-Quantitative Warning Modeling of Energy Consumption Processes in Inland Waterway Freight Transport on River Sections for Environmental Management. Energies 2022, 15, 4660. [Google Scholar] [CrossRef]
- Ishihara, S. Score-Based Likelihood Ratios for Linguistic Text Evidence with a Bag-of-Words Model. Forensic Sci. Int. 2021, 327, 110980. [Google Scholar] [CrossRef]
- Crain, S.P.; Zhou, K.; Yang, S.H.; Zha, H. Dimensionality Reduction and Topic Modeling: From Latent Semantic Indexing to Latent Dirichlet Allocation and Beyond. Mining Text Data 2012, 9781461432234, 129–161. [Google Scholar] [CrossRef]
- Dewangan, J.K.; Sharaff, A.; Pandey, S. Improving Topic Coherence Using Parsimonious Language Model and Latent Semantic Indexing. Lect. Notes Electr. Eng. 2020, 601, 823–830. [Google Scholar] [CrossRef]
- Colneric, N.; Demsar, J. Emotion Recognition on Twitter: Comparative Study and Training a Unison Model. IEEE Trans. Affect. Comput. 2020, 11, 433–446. [Google Scholar] [CrossRef]
- Szaruga, E.; Załoga, E. Machine Learning in Exploration the Decoupling Paradigm in Transport. Procedia Comput. Sci. 2022, 207, 3904–3914. [Google Scholar] [CrossRef]
- Sesana, M.M.; Cuca, B.; Iannaccone, G.; Brumana, R.; Caccavelli, D.; Gay, C. Geomapping Methodology for the GeoCluster Mapping Tool to Assess Deployment Potential of Technologies for Energy Efficiency in Buildings. Sustain. Cities Soc. 2015, 17, 22–34. [Google Scholar] [CrossRef]
- Reinhard, C.; Bachoud-Lévi, A.C.; Bäumer, T.; Bertini, E.; Brunelle, A.; Buizer, A.I.; Federico, A.; Gasser, T.; Groeschel, S.; Hermanns, S.; et al. The European Reference Network for Rare Neurological Diseases. Front. Neurol. 2021, 11, 616569. [Google Scholar] [CrossRef]
- Forman, J.; Taruscio, D.; Llera, V.A.; Barrera, L.A.; Coté, T.R.; Edfjäll, C.; Gavhed, D.; Haffner, M.E.; Nishimura, Y.; Posada, M.; et al. The Need for Worldwide Policy and Action Plans for Rare Diseases. Acta Paediatr. Int. J. Paediatr. 2012, 101, 805–807. [Google Scholar] [CrossRef]
- Schey, C.; Postma, M.J.; Krabbe, P.F.M.; Topachevskyi, O.; Volovyk, A.; Connolly, M. Assessing the Preferences for Criteria in Multi-Criteria Decision Analysis in Treatments for Rare Diseases. Front. Public Health 2020, 8, 162. [Google Scholar] [CrossRef]
- Hyry, H.I.; Roos, J.C.; Manuel, J.; Cox, T.M. The Legal Imperative for Treating Rare Disorders. Orphanet J. Rare Dis. 2013, 8, 1. [Google Scholar] [CrossRef]
- Münster, T.; Richards, E.; Dillow, J.; Percesepe, A. OrphanAnesthesia—A Common Project of the Scientific Working Group of Paediatric Anaesthesia of the German Society of Anaesthesiology and Intensive Care Medicine. Anasthesiol. Und Intensivmed. 2019, 60, S82–S94. [Google Scholar] [CrossRef]
- Detiček, A.; Locatelli, I.; Kos, M. Patient Access to Medicines for Rare Diseases in European Countries. Value Health 2018, 21, 553–560. [Google Scholar] [CrossRef]
- Stakisaitis, D.; Spokiene, I.; Jukevicius, J.; Valuckas, K.P.; Baiardi, P. Access to Information Supporting Availability of Medicines for Patients Suffering from Rare Diseases Looking for Possible Treatments: The EuOrphan Service. Medicina 2007, 43, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Martín, J.; Díaz-Rodríguez, L.; Piqueras-Sola, B.; Rodríguez-Blanque, R.; Bermejo-Fernández, A.; Sánchez-García, J.C. Hajdu-Cheney Syndrome: A Systematic Review of the Literature. Int. J. Environ. Res. Public Health 2020, 17, 6174. [Google Scholar] [CrossRef]
- Spokiene, I. Legal Assessment of Current Situation on Orphan Patients in Lithuania. Medicina 2008, 44, 571–576. [Google Scholar] [CrossRef]
- de Andres-Nogales, F.; Cruz, E.; Calleja, M.Á.A.; Delgado, O.; Gorgas, M.Q.; Espin, J.; Mestre-Ferrandiz, J.; Palau, F.; Ancochea, A.; Arce, R.; et al. A Multi-Stakeholder Multicriteria Decision Analysis for the Reimbursement of Orphan Drugs (FinMHU-MCDA Study). Orphanet J. Rare Dis. 2021, 16, 1–12. [Google Scholar] [CrossRef]
- Karpman, D.; Höglund, P. Orphan Drug Policies and Use in Pediatric Nephrology. Pediatr. Nephrol. 2017, 32, 1–6. [Google Scholar] [CrossRef]
- Wilson, C. Policies and Research Funding. Orphan Drugs: Underst. Rare Dis. Mark. Its Dyn. 2013, 145–186. [Google Scholar] [CrossRef]
- Pavan, S.; Rommel, K.; Marquina, M.E.M.; Höhn, S.; Lanneau, V.; Rath, A. Clinical Practice Guidelines for Rare Diseases: The Orphanet Database. PLoS ONE 2017, 12, e0170365. [Google Scholar] [CrossRef] [PubMed]
- Heard, J.M.; Vrinten, C.; Schlander, M.; Bellettato, C.M.; Van Lingen, C.; Scarpa, M.; Matthijs, G.; Nassogne, M.C.; Debray, F.G.; Roland, D.; et al. Availability, Accessibility and Delivery to Patients of the 28 Orphan Medicines Approved by the European Medicine Agency for Hereditary Metabolic Diseases in the MetabERN Network. Orphanet J. Rare Dis. 2020, 15, 1–10. [Google Scholar] [CrossRef]
- Almalki, Z.S.; Alahmari, A.K.; Guo, J.J.; Kelton, C.M.L. Access to Orphan Drugs in the Middle East: Challenge and Perspective. Intractable Rare Dis. Res. 2012, 1, 139–143. [Google Scholar] [CrossRef]
- Szegedi, M.; Molnár, M.J.; Boncz, I.; Kosztolányi, G. Focus shifts in the Hungarian reimbursement system: Funding of orphan medicinal products for rare disease patients in Hungary: Financing of orphan medicines. Orv Hetil. 2014, 155, 1735–1741. [Google Scholar] [CrossRef]
- Hanisch, M.; Hanisch, L.; Benz, K.; Kleinheinz, J.; Jackowski, J. Development of a Database to Record Orofacial Manifestations in Patients with Rare Diseases: A Status Report from the ROMSE (Recording of Orofacial Manifestations in People with Rare Diseases) Database. Br. J. Oral Maxillofac. Surg. 2017, 55, 500–503. [Google Scholar] [CrossRef] [PubMed]
- Mayrides, M.; Ruiz De Castilla, E.M.; Szelepski, S.; de Castilla, E.M.R.; Szelepski, S.; Ruiz De Castilla, E.M.; Szelepski, S.; de Castilla, E.M.R.; Szelepski, S.; Ruiz De Castilla, E.M.; et al. A Civil Society View of Rare Disease Public Policy in Six Latin American Countries. Orphanet J. Rare Dis. 2020, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hernández, A.M.B.; Almela, J.A.S. The Debate on Rare Diseases: A Look at Media Response. Metode 2016, 6, 209–213. [Google Scholar] [CrossRef]
- Kole, A.; Faurisson, F. Rare Diseases Social Epidemiology: Analysis of Inequalities. Adv. Exp. Med. Biol. 2010, 686, 223–250. [Google Scholar] [CrossRef] [PubMed]
- Zelei, T.; Molnár, M.J.; Szegedi, M.; Kaló, Z.; Molnar, M.J.; Szegedi, M.; Kalo, Z. Systematic Review on the Evaluation Criteria of Orphan Medicines in Central and Eastern European Countries. Orphanet J. Rare Dis. 2016, 11, 72. [Google Scholar] [CrossRef]
- Bogart, K.; Hemmesch, A.; Barnes, E.; Blissenbach, T.; Beisang, A.; Engel, P. Chloe Barnes Advisory Council on Rare Diseases Healthcare Access, Satisfaction, and Health-Related Quality of Life among Children and Adults with Rare Diseases. Orphanet J. Rare Dis. 2022, 17, 196. [Google Scholar] [CrossRef]
- Dunkle, M.; Pines, W.; Saltonstall, P.L. Advocacy Groups and Their Role in Rare Diseases Research. Adv. Exp. Med. Biol. 2010, 686, 515–525. [Google Scholar] [CrossRef]
- Kerpel-Fronius, S.; Baroutsou, V.; Becker, S.; Carlesi, R.; Collia, L.; Franke-Bray, B.; Kleist, P.; Kurihara, C.; Laranjeira, L.F.; Matsuyama, K.; et al. Development and Use of Gene Therapy Orphan Drugs—Ethical Needs for a Broader Cooperation Between the Pharmaceutical Industry and Society. Front. Med. 2020, 7, 608249. [Google Scholar] [CrossRef]
- Pearson, I.; Rothwell, B.; Olaye, A.; Knight, C. Economic Modeling Considerations for Rare Diseases. Value Health 2018, 21, 515–524. [Google Scholar] [CrossRef]
- Gupta, R.; Bollyky, T.J.; Cohen, M.; Ross, J.S.; Kesselheim, A.S. Affordability and Availability of Off-Patent Drugs in the United States—The Case for Importing from Abroad: Observational Study. BMJ 2018, 360, k381. [Google Scholar] [CrossRef]
- Martins, P. Orphan Anesthesia. Anasthesiol. Und Intensivmed. 2020, 61, S108–S118. [Google Scholar] [CrossRef]
- Pavlović, N.; Stanimirov, B.; Stojančević, M.; Paut-Kusturica, M.; Stoimenova, A.; Goločorbin-Kon, S.; Mikov, M. An Insight on Differences in Availability and Reimbursement of Orphan Medicines among Serbia, Bulgaria and Sweden. Biotechnol. Biotechnol. Equip. 2012, 26, 26–3236. [Google Scholar] [CrossRef]
- Bavisetty, S.; Grody, W.W.; Yazdani, S. Emergence of Pediatric Rare Diseases Review of Present Policies and Opportunities for Improvement. Rare Dis. 2013, 1, 1–5. [Google Scholar] [CrossRef]
- Kanters, T.A.; Hakkaart, L.; Rutten-Van Mölken, M.P.M.H.; Redekop, W.K. Access to Orphan Drugs in Western Europe: Can More Systematic Policymaking Really Help to Avoid Different Decisions about the Same Drug? Expert Rev. Pharmacoecon Outcomes Res. 2015, 15, 557–559. [Google Scholar] [CrossRef] [PubMed]
- Soon, S.S.; Lopes, G.; Lim, H.Y.; Wong-Rieger, D.; Bahri, S.; Hickinbotham, L.; Jha, A.; Ko, B.S.; MacDonell, D.; Pwu, J.R.F.; et al. A Call for Action to Improve Access to Care and Treatment for Patients with Rare Diseases in the Asia-Pacific Region. Orphanet J. Rare Dis. 2014, 9, 137. [Google Scholar] [CrossRef]
- Chalamon, I. Consumer Resistance between Conflict and Cooperation: The Extreme Case of Orphan Drugs. Eur. J. Mark 2011, 45, 1736–1745. [Google Scholar] [CrossRef]
- Robinson, S.W.; Brantley, K.; Liow, C.; Russell Teagarden, J. An Early Examination of Access to Select Orphan Drugs Treating Rare Diseases in Health Insurance Exchange Plans. J. Manag. Care Spec. Pharm. 2014, 20, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Derham, R. Rare Disease and Orphan Drug Leadership—CBI’s Seventh Annual Conference, Philadelphia, Pennsylvania, USA, 18–19 July 2012. Drugs Future 2012, 37, 829–831. [Google Scholar] [CrossRef]
- Gong, S.W.; Jin, S. Current Progress in the Management of Rare Diseases and Orphan Drugs in China. Intractable Rare Dis. Res. 2012, 1, 45–52. [Google Scholar] [CrossRef]
- Godeau, B. Objectives and organization of a reference center for adults. Bull. Acad. Natl. Med. 2009, 193, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Kamusheva, M.; Dimitrova, M.; Tachkov, K.; Petrova, G.; Mitkova, Z. Pharmacotherapeutic Patterns and Patients’ Access to Pharmacotherapy for Some Rare Diseases in Bulgaria—A Pilot Comparative Study. Front. Pharmacol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Hughes-Wilson, W.; Palma, A.; Schuurman, A.; Simoens, S. Paying for the Orphan Drug System: Break or Bend? Is It Time for a New Evaluation System for Payers in Europe to Take Account of New Rare Disease Treatments? Orphanet J. Rare Dis. 2012, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Pasquini, T.L.S.; Goff, S.L.; Whitehill, J.M. Navigating the U.S. Health Insurance Landscape for Children with Rare Diseases: A Qualitative Study of Parents’ Experiences. Orphanet J. Rare Dis. 2021, 16, 1–14. [Google Scholar] [CrossRef]
- Cannizzo, S.; Lorenzoni, V.; Palla, I.; Pirri, S.; Trieste, L.; Triulzi, I.; Turchetti, G. Rare Diseases under Different Levels of Economic Analysis: Current Activities, Challenges and Perspectives. RMD Open 2018, 4, 794. [Google Scholar] [CrossRef] [PubMed]
- Rixen, S. Rare diseases—A problem of healthcare-related social security law. Z Evid Qual Gesundhwes 2008, 102, 31–36. [Google Scholar] [CrossRef]
- Boudes, P.F. Clinical Studies in Lysosomal Storage Diseases Past, Present, and Future. Rare Dis. 2013, 1, e26690. [Google Scholar] [CrossRef]
- Blankart, C.R.; Stargardt, T.; Schreyögg, J. Availability of and Access to Orphan Drugs: An International Comparison of Pharmaceutical Treatments for Pulmonary Arterial Hypertension, Fabry Disease, Hereditary Angioedema and Chronic Myeloid Leukaemia. Pharmacoeconomics 2011, 29, 63–82. [Google Scholar] [CrossRef]
- Orofino, J.; Soto, J.; Casado, M.A.; Oyagez, I. Global Spending on Orphan Drugs in France, Germany, the UK, Italy and Spain during 2007. Appl. Health Econ. Health Policy 2010, 8, 301–315. [Google Scholar] [CrossRef]
- Winstone, J.; Chadda, S.; Ralston, S.; Sajosi, P. Review and Comparison of Clinical Evidence Submitted to Support European Medicines Agency Market Authorization of Orphan-Designated Oncological Treatments. Orphanet J. Rare Dis. 2015, 10, 1–7. [Google Scholar] [CrossRef]
- Bellgard, M.I.; Napier, K.R.; Bittles, A.H.; Szer, J.; Fletcher, S.; Zeps, N.; Hunter, A.A.; Goldblatt, J. Design of a Framework for the Deployment of Collaborative Independent Rare Disease-Centric Registries: Gaucher Disease Registry Model. Blood Cells Mol. Dis. 2018, 68, 232–238. [Google Scholar] [CrossRef]
- Ghedira, K.; Kouidhi, S.; Hamdi, Y.; Othman, H.; Kechaou, S.; Znaidi, S.; Haïtham, S.; Rabhi, I. Pathway Maps of Orphan and Complex Diseases Using an Integrative Computational Approach. Biomed Res. Int. 2020, 2020, 1–11. [Google Scholar] [CrossRef]
- Witkowska, J. Corporate Social Responsibility (CSR) of Innovative Pharmaceutical Corporations. The Case of BIOGEN. Comp. Econ. Res. 2018, 21, 45–62. [Google Scholar] [CrossRef]
- Thompson, R.; Abicht, A.; Beeson, D.; Engel, A.G.; Eymard, B.; Maxime, E.; Lochmüller, H. A Nomenclature and Classification for the Congenital Myasthenic Syndromes: Preparing for FAIR Data in the Genomic Era. Orphanet J. Rare Dis. 2018, 13, 211. [Google Scholar] [CrossRef] [PubMed]
- Drummond, M.; Towse, A. Orphan Drugs Policies: A Suitable Case for Treatment. Eur. J. Health Econ. 2014, 15, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Nestler-Parr, S.; Korchagina, D.; Toumi, M.; Pashos, C.L.; Blanchette, C.; Molsen, E.; Morel, T.; Simoens, S.; Kaló, Z.; Gatermann, R.; et al. Challenges in Research and Health Technology Assessment of Rare Disease Technologies: Report of the ISPOR Rare Disease Special Interest Group. Value Health 2018, 21, 493–500. [Google Scholar] [CrossRef]
- Annemans, L.; Makady, A. TRUST4RD: Tool for Reducing Uncertainties in the Evidence Generation for Specialised Treatments for Rare Diseases. Orphanet J. Rare Dis. 2020, 15, 1–9. [Google Scholar] [CrossRef]
- Sachs-Barrable, K.; Conway, J.; Gershkovich, P.; Ibrahim, F.; Wasan, K.M. The Use of the United States FDA Programs as a Strategy to Advance the Development of Drug Products for Neglected Tropical Diseases. Drug Dev. Ind. Pharm. 2014, 40, 1429–1434. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Chen, L. Orphan Drug Development in China—Turning Challenges into Opportunities. Intractable Rare Dis. Res. 2016, 5, 308–313. [Google Scholar] [CrossRef]
- Logviss, K.; Krievins, D.; Purvina, S. Rare Diseases and Orphan Drugs: Latvian Story. Orphanet J. Rare Dis. 2014, 9, 147. [Google Scholar] [CrossRef]
- Giugliani, L.; Vanzella, C.; Zambrano, M.B.; Donis, K.C.; Wallau, T.K.W.; Da Costa, F.M.; Giugliani, R. Clinical Research Challenges in Rare Genetic Diseases in Brazil. Genet. Mol. Biol. 2019, 42, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Luisetti, M.; Balfour-Lynn, I.M.; Johnson, S.R.; Miravitlles, M.; Strange, C.; Trapnell, B.C.; Van Bronswijk, H.; Vogelmeier, C. Perspectives for Improving the Evaluation and Access of Therapies for Rare Lung Diseases in Europe. Respir. Med. 2012, 106, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Potter, B.K.; Chakraborty, P.; Kronick, J.B.; Wilson, K.; Coyle, D.; Feigenbaum, A.; Geraghty, M.T.; Karaceper, M.D.; Little, J.; Mhanni, A.; et al. Achieving the “Triple Aim” for Inborn Errors of Metabolism: A Review of Challenges to Outcomes Research and Presentation of a New Practice-Based Evidence Framework. Genet. Med. 2013, 15, 415–422. [Google Scholar] [CrossRef]
- Simoens, S.; Cassiman, D.; Dooms, M.; Picavet, E. Orphan Drugs for Rare Diseases: Is It Time to Revisit Their Special Market Access Status? Drugs 2012, 72, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- Park, T.; Griggs, S.K.; Suh, D.C. Cost Effectiveness of Monoclonal Antibody Therapy for Rare Diseases: A Systematic Review. BioDrugs 2015, 29, 259–274. [Google Scholar] [CrossRef]
- Czech, M.; Baran-Kooiker, A.; Holownia-Voloskova, M.; Kooiker, C.; Sykut-Cegielska, J. Bridging East with West of Europe—A Comparison of Orphan Drugs Policies in Poland, Russia and the Netherlands. Acta Pol. Pharm. —Drug Res. 2018, 75, 1409–1422. [Google Scholar] [CrossRef]
- Yang, Y.; Kang, Q.; Hu, J.; Kong, F.; Tang, M.; He, J.; Jin, C. Accessibility of Drugs for Rare Diseases in China: Policies and Current Situation. Intractable Rare Dis. Res. 2019, 8, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Feltmate, K.; Janiszewski, P.M.; Gingerich, S.; Cloutier, M. Delayed Access to Treatments for Rare Diseases: Who’s to Blame? Respirology 2015, 20, 361–369. [Google Scholar] [CrossRef]
- Carr, D.R.; Bradshaw, S.E. Gene Therapies: The Challenge of Super-High-Cost Treatments and How to Pay for Them. Regen. Med. 2016, 11, 381–393. [Google Scholar] [CrossRef]
- Dunoyer, M. Accelerating Access to Treatments for Rare Diseases. Nat. Rev. Drug Discov. 2011, 10, 475–476. [Google Scholar] [CrossRef]
- Adkins, E.M.; Nicholson, L.; Floyd, D.; Ratcliffe, M.; Chevrou-Severac, H. Oncology Drugs for Orphan Indications: How Are HTA Processes Evolving for This Specific Drug Category? Clin. Outcomes Res. 2017, 9, 327–342. [Google Scholar] [CrossRef] [PubMed]
- Bogart, K.R.; Irvin, V.L. Health-Related Quality of Life among Adults with Diverse Rare Disorders. Orphanet J. Rare Dis. 2017, 12, 177. [Google Scholar] [CrossRef] [PubMed]
- Min, R.; Zhang, X.; Fang, P.; Wang, B.; Wang, H. Health Service Security of Patients with 8 Certain Rare Diseases: Evidence from China’s National System for Health Service Utilization of Patients with Healthcare Insurance. Orphanet J. Rare Dis. 2019, 14, 1–18. [Google Scholar] [CrossRef]
- Hyry, H.I.; Manuel, J.; Cox, T.M.; Roos, J.C.P. Compassionate Use of Orphan Drugs. Orphanet J. Rare Dis. 2015, 10, 100. [Google Scholar] [CrossRef] [PubMed]
- Medic, G.; Korchagina, D.; Young, K.E.; Toumi, M.; Postma, M.J.; Wille, M.; Hemels, M.; Postma, J.; Wille, M.; Hemels, M.; et al. Do Payers Value Rarity? An Analysis of the Relationship between Disease Rarity and Orphan Drug Prices in Europe. J. Mark. Access Health Policy 2017, 5, 1299665. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, L.; Patris, J.; Hutchings, A.; Cowell, W. Principles for Consistent Value Assessment and Sustainable Funding of Orphan Drugs in Europe. Orphanet J. Rare Dis. 2015, 10, 1–9. [Google Scholar] [CrossRef]
- Logviss, K.; Krievins, D.; Purvina, S. Impact of Orphan Drugs on Latvian Budget. Orphanet J. Rare Dis. 2016, 11, 1–13. [Google Scholar] [CrossRef]
- Hanisch, M.; Hanisch, L.; Kleinheinz, J.; Danesh, G.; Benz, K.; Jackowski, J. Orthodontically Relevant Manifestations in People with Rare Diseases. Med. Princ. Pract. 2019, 28, 216–221. [Google Scholar] [CrossRef]
- Alfaro, T.M.; Wijsenbeek, M.S.; Powell, P.; Stolz, D.; Hurst, J.R.; Kreuter, M.; Moor, C.C. Educational Aspects of Rare and Orphan Lung Diseases. Respir Res. 2021, 22, 1–8. [Google Scholar] [CrossRef]
- Vanhoorne, V.; Peeters, E.; Van Tongelen, I.; Boussery, K.; Wynendaele, E.; De Spiegeleer, B.; Remon, J.P.; Vervaet, C. Pharmaceutical Compounding of Orphan Active Ingredients in Belgium: How Community and Hospital Pharmacists Can Address the Needs of Patients with Rare Diseases. Orphanet J. Rare Dis. 2019, 14, 1–9. [Google Scholar] [CrossRef]
- Hughes, D.A.; Tunnage, B.; Yeo, S.T. Drugs for Exceptionally Rare Diseases: Do They Deserve Special Status for Funding? QJM 2005, 98, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.Y.L.; Chan, V.K.Y.; Olsson, S.; Fan, M.; Jit, M.; Gong, M.; Zhang, S.; Ge, M.; Pathadka, S.; Chung, C.C.Y.; et al. Access and Unmet Needs of Orphan Drugs in 194 Countries and 6 Areas: A Global Policy Review With Content Analysis. Value Health 2020, 23, 1580–1591. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Song, P.; Kang, Q.; Zhang, X.; Hu, J.; Yang, Y.; Tang, M.; Chen, D.; Hu, S.; Jin, C. Overview on Social Security System of Rare Diseases in China. Biosci. Trends 2019, 13, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Teagarden, J.R.; Unger, T.F.; Hirsch, G. Access and Availability of Orphan Drugs in the United States: Advances or Cruel Hoaxes? Expert Opin. Orphan Drugs 2014, 2, 1147–1150. [Google Scholar] [CrossRef]
- Gong, S.; Wang, Y.; Pan, X.; Zhang, L.; Huang, R.; Chen, X.; Hu, J.; Xu, Y.; Jin, S. The Availability and Affordability of Orphan Drugs for Rare Diseases in China. Orphanet J. Rare Dis. 2016, 11, 1–12. [Google Scholar] [CrossRef]
- Korchagina, D.; Millier, A.; Vataire, A.L.; Aballea, S.; Falissard, B.; Toumi, M. Determinants of Orphan Drugs Prices in France: A Regression Analysis. Orphanet J. Rare Dis. 2017, 12, 75. [Google Scholar] [CrossRef]
- Choudhury, M.C.; Saberwal, G. The Work, Goals, Challenges, Achievements, and Recommendations of Orphan Medicinal Product Organizations in India: An Interview-Based Study. Orphanet J. Rare Dis. 2019, 14, 241. [Google Scholar] [CrossRef]
- Li, X.; Liu, M.; Lin, J.; Li, B.; Zhang, X.; Zhang, S.; Lu, Z.; Zhang, J.; Zhou, J.; Ou, L. A Questionnaire-Based Study to Comprehensively Assess the Status Quo of Rare Disease Patients and Care-Givers in China. Orphanet J. Rare Dis. 2021, 16, 327. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.E.; Neidle, S.; Taylor, D.G. Developing and Paying for Medicines for Orphan Indications in Oncology: Utilitarian Regulation vs Equitable Care? Br. J. Cancer 2012, 106, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Yong, J.; Moffett, M.; Lucas, S. Implementing a Global Expanded Access Program (EAP) for Infantile-Onset Spinal Muscular Atrophy (Type I): Understanding the Imperative, Impact and Challenges. J. Neuromuscul. Dis. 2019, 6, 227–231. [Google Scholar] [CrossRef]
- Nicod, E.; Whittal, A.; Drummond, M.; Facey, K. Are Supplemental Appraisal/Reimbursement Processes Needed for Rare Disease Treatments? An International Comparison of Country Approaches. Orphanet J. Rare Dis. 2020, 15, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Dharssi, S.; Wong-Rieger, D.; Harold, M.; Terry, S. Review of 11 National Policies for Rare Diseases in the Context of Key Patient Needs. Orphanet J. Rare Dis. 2017, 12, 1303–1304. [Google Scholar] [CrossRef] [PubMed]
- Watson, M.; Directors, A.B. Insuring Patient Access and Affordability for Treatments for Rare and Ultrarare Diseases: A Policy Statement of the American College of Medical Genetics and Genomics. Genet. Med. 2018, 20, 1303–1304. [Google Scholar] [CrossRef]
- So, D.; Joly, Y.; Knoppers, B.M. Clinical Trial Transparency and Orphan Drug Development: Recent Trends in Data Sharing by the Pharmaceutical Industry. Public Health Genom. 2013, 16, 322–335. [Google Scholar] [CrossRef]
- Stein, S.; Bogard, E.; Boice, N.; Fernandez, V.; Field, T.; Gilstrap, A.; Kahn, S.R.; Larkindale, J.; Mathieson, T. Principles for Interactions with Biopharmaceutical Companies: The Development of Guidelines for Patient Advocacy Organizations in the Field of Rare Diseases. Orphanet J. Rare Dis. 2018, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Monguio, R.; Spargo, T.; Seoane-Vazquez, E. Ethical Imperatives of Timely Access to Orphan Drugs: Is Possible to Reconcile Economic Incentives and Patients’ Health Needs? Orphanet J. Rare Dis. 2017, 12, 1–8. [Google Scholar] [CrossRef]
- Blonda, A.; Barcina Lacosta, T.; Toumi, M.; Simoens, S. Assessing the Value of Nusinersen for Spinal Muscular Atrophy: A Comparative Analysis of Reimbursement Submission and Appraisal in European Countries. Front. Pharmacol. 2022, 12, 750742. [Google Scholar] [CrossRef]
- Hsiao, E.C.; Di Rocco, M.; Cali, A.; Zasloff, M.; Al Mukaddam, M.; Pignolo, R.J.; Grunwald, Z.; Netelenbos, C.; Keen, R.; Baujat, G.; et al. Special Considerations for Clinical Trials in Fibrodysplasia Ossificans Progressiva (FOP). Br. J. Clin. Pharmacol. 2019, 85, 1199–1207. [Google Scholar] [CrossRef]
- Liu, X.; Tang, Y.; Zhang, B.; Li, J.T.; Mei, D. Off-Label Drug Use in the T88888reatment of Rare Diseases:The Current Situation. J. Int. Pharm. Res. 2019, 46, 685–690. [Google Scholar] [CrossRef]
- Tafuri, G.; Bracco, A.; Grueger, J. Access and Pricing of Medicines for Patients with Rare Diseases in the European Union: An Industry Perspective. Expert Rev. Pharm. Outcomes Res. 2022, 22, 381–389. [Google Scholar] [CrossRef]
- Weerasooriya, S.U. The Impact of Orphan Drug Policies in Treating Rare Diseases. Health Inf. Libr. J. 2019, 36, 179–184. [Google Scholar] [CrossRef] [PubMed]
- McCormack, P.; Woods, S.; Aartsma-Rus, A.; Hagger, L.; Herczegfalvi, A.; Heslop, E.; Irwin, J.; Kirschner, J.; Moeschen, P.; Muntoni, F.; et al. Guidance in Social and Ethical Issues Related to Clinical, Diagnostic Care and Novel Therapies for Hereditary Neuromuscular Rare Diseases: “Translating” the Translational. PLoS Curr. 2013, 5, 259–276. [Google Scholar] [CrossRef] [PubMed]
- Fontrier, A.-M. Market Access for Medicines Treating Rare Diseases: Association between Specialised Processes for Orphan Medicines and Funding Recommendations. Soc. Sci. Med. 2022, 306, 115119. [Google Scholar] [CrossRef] [PubMed]
- Calleri, G.; Angheben, A.; Albonico, M. Neglected Tropical Diseases in Europe: Rare Diseases and Orphan Drugs? Infection 2019, 47, 3–5. [Google Scholar] [CrossRef]
- Zurynski, Y.; Deverell, M.; Dalkeith, T.; Johnson, S.; Christodoulou, J.; Leonard, H.; Elliott, E.J. Australian Children Living with Rare Diseases: Experiences of Diagnosis and Perceived Consequences of Diagnostic Delays. Orphanet J. Rare Dis. 2017, 12, 68. [Google Scholar] [CrossRef]
- Rawson, N.S.B. Alignment of Health Technology Assessments and Price Negotiations for New Drugs for Rare Disorders in Canada: Does It Lead to Improved Patient Access? J. Popul. Ther. Clin. Pharmacol. 2020, 27, e48–e64. [Google Scholar] [CrossRef]
- Benson, M.; Albanese, A.; Bhatia, K.P.; Cavillon, P.; Cuffe, L.; König, K.; Reinhard, C.; Graessner, H. Development of a Patient Journey Map for People Living with Cervical Dystonia. Orphanet J. Rare Dis. 2022, 17, 1–9. [Google Scholar] [CrossRef]
- Bienstock, R.J. Data Sharing Advances Rare and Neglected Disease Clinical Research and Treatments. ACS Pharmacol. Transl. Sci. 2019, 2, 491–496. [Google Scholar] [CrossRef]
- Foltanova, T.; Majernik, A.; Malikova, E.; Kosirova, S. Availability and Accessibility of Orphan Medicinal Products to Patients in Slovakia in the Years 2010–2019. Front. Pharmacol. 2022, 13, 768325. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, G.; de Andrade, P.V.; Dos Santos, J.M.; do Amaral, J.L.G.; da Silva, H.C.A. Impact of a Digital Manual for Guidance on Malignant Hyperthermia: Patient Education. Orphanet J. Rare Dis. 2022, 17, 265. [Google Scholar] [CrossRef]
- Sardella, M.; Lungu, C. Evaluation of Quantitative Signal Detection in EudraVigilance for Orphan Drugs: Possible Risk of False Negatives. Ther. Adv. Drug Saf. 2019, 10, 2042098619882819. [Google Scholar] [CrossRef] [PubMed]
- Schroader, B.; Kong, S.; Anderson, S.; Williamson, T.; Sireci, A.; Shields, K. Current Status of Biomarker Testing in Historically Rare, High-Unmet-Need Tumors: Soft Tissue Sarcomas and Thyroid Cancers. Expert Rev. Anticancer. Ther. 2019, 19, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Sisk, B.A.; Kerr, A.; King, K.A. Factors Affecting Pathways to Care for Children and Adolescents with Complex Vascular Malformations: Parental Perspectives. Orphanet J. Rare Dis. 2022, 17, 271. [Google Scholar] [CrossRef] [PubMed]
- Koçkaya, G.; Atalay, S.; Oğuzhan, G.; Kurnaz, M.; Ökçün, S.; Sar Gedik, Ç.; Şaylan, M.; Şencan, N. Analysis of Patient Access to Orphan Drugs in Turkey. Orphanet J. Rare Dis. 2021, 16, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Richter, T.; Janoudi, G.; Amegatse, W.; Nester-Parr, S. Characteristics of Drugs for Ultra-Rare Diseases versus Drugs for Other Rare Diseases in HTA Submissions Made to the CADTH CDR. Orphanet J. Rare Dis. 2018, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tomeo, F.; Mariz, S.; Brunetta, A.L.; Stoyanova-Beninska, V.; Penttila, K.; Magrelli, A. Haemophilia, State of the Art and New Therapeutic Opportunities, a Regulatory Perspective. Br. J. Clin. Pharmacol. 2021, 87, 4183–4196. [Google Scholar] [CrossRef]
- Lei, X.; Dai, G.; Chen, Q.; Hu, H. Design of the Knowledge Database and Model Database for Rare Diseases. Chin. Gen. Pract. 2021, 24, 3634. [Google Scholar] [CrossRef]
- Bouwman, M.L.; Sousa, J.J.S.; Pina, M.E.T. Regulatory Issues for Orphan Medicines: A Review. Health Policy Technol. 2020, 9, 115–121. [Google Scholar] [CrossRef]
- Menon, D.; Clark, D.; Stafinski, T. Reimbursement of Drugs for Rare Diseases through the Public Healthcare System in Canada: Where Are We Now? Healthcare Policy 2015, 11, 15–32. [Google Scholar] [CrossRef]
- Lumry, W.R. Pharmacoeconomics of Orphan Disease Treatment with a Focus on Hereditary Angioedema. Immunol. Allergy Clin. N. Am. 2017, 37, 617–628. [Google Scholar] [CrossRef]
- Lam, B.L.; Leroy, B.P.; Black, G.; Ong, T.; Yoon, D.; Trzupek, K. Genetic Testing and Diagnosis of Inherited Retinal Diseases. Orphanet J. Rare Dis. 2021, 16, 1–9. [Google Scholar] [CrossRef]
- Nuijten, M.; Capri, S. Pricing of Orphan Drugs in Oncology and Rare Diseases. J. Mark. Access Health Policy 2020, 8, 1838191. [Google Scholar] [CrossRef]
- Low, Z.Y.; Farouk, I.A.; Lal, S.K. Drug Repositioning: New Approaches and Future Prospects for Life-Debilitating Diseases and the COVID-19 Pandemic Outbreak. Viruses 2020, 12, 1058. [Google Scholar] [CrossRef]
- Kakkis, E.D.; O’Donovan, M.; Cox, G.; Hayes, M.; Goodsaid, F.; Tandon, P.K.; Furlong, P.; Boynton, S.; Bozic, M.; Orfali, M.; et al. Recommendations for the Development of Rare Disease Drugs Using the Accelerated Approval Pathway and for Qualifying Biomarkers as Primary Endpoints. Orphanet J. Rare Dis. 2015, 10, 16. [Google Scholar] [CrossRef]
- Chorostowska-Wynimko, J.; Barrecheguren, M.; Ferrarotti, I.; Greulich, T.; Sandhaus, R.A.; Campos, M. New Patient-Centric Approaches to the Management of Alpha-1 Antitrypsin Deficiency. Int. J. COPD 2020, 15, 345–355. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.Y.; Yue, X.M.; Wu, J.H. Considerations on the Health Techology Assessment for Rare Diseases and Orphan Drugs in China. J. Int. Pharm. Res. 2019, 46, 666–672. [Google Scholar] [CrossRef]
- Arnold, R.J.G.; Bighash, L.; Bryón Nieto, A.; Tannus Branco de Araújo, G.; Gay-Molina, J.G.; Augustovski, F. The Role of Globalization in Drug Development and Access to Orphan Drugs: Orphan Drug Legislation in the US/EU and in Latin America. F1000Res 2015, 4, 57. [Google Scholar] [CrossRef]
- Abdallah, K.; De Boeck, K.; Dooms, M.; Simoens, S. A Comparative Analysis of Pricing and Reimbursement of Cystic Fibrosis Transmembrane Conductance Regulator Modulators in Europe. Front. Pharmacol. 2021, 12, 746710. [Google Scholar] [CrossRef] [PubMed]
- Tylki-Szymańska, A.; Almássy, Z.; Christophidou-Anastasiadou, V.; Avdjieva-Tzavella, D.; Barisic, I.; Cerkauskiene, R.; Cuturilo, G.; Djiordjevic, M.; Gucev, Z.; Hlavata, A.; et al. The Landscape of Mucopolysaccharidosis in Southern and Eastern European Countries: A Survey from 19 Specialistic Centers. Orphanet J. Rare Dis. 2022, 17, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Oro-Ayude, M.; Batalla, A.; Dávila-Pousa, C.; González-Freire, L.; Flórez, Á. Effect of Drug Compounding on Quality of Life in Patients With Genodermatoses: A Cross-Sectional Study. Actas Dermosifiliogr. 2022, 113, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Dahir, K.M.; Seefried, L.; Kishnani, P.S.; Petryk, A.; Högler, W.; Linglart, A.; Martos-Moreno, G.Á.; Ozono, K.; Fang, S.; Rockman-Greenberg, C. Clinical Profiles of Treated and Untreated Adults with Hypophosphatasia in the Global HPP Registry. Orphanet J. Rare Dis. 2022, 17, 277. [Google Scholar] [CrossRef] [PubMed]
- Magner, M.; Almássy, Z.; Gucev, Z.; Kieć-Wilk, B.; Plaiasu, V.; Tylki-Szymańska, A.; Zafeiriou, D.; Zaganas, I.; Lampe, C. Consensus Statement on Enzyme Replacement Therapy for Mucopolysaccharidosis IVA in Central and South-Eastern European Countries. Orphanet J. Rare Dis. 2022, 17, 1–10. [Google Scholar] [CrossRef]
- Laimer, M.; Pohla-Gubo, G.; Diem, A.; Prodinger, C.; Bauer, J.W.; Hintner, H. Epidermolysis Bullosa House Austria and Epidermolysis Bullosa Clinical Network: Example of a Centre of Expertise Implemented in a European Reference Network to Face the Burden of a Rare Disease. Wien. Klin. Wochenschr. 2017, 129, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Di Paolo, A.; Arrigoni, E. Generic Substitution of Orphan Drugs for the Treatment of Rare Diseases: Exploring the Potential Challenges. Drugs 2018, 78, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Jyothi, M.S.; Balamuralidhara, V.; Nagaraju, S. Regulatory Overview on Rare Diseases and Orphan Drugs in India. Int. J. Pharm. Res. 2020, 12, 1957–1965. [Google Scholar] [CrossRef]
- Mortensen, A.; Raebel, E.M.; Wiseman, S. Impact of the COVID-19 Pandemic on Access to the Cerliponase Alfa Managed Access Agreement in England for CLN2 Treatment. Orphanet J. Rare Dis. 2022, 17, 1–15. [Google Scholar] [CrossRef]
- Bang, J.S.; Lee, J.H. The National Drug Formulary Listing Process for Orphan Drugs in South Korea: Narrative Review Focused on Pricing and Reimbursement Pathways. Expert Opin. Orphan Drugs 2021, 9, 105–112. [Google Scholar] [CrossRef]
- Varghese, M.S.; Liu, C.L.; Kazi, D.S. The Price of Progress: Cost, Access, and Adoption of Novel Cardiovascular Drugs in Clinical Practice. Curr. Cardiol. Rep. 2021, 23, 1–7. [Google Scholar] [CrossRef]
- Solís, L.; Nordin, J.; Prevot, J.; Mahlaoui, N.; Sánchez-Ramón, S.; Ali, A.; Cassignol, E.; Seymour, J.W.; Pergent, M. The PID Life Index: An Interactive Tool to Measure the Status of the PID Healthcare Environment in Any given Country. Orphanet J. Rare Dis. 2022, 17, 1–8. [Google Scholar] [CrossRef]
- Jagadeesan, M.; Harini, R.R.; Chinnasami, B. Rational Prescribing Challenges in Paediatrics: A Evidence Based Review. Res. J. Pharm. Technol. 2020, 13, 5551–5556. [Google Scholar] [CrossRef]
- Kamusheva, M.; Milushewa, P. Rare Disease Patients’ Needs: An up-to-Date Analysis and Future Directions. Pharmacia 2021, 68, 763–770. [Google Scholar] [CrossRef]
- Groft, S.C.; Posada, M.; Taruscio, D. Progress, Challenges and Global Approaches to Rare Diseases. Acta Paediatr. Int. J. Paediatr. 2021, 110, 2711–2716. [Google Scholar] [CrossRef] [PubMed]
- Leyens, J.; Bender, T.T.A.; Mücke, M.; Stieber, C.; Kravchenko, D.; Dernbach, C.; Seidel, M.F. The Combined Prevalence of Classified Rare Rheumatic Diseases Is Almost Double That of Ankylosing Spondylitis. Orphanet J. Rare Dis. 2021, 16, 1–43. [Google Scholar] [CrossRef] [PubMed]
- Reardon, G. Pharmacoeconomics of Biologic Medicines and Biosimilars. Biol. Biosimilars Biobetters Introd. Pharm. Physicians Other Health Pract. 2021, 47–69. [Google Scholar] [CrossRef]
- Schey, C.; Postma, M.; Krabbe, P.; Medic, G.; Connolly, M. The Application of Multi-Criteria Decision Analysis to Inform in Resource Allocation. F1000Res 2020, 9, 445. [Google Scholar] [CrossRef]
- Campa, C.; Gallenga, C.E.; Bolletta, E.; Perri, P. The Role of Gene Therapy in the Treatment of Retinal Diseases: A Review. Curr. Gene Ther. 2017, 17, 194–213. [Google Scholar] [CrossRef]
- Guien, C.; Blandin, G.; Lahaut, P.; Sanson, B.; Nehal, K.; Rabarimeriarijaona, S.; Bernard, R.; Lévy, N.; Sacconi, S.; Béroud, C. The French National Registry of Patients with Facioscapulohumeral Muscular Dystrophy. Orphanet J. Rare Dis. 2018, 13, 318. [Google Scholar] [CrossRef]
- Encina, G.; Castillo-Laborde, C.; Lecaros, J.A.; Dubois-Camacho, K.; Calderón, J.F.; Aguilera, X.; Klein, A.D.; Repetto, G.M. Rare Diseases in Chile: Challenges and Recommendations in Universal Health Coverage Context. Orphanet J. Rare Dis. 2019, 14, 1–8. [Google Scholar] [CrossRef]
- Mulberg, A.E.; Bucci-Rechtweg, C.; Giuliano, J.; Jacoby, D.; Johnson, F.K.; Liu, Q.; Marsden, D.; McGoohan, S.; Nelson, R.; Patel, N.; et al. Regulatory Strategies for Rare Diseases under Current Global Regulatory Statutes: A Discussion with Stakeholders. Orphanet J. Rare Dis. 2019, 14, 1–10. [Google Scholar] [CrossRef]
- Al-Attar, M. TRAPPED—An Insight into Two Sisters’ Struggle to Access Treatment for a Rare Genetic Disease. Orphanet J. Rare Dis. 2018, 13, 1–3. [Google Scholar] [CrossRef]
- Barak, A.; Shankar Nandi, J. Orphan Drugs: Pricing, Reimbursement and Patient Access. Int. J. Pharm. Healthc Mark. 2011, 5, 299–317. [Google Scholar] [CrossRef]
- Giannetta, N.; Cianciulli, A.; Dionisi, S.; Figura, M.; Di Simone, E.; Di Muzio, M. Orphan Drugs: An European Production, Research and Development Policies. G. Ital. Di Farm. Clin. 2019, 33, 29–34. [Google Scholar] [CrossRef]
- Weinstein, N.; Martin, M.; Campbell, R. Orphan Drugs In The Uk, Do They Meet The Nice Highly Specialised Technology Threshold? Value Health 2017, 20, A660. [Google Scholar] [CrossRef]
- Friedlander, L.; Berdal, A.; Boizeau, P.; Licht, B.A.; Manière, M.C.; Picard, A.; Azzis, O.; Vazquez, M.P.; Alberti, C.; Molla, M.D.L.D. Oral Health Related Quality of Life of Children and Adolescents Affected by Rare Orofacial Diseases: A Questionnaire-Based Cohort Study. Orphanet J. Rare Dis. 2019, 14, 124. [Google Scholar] [CrossRef]
- Shih, D.Y.; Jarrett, J. Orphan Drug Policy: Approaches To Market Access In Multiple Countries. Value Health 2014, 17, A539. [Google Scholar] [CrossRef]
- Ozbek, U. Molecular Analysis of Consanguineous Rare Syndromes in Turkey. J. Biotechnol. 2014, 185, S16. [Google Scholar] [CrossRef]
- Painous, C.; van Os, N.J.H.; Delamarre, A.; Michailoviene, I.; Marti, M.J.; van de Warrenburg, B.P.; Meissner, W.G.; Utkus, A.; Reinhard, C.; Graessner, H.; et al. Management of Rare Movement Disorders in Europe: Outcome of Surveys of the European Reference Network for Rare Neurological Diseases. Eur. J. Neurol. 2020, 27, 1493–1500. [Google Scholar] [CrossRef]
- Bax, B.E. Mitochondrial Neurogastrointestinal Encephalomyopathy: Approaches to Diagnosis and Treatment. J. Transl. Genet. Genom. 2020, 4, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Margaretos, N.M.; Bawa, K.; Engmann, N.J.; Chambers, J.D. Patients’ Access to Rare Neuromuscular Disease Therapies Varies across US Private Insurers. Orphanet J. Rare Dis. 2022, 17, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mikami, K.; Sturdy, S. Patient Organization Involvement and the Challenge of Securing Access to Treatments for Rare Diseases: Report of a Policy Engagement Workshop. Res. Involv Engagem. 2017, 3, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Macaulay, R.; Liu, L.W.; Turkstra, E. PRO112 THE HST TEST: GOOD, BETTER, BEST? Value Health 2019, 22, S861–S862. [Google Scholar] [CrossRef]
- Boncheva, E.; Benisheva, T.; Cherneva, D. PRO74 Is IT Possible to Accelerate the Access of Patients with RARE Diseases to New Therapy—the Case with IPF Medicines. Value Health 2020, 23, S702. [Google Scholar] [CrossRef]
- Kennedy, C.; Janwar, J.; McBride, M.; Connolly, E.; Tilson, L.; Barry, M. Pns164 Pharmacoeconomic Assessment And Drug Costs of Treatments for Cancer and Rare Diseases in Ireland. Value Health 2019, 22, S788–S789. [Google Scholar] [CrossRef]
- Godinas, L.; Iyer, K.; Meszaros, G.; Quarck, R.; Escribano-Subias, P.; Vonk Noordegraaf, A.; Jansa, P.; D’Alto, M.; Luknar, M.; Milutinov Ilic, S.; et al. PH CARE COVID Survey: An International Patient Survey on the Care for Pulmonary Hypertension Patients during the Early Phase of the COVID-19 Pandemic. Orphanet J. Rare Dis. 2021, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.Y.L.; Chan, V.K.Y.; Olsson, S.; Fan, M.; Jit, M.; Gong, M.; Zhang, S.; Ge, M.; Pathadka, S.; Chui, C.S.L.; et al. Orphan Drugs—Access and Unmet Needs in 194 Countries and Six Regions: A Comprehensive Policy Review with Content Analysis. Lancet 2019, 394, S72. [Google Scholar] [CrossRef]
- Aldosari, M.H.; den Hartog, M.; Ganizada, H.; Evers, M.J.W.; Mastrobattista, E.; Schellekens, H. Feasibility Study for Bedside Production of Recombinant Human Acid α-Glucosidase: Technical and Financial Considerations. Curr. Pharm. Biotechnol. 2020, 21, 467–479. [Google Scholar] [CrossRef]
- Hernberg-Ståhl, E.; Reljanovic, M. Orphan Drugs: Understanding the Rare Disease Market and Its Dynamics; Elsevier: Amsterdam, The Netherlands, 2013; pp. 1–317. [Google Scholar] [CrossRef]
- Gahl, W.A.; Wong-Rieger, D.; Hivert, V.; Yang, R.; Zanello, G.; Groft, S. Essential List of Medicinal Products for Rare Diseases: Recommendations from the IRDiRC Rare Disease Treatment Access Working Group. Orphanet J. Rare Dis. 2021, 16, 1–11. [Google Scholar] [CrossRef]
- Stevens, B.; Kenny, T.; Thomas, S.; Morrison, A.; Jarrett, J.; Jain, M. Elosulfase Alfa in the Treatment of Mucopolysaccharidosis Type IVA: Insights from the First Managed Access Agreement. Orphanet J. Rare Dis. 2021, 16, 1–8. [Google Scholar] [CrossRef]
- McCabe, C.; Edlin, R.; Round, J. Economic Considerations in the Provision of Treatments for Rare Diseases. Adv. Exp. Med. Biol. 2010, 686, 211–222. [Google Scholar] [CrossRef]
- Costa, E.; Schieppati, A.; Luzzatto, L.; Remuzzi, G. Drugs for Rare Diseases: The Blessing of Being Orphans. Recenti Prog. Med. 2019, 110, 221–229. [Google Scholar] [CrossRef]
- Qiu, T.; Wang, Y.; Dabbous, M.; Hanna, E.; Han, R.; Liang, S.; Toumi, M. Current State of Developing Advanced Therapies for Rare Diseases in the European Union. Expert Opin. Orphan Drugs 2020, 8, 417–429. [Google Scholar] [CrossRef]
- Jarosławski, S.; Auquier, P.; Borissov, B.; Dussart, C.; Toumi, M. Low Rates of Patient-Reported Outcome Claims for Orphan Drugs Approved by the Us Food and Drug Administration. J. Mark. Access Health Policy 2018, 6, 1433426. [Google Scholar] [CrossRef] [PubMed]
- Cleary, M.; Davison, J.; Gould, R.; Geberhiwot, T.; Hughes, D.; Mercer, J.; Morrison, A.; Murphy, E.; Santra, S.; Jarrett, J.; et al. Impact of Long-Term Elosulfase Alfa Treatment on Clinical and Patient-Reported Outcomes in Patients with Mucopolysaccharidosis Type IVA: Results from a Managed Access Agreement in England. Orphanet J. Rare Dis. 2021, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Ramaswami, U.; Muenzer, J.; Giugliani, R.; Ullrich, K.; Collin-Histed, T.; Panahloo, Z.; Wellhoefer, H.; Frader, J. A Charitable Access Program for Patients with Lysosomal Storage Disorders in Underserved Communities Worldwide. Orphanet J. Rare Dis. 2021, 16, 8. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.; Schlander, M. Health Technology Assessment (HTA) and Economic Evaluation: Efficiency or Fairness First. J. Mark. Access Health Policy 2019, 7, 1557981. [Google Scholar] [CrossRef]
- Café, A.; Carvalho, M.; Crato, M.; Faria, M.; Kjollerstrom, P.; Oliveira, C.; Pinto, P.R.; Salvado, R.; Dos Santos, A.A.; Silva, C. Haemophilia A: Health and Economic Burden of a Rare Disease in Portugal. Orphanet J. Rare Dis. 2019, 14, 1–11. [Google Scholar] [CrossRef]
- Petrongonas, M.; Rinaki, E.; Fragiadaki, M.; Tzimis, L. GM-005 Orphan Drugs’ Pharmacoeconomic Data and the Impact on a County Hospital’s Budget. Eur. J. Hosp. Pharm. 2017, 24 (Suppl. S1), A159.2–A161.2. [Google Scholar] [CrossRef]
- Kaufmann, P.; Pariser, A.R.; Austin, C. From Scientific Discovery to Treatments for Rare Diseases—The View from the National Center for Advancing Translational Sciences—Office of Rare Diseases Research. Orphanet J. Rare Dis. 2018, 13, 196. [Google Scholar] [CrossRef]
- Kaiser, K.; Yount, S.E.; Martens, C.E.; Webster, K.A.; Shaunfield, S.; Sparling, A.; Peipert, J.D.; Cella, D.; Rottinghaus, S.T.; Donato, B.M.K.; et al. Assessing Preferences for Rare Disease Treatment: Qualitative Development of the Paroxysmal Nocturnal Hemoglobinuria Patient Preference Questionnaire (PNH-PPQ©). Patient Prefer. Adherence 2020, 14, 705–715. [Google Scholar] [CrossRef]
- Tosi, L.L.; Floor, M.K.; Dollar, C.M.; Gillies, A.P.; Lee, B.; Nagamani, S.C.S.; Rauch, F.; Glorieux, F.; Retrouvey, J.M.; Esposito, P.; et al. Assessing Disease Experience across the Life Span for Individuals with Osteogenesis Imperfecta: Challenges and Opportunities for Patient-Reported Outcomes (PROs) Measurement: A Pilot Study. Orphanet J. Rare Dis. 2019, 14, 1–12. [Google Scholar] [CrossRef]
- Badia, X.; Gil, A.; Poveda-Andrés, J.L.; Shepherd, J.; Tort, M. Analysing Criteria for Price and Reimbursement of Orphan Drugs in Spain. Farm. Hosp. 2019, 43, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Atalaia, A.; Thompson, R.; Corvo, A.; Carmody, L.; Piscia, D.; Matalonga, L.; Macaya, A.; Lochmuller, A.; Fontaine, B.; Zurek, B.; et al. A Guide to Writing Systematic Reviews of Rare Disease Treatments to Generate FAIR-Compliant Datasets: Building a Treatabolome. Orphanet J. Rare Dis. 2020, 15, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Krajnović, D.; Arsić, J.; Tasić, L.; Petrova, G.; Milijić, S. Access To Orphan Drugs: A Cross Country Comparison Of Legislative Approach Among Serbia, Croati A And Macedonia. Acta Med. Median. 2018, 57, 43–51. [Google Scholar] [CrossRef]
- Young, K.E.; Soussi, I.; Hemels, M.; Toumi, M. A Comparative Study of Orphan Drug Prices in Europe. J. Mark. Access Health Policy 2017, 5, 1297886. [Google Scholar] [CrossRef] [PubMed]
- Horgan, D.; Moss, B.; Boccia, S.; Genuardi, M.; Gajewski, M.; Capurso, G.; Fenaux, P.; Gulbis, B.; Pellegrini, M.; Mañú Pereira, M.d.M.; et al. Time for Change? The Why, What and How of Promoting Innovation to Tackle Rare Diseases—Is It Time to Update the EU’s Orphan Regulation? And If so, What Should Be Changed? Biomed Hub. 2020, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.; Redmond, S. To HTA or Not to HTA: Identifying the Factors Influencing the Rapid Review Outcome in Ireland. Value Health 2019, 22, 385–390. [Google Scholar] [CrossRef]
- Miyamoto, B.E.; Kakkis, E.D. The Potential Investment Impact of Improved Access to Accelerated Approval on the Development of Treatments for Low Prevalence Rare Diseases. Orphanet J. Rare Dis. 2011, 6, 49. [Google Scholar] [CrossRef]
- Foteeva, A.V.; Rostova, N.B.; Isupova, A.D.; Gerbergagen, E.S. The Orphan Preparations: International Approaches and National Regulation. Probl. Sotsialnoi Gig Zdravookhranenniiai Istor Med. 2021, 29, 1490–1497. [Google Scholar] [CrossRef]
- Bojakowski, S.; Spoors, J. The Funding of Orphan Medicines in the UK. Br. J. Health Care Manag. 2014, 20, 384–391. [Google Scholar] [CrossRef]
- Young, K.E.; Soussi, I.; Toumi, M. The Perverse Impact of External Reference Pricing (ERP): A Comparison of Orphan Drugs Affordability in 12 European Countries. A Call for Policy Change. J. Mark. Access Health Policy 2017, 5, 1369817. [Google Scholar] [CrossRef]
- Lampe, C.; Dionisi-Vici, C.; Bellettato, C.M.; Paneghetti, L.; van Lingen, C.; Bond, S.; Brown, C.; Finglas, A.; Francisco, R.; Sestini, S.; et al. The Impact of COVID-19 on Rare Metabolic Patients and Healthcare Providers: Results from Two MetabERN Surveys. Orphanet J. Rare Dis. 2020, 15, 341. [Google Scholar] [CrossRef] [PubMed]
- Kanavos, P.; Nicod, E. What Is Wrong with Orphan Drug Policies? Suggestions for Ways Forward. Value Health 2012, 15, 1182–1184. [Google Scholar] [CrossRef]
- Murphy, S.M.; Puwanant, A.; Griggs, R.C. Unintended Effects of Orphan Product Designation for Rare Neurological Diseases. Ann. Neurol. 2012, 72, 481–490. [Google Scholar] [CrossRef] [PubMed]
- de Dios García-Díaz, J.; López-Rodríguez, M.; Morales-Conejo, M.; Riera-Mestre, A. Understanding the Ecosystem of Patients with Lysosomal Storage Diseases in Spain: A Qualitative Research with Patients and Health Care Professionals. Orphanet J. Rare Dis. 2022, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Berdud, M.; Drummond, M.; Towse, A. Establishing a Reasonable Price for an Orphan Drug. Cost Eff. Resour. Alloc. 2020, 18, 31. [Google Scholar] [CrossRef]
- Luzzatto, L.; Makani, J. Treating Rare Diseases in Africa: The Drugs Exist but the Need Is Unmet. Front. Pharmacol. 2022, 12, 770640. [Google Scholar] [CrossRef]
- Cousyn, C.; Bouchard, K.; Gaboury, S.; Bouchard, B. Towards Using Scientific Publications to Automatically Extract Information on Rare Diseases. Mob. Netw. Appl. 2020, 25, 953–960. [Google Scholar] [CrossRef]
- Jarosławski, S.; Auquier, P.; Borissov, B.; Dussart, C.; Toumi, M. Patient-Reported Outcome Claims in European and United States Orphan Drug Approvals. J. Mark. Access Health Policy 2018, 6, 1542920. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, K.; Vernon, M.K.; Patrick, D.L.; Perfetto, E.; Nestler-Parr, S.; Burke, L. Patient-Reported Outcome and Observer-Reported Outcome Assessment in Rare Disease Clinical Trials: An ISPOR COA Emerging Good Practices Task Force Report. Value Health 2017, 20, 838–855. [Google Scholar] [CrossRef] [PubMed]
- Groft, S.C.; Posada De La Paz, M. Rare Diseases: Joining Mainstream Research and Treatment Based on Reliable Epidemiological Data. Adv. Exp. Med. Biol. 2017, 1031, 3–21. [Google Scholar] [CrossRef]
- Hu, S.L. Characteristics of Drug Policy and Pharmacoeconomics Study on Rare Diseases. J. Int. Pharm. Res. 2019, 46, 652–658. [Google Scholar] [CrossRef]
- Tingley, K.; Coyle, D.; Graham, I.D.; Chakraborty, P.; Wilson, K.; Potter, B.K. Stakeholder Perspectives on Clinical Research Related to Therapies for Rare Diseases: Therapeutic Misconception and the Value of Research. Orphanet J. Rare Dis. 2021, 16, 1–11. [Google Scholar] [CrossRef]
- Gulliver, W.; Landells, I.D.R.; Morgan, D.; Pirzada, S. Hidradenitis Suppurativa: A Novel Model of Care and an Integrative Strategy to Adopt an Orphan Disease. J. Cutan. Med. Surg. 2018, 22, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Chung, R.Y.N.; Chan, R.H.W.; Gong, S.; Xu, R.H. Why Is Misdiagnosis More Likely among Some People with Rare Diseases than Others? Insights from a Population-Based Cross-Sectional Study in China. Orphanet J. Rare Dis. 2020, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; An, Z.; Ming, Y.; Guo, Y.; Li, W.; Liang, Y.; Guo, D.; Li, X.; Tai, J.; Chen, G.; et al. ERAM: Encyclopedia of Rare Disease Annotations for Precision Medicine. Nucleic Acids Res. 2018, 46, D937–D943. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.; Goodson, N.; Wicks, P.; Reites, J. What Role Can Decentralized Trial Designs Play to Improve Rare Disease Studies? Orphanet J. Rare Dis. 2022, 17, 240. [Google Scholar] [CrossRef]
- Balasopoulou, A.; Κokkinos, P.; Pagoulatos, D.; Plotas, P.; Makri, O.E.; Georgakopoulos, C.D.; Vantarakis, A.; Li, Y.; Liu, J.J.; Qi, P.; et al. Symposium Recent Advances and Challenges in the Management of Retinoblastoma Globe—Saving Treatments. BMC Ophthalmol. 2017, 17, 1. [Google Scholar] [CrossRef]
- O’Connor, O.A.; Marchi, E.; Volinn, W.; Kim, W.S. Case Match Control Analysis of Propel Reveals Survival Advantage for Patients with Relapsed/Refractory (R/R) Peripheral T-Cell Lymphoma (PTCL) Treated with Pralatrexate. Blood 2016, 128, 4149. [Google Scholar] [CrossRef]
- Fidanci, B.; Ozen, S.; Acikel, C.; Fidanci, K.; Yildiz, D.; Basbozkurt, G.; Ravelli, A.; Demirkaya, E. THU0473-HPR Living with Childhood Vasculitis; a Qualitative Study. Ann. Rheum. Dis. 2013, 71, 738. [Google Scholar] [CrossRef]
- Reddy, D.S. Clinical Overview of Innovative New Drug Approvals in 2017. Int. J. Pharm. Sci. Nanotechnol. 2018, 11, 4225–4230. [Google Scholar] [CrossRef]
- Martínez-Rodríguez, I.; Uría, E.; Pomares, E.; Cuesta, M. PHP34—TIME ANALYSIS OF THE DRUG APPROVAL PROCESS IN EUROPE. Value Health 2018, 21, S156. [Google Scholar] [CrossRef]
- Ogden, K.; Thompson, J.; Halfpenny, N.; Scott, D. A Conceptual Search Filter to Identify Real-World Evidence. Value Health 2015, 18, A728. [Google Scholar] [CrossRef]
- O’Connor, O.A.; Marchi, E.; Kim, W.S. Case match control analysis of propel reveals a survival advantage for patients with relapsed PTCL receiving pralatrexate: A novel approach to benchmark drugs in rare diseases. Hematol. Oncol. 2017, 35, 250. [Google Scholar] [CrossRef]
- Valinotti, G.; Scaldaferri, M.; Tonelli, M.; Crosasso, P.; Chiappetta, M.; Cattel, F. 4CPS-215 Access to ‘Fondo AIFA 5%’ as an Instrument Supporting the Sustainability in a Shared Clinical Management of Rare and Difficult-to-Treat Diseases. Eur. J. Hosp. Pharm. 2019, 26, A169–A170. [Google Scholar] [CrossRef]
- Wang, G.; Macaulay, R. The Cost-Effectiveness of Manufacturer Fees for Htas: Are They Promoting or Hindering Innovation? Value Health 2017, 20, A696. [Google Scholar] [CrossRef]
- Nayroles, G.; Gabriel, S.; Tolley, K.; Espin, J.; Toumi, M.; Kornfeld, A.; Frybourg, S. Extension of Indication With Mature Products: Toward More Incentives Rewarding Innovation? Value Health 2015, 18, A570. [Google Scholar] [CrossRef]
- Zhuravleva, M.V.; Lebedeva, A.Y. Organization of Pharmacological Support of Patients with Rare Diseases in Moscow as Exemplified by Pulmonary Arterial Hypertension. Med. Counc. 2018, 24–31. [Google Scholar] [CrossRef]
- Flostrand, S.; Rodriguez, I.; Maddox, B.; Finch, L.; Belulaj, S.; Gould, A. Is the Orphanage Filling Up? Projecting the Growth and Budget Impact of Orphan Drugs in Europe. Value Health 2016, 19, A599. [Google Scholar] [CrossRef]
- Mwamburi, M.; Nanavaty, M.; Gala, S.; Nyandege, A.; Ramesh, V. Special Considerations and Patient Access Schemes for Oncology by Health Technology Assessment (HTA) Bodies: Systematic Evaluation in 25 Countries. Value Health 2016, 19, A761. [Google Scholar] [CrossRef]
- Mardiguian, S.; Stefanidou, M.; Sheppard, F. Trends and Key Decision Drivers For Rejecting An Orphan Drug Submission Across Five Different HTA Agencies. Value Health 2014, 17, A438. [Google Scholar] [CrossRef]
- Prada, M.; Bertozzi, C.; Proietti, B.; Urbinati, D. Italian Law 326/2003 for The Reimbursement of Orphan and Life Saving Drugs Awaiting Market Entry: Approvals, Rejections And Methods In Aifa’s Evaluation Process Between January 2013 And May 2015. Value Health 2015, 18, A544. [Google Scholar] [CrossRef]
- Almutairi, R.D.; Alghamdi, A.A.; Felemban, D.F.; Seoane-Vazquez, E.; Rodriguez-Monguio, R.; Szeinbach, S.L. Analysis of Orphan Drug Designations and Approvals in the United States and the European Union. Value Health 2013, 16, A124. [Google Scholar] [CrossRef]
- Zhang, A.; Weisse, S.; Chen, X. Health Technology Assessment (HTA) for Orphan Drugs in Cost-Effectiveness (CE) Markets: Current Development and Future Trends. Value Health 2016, 19, A601. [Google Scholar] [CrossRef]
- Allen, G.; Hall, A.; Hanman, K.; Le Fevre, R.; Griffiths, A. Do EU5 Countries with Favourable Healthcare Expenditure and Reimbursement Indicators Have Better Patient-Reported Access to Treatments for Rare Diseases? Value Health 2017, 20, A409–A410. [Google Scholar] [CrossRef]
- Marshall, J.; Clayton, J. Regional Variations In Appraisal And Uptake Of New Treatments For Ultra Rare Diseases In The UK: A Case Study Of Ataluren For Nonsense Mutation Duchenne Muscular Dystrophy (NMDMD). Value Health 2017, 20, A562–A563. [Google Scholar] [CrossRef]
- Nicolodi, C. Orphan Drug Designation in Europe-Procedural Guidance and Challenges. Int. J. Drug Regul. Aff. 2019, 7, 1–7. [Google Scholar] [CrossRef]
- Skeldon, G.; Adkins, E.; Ayre, S.; Langham, S.; Nicholson, L. PRO95 Patient Engagement Process in Rare Disease: How Does It Differ between Countries? Value Health 2020, 23, S706. [Google Scholar] [CrossRef]
- Macaulay, R. Encouraging Orphan Designation for New Ebola Treatments—Could This Do More Harm than Good? Value Health 2015, 18, A243–A244. [Google Scholar] [CrossRef]
- Dastan, S.; Kalieva, S.; Yesbatyrova, L.; Myasnikova, Z.; Kayupova, G. Comparative Analysis of the Availability of Medicines for Patients with Orphan Diseases in the Republic of Kazakhstan for 2015 and 2020. J. Health Dev. 2021, 1, 54–60. [Google Scholar] [CrossRef]
- Nicholson, L.; Fountain, D.; Longworth, L.; Adkins, E. How Will Proposed Changes To The Nice Highly Specialised Technology Evaluation Process Impact Patient Access To Innovative Drugs for Rare Diseases? Value Health 2017, 20, A694. [Google Scholar] [CrossRef]
- Palmer, M.; Hughes, D.A. Orphan Drug Legislations: Heyday or Had Their Day? Value Health 2013, 16, A491. [Google Scholar] [CrossRef]
- Office, E. Letter of Editor. J. Rare Dis. Orphan Drugs 2020, 1. [Google Scholar] [CrossRef]
- Koury, C.D.N.; Silva, M.T. Rapid Economic Evaluation Review for Rare Diseases Treatments—The Case of Pegvisomant for Acromegaly. Value Health 2013, 16, A119. [Google Scholar] [CrossRef]
- Tsiantou, V.; Mylona, K.; Karampli, E.; Boubouchairopoulou, N.; Kyriopoulos, I.I.; Athanasakis, K.; Gabriel, E.; Makridaki, D.; Kyriopoulos, J. Access To Orphan Drugs In Greece During Economic Crisis. Value Health 2014, 17, A541. [Google Scholar] [CrossRef] [PubMed]
- Macaulay, R.; Roibu, C.; Ivanova, H.; Campbell, J. PSY136—A comparison of p&r requirements for orphan drugs in 25 markets. Value Health 2018, 21, S459. [Google Scholar] [CrossRef]
- Franceschini, M.; Soon, J.; Han, Y. PRO99 Real-World Application of Multiple Criteria Decision Analysis (MCDA) for Evaluating Orphan Drugs (ODS) in Europe. Value Health 2020, 23, S707. [Google Scholar] [CrossRef]
- Pavlović, N.; Stanimirov, B.; Stojančević, M.; Paut Kusturica, M.; Goloc orbin-Kon, S.; Mikov, M. Low Availability of Orphan Medicines in Serbia. Value Health 2013, 16, A123–A124. [Google Scholar] [CrossRef]
- Montilva, J.; Xue, Y.; Degun, R. Impact of National Orphan Drug Policy and Reimbursement Mechanisms Over the Implementation of Managed Entry Agreements in Select Asia-Pacific Countries. Value Health 2016, 19, A884. [Google Scholar] [CrossRef]
- Sburlan, E.A.; Voinea, L.-M.; Alexandrescu, C.; Istrate, S.; Iancu, R.; Pirvulescu, R.; Geamanu, A.; Ghita, M.; Ungureanu, E.; Radu, C. Rare Ophthalmology Diseases. Rom. J. Ophthalmol. 2019, 63, 10–14. [Google Scholar] [CrossRef]
- Mwamburi, M.; Nanavaty, M.; Gala, S.; Nyandege, A. Ramesh Provisions and Special Considerations for Rare Diseases / Orphan Drugs by Health Technology Assessment (HTA) Bodies: Systematic Evaluation in 25 Countries. Value Health 2016, 19, A602. [Google Scholar] [CrossRef]
- Hutchings, A.; Palaska, C.; Schey, C.; Pericleous, L.; Petersen, J. Payer Assessment and Reimbursement Policy for Rare Diseases: A Review of the Literature. Value Health 2013, 16, A391. [Google Scholar] [CrossRef]
- Macaulay, R.; Wang, G.; Fernandez Dacosta, R. PRO41 The Omar Era—Fate Of Orphan Designations In Europe. Value Health 2019, 22, S342–S343. [Google Scholar] [CrossRef]
- Paz, S.; Torrent, J.; Poveda, J.; Perez, J.; Moreno, J.; Martin, A.; Gonzalez, L.; Cruz, J.; Comellas, M.; Abaitua, I.; et al. Experts Consensus on The Future of Rare Diseases Care and Orphan Drugs Access In Spain: A Delphi Study. Value Health 2015, 18, A679. [Google Scholar] [CrossRef]
- Wang, G.; Macaulay, R. Orphan Legislation—Leave No One Behind? Value Health 2018, 21, S260. [Google Scholar] [CrossRef]
- Lise Aagaard, L.; Kristensen, K. Access to Cross-Border Health Care Services for Patients with Rare Diseases in the European Union. Orphan Drugs Res. Rev. 2014, 4, 39. [Google Scholar] [CrossRef]
- Lee, C.; Proudfoot, E.; O’Leary, B. Access To Medicines For Rare Diseases In Australia: The Current Climate For Reimbursement. Value Health 2017, 20, A567–A568. [Google Scholar] [CrossRef]
- Dodman, S.; Iheanacho, I.; Koufopoulou, M. PRO67 Does Society Support The Prioritisation Of High-Cost Treatments For Rare Diseases? A Systematic Literature Review. Value Health 2020, 23, S701–S702. [Google Scholar] [CrossRef]
- Schey, C.; Irwin, J.; Teneishvili, M.; Krabbe, P.F.M.; Connolly, M. Assessing The Relationship Between Individual Attributes Identified In Review Of Multi-Criteria Decision Analysis (MCDA) Of Rare Diseases And Annual Treatment Costs In Rare Endocrine Disorders. Value Health 2014, 17, A562. [Google Scholar] [CrossRef] [PubMed]
- Mennezein, L.; Avot, D.; Laigle, V. Orphan Drugs In France: Key Market Access Incentives. Value Health 2017, 20, A565. [Google Scholar] [CrossRef]
- Garcia Sanchez, J.; Sattar, S.; Hill, C. Review of Health Technology Assessment (HTA) Requirements for Rare Diseases Across European Countries. Value Health 2016, 19, A491. [Google Scholar] [CrossRef]
- Shah, R.R. Regulatory Framework for the Treatment of Orphan Diseases. In Fabry Disease: Perspectives from 5 Years of FOS; Oxford PharmaGenesis: Oxford, UK, 2006. [Google Scholar]
- Nagore Induráin, C.; Lacalle, E.; Arteche, L. El Farmacéutico En El Contexto de Las Enfermedades Raras y Los Medicamentos Huérfanos. An. Sist. Sanit. Navar. 2008, 31, 127–143. [Google Scholar] [CrossRef] [PubMed]
- Deegan, P.B.; Cox, T.M. Imiglucerase in the Treatment of Gaucher Disease: A History and Perspective. Drug Des Devel Ther 2012, 6, 81–106. [Google Scholar] [CrossRef] [PubMed]
- Coles, S.; Haire, K.; Kenny, T.; Jessop, E.G. Monitoring Access to Nationally Commissioned Services in England. Orphanet J. Rare Dis. 2012, 7, 85. [Google Scholar] [CrossRef] [PubMed]
- Colombatti, R.; Perrotta, S.; Samperi, P.; Casale, M.; Masera, N.; Palazzi, G.; Sainati, L.; Russo, G. Organizing National Responses for Rare Blood Disorders: The Italian Experience with Sickle Cell Disease in Childhood. Orphanet J. Rare Dis. 2013, 8, 169. [Google Scholar] [CrossRef]
- Žnidar, I.; Collin-Histed, T.; Niemeyer, P.; Parkkinen, J.; Lauridsen, A.G.; Zariņa, S.; Cohen, Y.; Manuel, J. The European Gaucher Alliance: A Survey of Member Patient Organisations’ Activities, Healthcare Environments and Concerns. Orphanet J. Rare Dis. 2014, 9, 1–14. [Google Scholar] [CrossRef]
- Schreiber-Katz, O.; Klug, C.; Thiele, S.; Schorling, E.; Zowe, J.; Reilich, P.; Nagels, K.H.; Walter, M.C. Comparative Cost of Illness Analysis and Assessment of Health Care Burden of Duchenne and Becker Muscular Dystrophies in Germany. Orphanet J. Rare Dis. 2014, 9, 210. [Google Scholar] [CrossRef]
- Bosch, A.M.; Burlina, A.; Cunningham, A.; Bettiol, E.; Moreau-Stucker, F.; Koledova, E.; Benmedjahed, K.; Regnault, A. Assessment of the Impact of Phenylketonuria and Its Treatment on Quality of Life of Patients and Parents from Seven European Countries. Orphanet J. Rare Dis. 2015, 10, 80. [Google Scholar] [CrossRef]
- Van Groenendael, S.; Giacovazzi, L.; Davison, F.; Holtkemper, O.; Huang, Z.; Wang, Q.; Parkinson, K.; Barrett, T.; Geberhiwot, T. High Quality, Patient Centred and Coordinated Care for Alstrom Syndrome: A Model of Care for an Ultra-Rare Disease Rare Systemic Diseases. Orphanet J. Rare Dis. 2015, 10, 149. [Google Scholar] [CrossRef] [PubMed]
- Garau, R. The Medical Experience of a Patient with a Rare Disease and Her Family. Orphanet J. Rare Dis. 2016, 11, 19. [Google Scholar] [CrossRef]
- Dalal, P.G. The project OrphanAnesthesia is internationally oriented. Thus all recommendations will be published in English. Anasthesiol. Intensivmed. 2019, 60, S26–S34. [Google Scholar]
- Wong-Rieger, D.; Rieger, F. Health Policies for Orphan Diseases: International Comparison of Regulatory, Reimbursement and Health Services Policies. Rare Dis. Age Health 2.0 2014, 4, 267–277. [Google Scholar] [CrossRef]
- Maresova, P.; Mohelska, H.; Kuca, K. Cooperation Policy of Rare Diseases in the European Union. 5th Iceepsy Int. Conf. Educ. Educ. Psychol. 2015, 171, 1302–1308. [Google Scholar] [CrossRef]
- Kogushi, K.; Ogawa, T.; Ikeda, S. An Impact Analysis of the Implementation of Health Technology Assessment for New Treatment of Orphan Diseases in Japan. Expert Rev. Pharm. Outcomes Res. 2020, 20, 455–471. [Google Scholar] [CrossRef] [PubMed]
- Pasceri, E. Analyzing Rare Diseases Terms in Biomedical Terminologies. Ital. J. Libr. Inf. Sci. 2012, 3, 1–15. [Google Scholar] [CrossRef]
- Bannister, J.B. Regulating rare disease: Safely facilitating access to orphan drugs. Fordham Law Rev. 2018, 86, 1889–1921. [Google Scholar]
- Xoxi, E.; Facey, K.M.; Cicchetti, A. The Evolution of AIFA Registries to Support Managed Entry Agreements for Orphan Medicinal Products in Italy. Front. Pharmacol. 2021, 12, 699466. [Google Scholar] [CrossRef]
- Mattingly, T.J.; Simoni-Wastila, L. Patient-Centered Drug Approval: The Role of Patient Advocacy in the Drug Approval Process. J. Manag. Care Spec. Pharm. 2017, 23, 1078–1082. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, I.A. Medical care to help improve the quality of life for children with rare diseases and their families. Rev. De Educ. Inclusiva 2014, 7, 14–32. [Google Scholar]
- Künnemann, K. 10th World Orphan Drug Congress (WODC) (November 12-14, 2019-Barcelona, Spain). Drugs Today 2019, 55, 753–758. [Google Scholar] [CrossRef]
- Picavet, E.; Annemans, L.; Cleemput, I.; Cassiman, D.; Simoens, S. Market Uptake of Orphan Drugs—A European Analysis. J. Clin. Pharm. Ther. 2012, 37, 664–667. [Google Scholar] [CrossRef]
- Iskrov, G.; Miteva-Katrandzhieva, T.; Stefanov, R. Health Technology Assessment and Appraisal of Therapies for Rare Diseases. Rare Dis. Epidemiol. Update Overv. 2nd Ed. 2017, 1031, 221–231. [Google Scholar] [CrossRef]
- Nagore, C.; Lacalle, E.; Arteche, L. The pharmacist, rare diseases and orphan medicines. An. Sist. Sanit. Navar. 2008, 31, 127–143. [Google Scholar]
- Ho, L.S.; Zhang, T.L.; Kwok, T.C.T.; Wat, K.P.; Lai, F.T.T.; Li, S.C. Financing Orphan Drugs Through a Blockchain-Supported Insurance Model. Front. Blockchain 2022, 5, 818807. [Google Scholar] [CrossRef]
- Ankit, T.; Shrikalp, D.; Maitreyi, Z.; Kumar, J.P.; Kiran, K. Transition of Pharmaceutical Regulations: The New Regulatory Era after Brexit. J. Pharm. Res.Int. 2021, 33, 804–817. [Google Scholar] [CrossRef]
- Sarpatwari, A.; Beall, R.F.; Abdurrob, A.; He, M.D.; Kesselheim, A.S. Evaluating The Impact Of The Orphan Drug Act’s Seven-Year Market Exclusivity Period. Health Aff. 2018, 37, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Yamoah, L.; Dragojlovic, N.; Smith, A.; Lynd, L.D.; Marra, C.A. Evaluating New Zealanders’ Values for Drug Coverage Decision Making: Trade-Offs between Treatments for Rare and Common Conditions. Pharmacoeconomics 2021, 39, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Dupont, A.G.; Van Wilder, P.B. Access to Orphan Drugs despite Poor Quality of Clinical Evidence. Br. J. Clin. Pharmacol. 2011, 71, 488–496. [Google Scholar] [CrossRef]
- Lee, B.; Bae, E.Y.; Bae, S.; Choi, H.J.; Son, K.B.; Lee, Y.S.; Jang, S.; Lee, T.J. How Can We Improve Patients’ Access to New Drugs under Uncertainties? South Korea’s Experience with Risk Sharing Arrangements. BMC Health Serv. Res. 2021, 21, 967. [Google Scholar] [CrossRef]
- Huber, J.O.; Schmidt, M. The contribution of the pharmaceutical industry to the diagnosis and treatment of children and adolescents, in particular those with rare and complex diseases. An overview on the economic and regulatory challenges to examples of successful implementation. Padiatr. Padol. 2015, 50, S103–S110. [Google Scholar] [CrossRef]
- Shafie, A.A.; Supian, A.; Hassali, M.A.A.; Ngu, L.H.; Thong, M.K.; Ayob, H.; Chaiyakunapruk, N. Rare Disease in Malaysia: Challenges and Solutions. PLoS ONE 2020, 15, e0230850. [Google Scholar] [CrossRef]
- Thaker, Z.; Jethva, K.; Bhatt, D.; Zaveri, M.; Deshpande, S. Orphan drugs: Overview and regulatory review process. Int. J. Pharm. Sci. Res. 2019, 10, 505–518. [Google Scholar] [CrossRef]
- Pogany, G. Rare diseases and their patient organization: The Hungarian Federation of People with Rare and Congenital Diseases. Orv Hetil 2014, 155, 329–333. [Google Scholar] [CrossRef]
- Pascarelli, D.B.N.; Pereira, E.L. Rare diseases in the Brazilian National Congress: Analysis of parliamentary action. Cad. Saude Publica 2022, 38, e00167721. [Google Scholar] [CrossRef]
- Spagnolo, P.; du Bois, R.M.; Cottin, V. Rare Lung Disease and Orphan Drug Development. Lancet Respir. Med. 2013, 1, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Enzmann, H.; Broich, K. Cancer: Is it really so different? Particularities of oncologic drugs from the perspective of the pharmaceutical regulatory agency. Z. Fur Evidenz Fortbild. Und Qual. Im Gesundh. 2013, 107, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Hogerzeil, H.V. Rare Diseases and Essential Medicines: A Global Perspective. Int. J. Pharm. Med. 2005, 19, 285–288. [Google Scholar] [CrossRef]
- Syrop, J. The World’s Costliest Drug: Conflict Overshadows Cure. P T 1994, 19, 286–287+290. [Google Scholar]
- Thielke, D.; Thyssen, J.P.; Hansen, B.J. Orphan Drugs: Medications for Patients with Rare Diseases. Ugeskr. Laeger 2006, 168, 2236–2238. [Google Scholar] [PubMed]
- Taruscio, D.; Trama, A.; Stefanov, R. Tackling Rare Diseases at European Level: Why Do We Need a Harmonized Framework? Folia Med. (Plovdiv) 2007, 49, 59–67. [Google Scholar]
- Malcolm, L.M. Information Service on Treatment for Rare Diseases: The National Information Center for Orphan Drugs and Rare Diseases. Am. Pharm. 1989, NS29, 32. [Google Scholar] [CrossRef]
- Vassal, G.; Méry-Mignard, D.; Caulin, C. Clinical Trials in Paediatric Oncology: Recommendations for the Development of New Anticancer Agents. Therapie 2003, 58, 239–246. [Google Scholar] [CrossRef]
- Drummond, M.F.; Wilson, D.A.; Kanavos, P.; Ubel, P.; Rovira, J. Assessing the Economic Challenges Posed by Orphan Drugs. Int. J. Technol. Assess Health Care 2007, 23, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liang, Y.Q.; Xu, H. Analysis of Orphan Drugs Approved by FDA in 2015. Chin. J. New Drugs 2017, 26, 841–844. [Google Scholar]
- Schlander, M.; Beck, M. Expensive Drugs for Rare Disorders: To Treat or Not to Treat? The Case of Enzyme Replacement Therapy for Mucopolysaccharidosis VI. Curr. Med. Res. Opin. 2009, 25, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Zaric, G.S. Optimal Drug Pricing, Limited Use Conditions and Stratified Net Benefits for Markov Models of Disease Progression. Health Econ. 2008, 17, 1277–1294. [Google Scholar] [CrossRef] [PubMed]
- Brecht, I.B.; Graf, N.; Von Schweinitz, D.; Frühwald, M.C.; Bielack, S.S.; Schneider, D.T. Networking for Children and Adolescents with Very Rare Tumors: Foundation of the Gpoh Pediatric Rare Tumor Group. Klin. Padiatr. 2009, 221, 181–185. [Google Scholar] [CrossRef] [PubMed]
- DeVincenzo, J.P. Harnessing RNA Interference to Develop Neonatal Therapies: From Nobel Prize Winning Discovery to Proof of Concept Clinical Trials. Early Hum. Dev. 2009, 85, S31–S35. [Google Scholar] [CrossRef] [PubMed]
- Beck, M. Emerging Drugs for Lysosomal Storage Diseases. Expert Opin. Emerg. Drugs 2010, 15, 495–507. [Google Scholar] [CrossRef]
- Butlen-Ducuing, F.; Rivière, F.; Aarum, S.; Llnares-Garcia, J. European Medicines Agency Support Mechanisms Fostering Orphan Drug Development. Drug News Perspect 2010, 23, 71–81. [Google Scholar] [CrossRef]
- McCabe, C.; Stafinski, T.; Menon, D. Is It Time to Revisit Orphan Drug Policies? BMJ 2010, 341, 614. [Google Scholar] [CrossRef]
- Liang, B.A.; Mackey, T. Reforming Off-Label Promotion to Enhance Orphan Disease Treatment. Science 2010, 327, 273–274. [Google Scholar] [CrossRef]
- Christensen, S.B.; Bygbjerg, I.C. Drugs for the Neglected Disease Malaria Based on Natural Products. Bioact. Compd. Nat. Sources: Nat. Prod. Lead Compd. Drug Discov. Second. Ed. 2011, 525–550. [Google Scholar] [CrossRef]
- Picavet, E.; Dooms, M.; Cassiman, D.; Simoens, S. Orphan Drugs for Rare Diseases: Grounds for Special Status. Drug Dev. Res. 2012, 73, 115–119. [Google Scholar] [CrossRef]
- Pinxten, W.; Denier, Y.; Dooms, M.; Cassiman, J.J.; Dierickx, K. A Fair Share for the Orphans: Ethical Guidelines for a Fair Distribution of Resources within the Bounds of the 10-Year-Old European Orphan Drug Regulation. J. Med. Ethics 2012, 38, 148–153. [Google Scholar] [CrossRef]
- Webb, S.M.; Santos, A.; Valassi, E. The Value of a European Registry for Pituitary Adenomas: The Example of Cushing’s Syndrome Registry. Ann. Endocrinol. 2012, 73, 83–89. [Google Scholar] [CrossRef]
- Van Herle, K.; Behne, J.M.; Van Herle, A.; Blaschke, T.F.; Smith, T.J.; Yeaman, M.R. Integrative Continuum: Accelerating Therapeutic Advances in Rare Autoimmune Diseases. Annu. Rev. Pharmacol. Toxicol. 2012, 52, 523–547. [Google Scholar] [CrossRef]
- Maiella, S.; Rath, A.; Angin, C.; Mousson, F.; Kremp, O. Orphanet et Son Réseau: Où Trouver Une Information Validée Sur Les Maladies Rares. Rev. Neurol. 2013, 169, S3–S8. [Google Scholar] [CrossRef]
- Storf, H.; Hartz, T.; Tegtbauer, N.; Pfeiffer, W.; Schmidtke, J.; Graessner, H.; Wagner, T.; Ückert, F. Vision and Challenges of a Cartographic Representation of Expert Medical Centres for Rare Diseases. Stud. Health Technol. Inform. 2014, 205, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Bowlus, C.L.; Kenney, J.T.; Rice, G.; Navarro, R. Primary Biliary Cholangitis: Medical and Specialty Pharmacy Management Update. J. Manag. Care Spec. Pharm. 2016, 22, S3–S15. [Google Scholar] [CrossRef] [PubMed]
- Girard, N. Rare Thoracic Tumors. Rev. Malad Respir. Actual. 2014, 6, 540–551. [Google Scholar] [CrossRef]
- Shameer, K.; Readhead, B.T.; Dudley, J. Computational and Experimental Advances in Drug Repositioning for Accelerated Therapeutic Stratification. Curr. Top. Med. Chem. 2015, 15, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Roberts, E.A.; Herder, M.; Hollis, A. Fair Pricing of Old Orphan Drugs: Considerations for Canada’s Orphan Drug Policy. CMAJ 2015, 187, 422–425. [Google Scholar] [CrossRef] [PubMed]
- Hyry, H.I.; Roos, J.C.P.; Cox, T.M. Orphan Drugs: Expensive yet Necessary. QJM 2015, 108, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.R.; Xiang, W.; Zhang, F. International Reimbursement Policies on Drugs for Pulmonary Arterial Hypertension and Enlightenment to China. Chin. Pharm. J. 2015, 50, 1639–1642. [Google Scholar] [CrossRef]
- Bradford, D.; Reilly, K.M.; Widemann, B.C.; Sandler, A.; Kummar, S. Developing Therapies for Rare Tumors: Opportunities, Challenges and Progress. Expert Opin. Orphan Drugs 2016, 4, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Speid, L. Don’t Do Different Things—Do Things Differently! Drug Development in Rare Diseases: The Patient’s Perspective. Clin. Pharmacol. Ther. 2016, 100, 336–338. [Google Scholar] [CrossRef]
- Rhizobium, G.E. Complete Genome Sequence of the Sesbania Symbiont and Rice. Nucleic Acids Res. 2013, 1, 13–14. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, J.; Wang, J. PHP3 rare diseases, orphan drugs, and the legislation in China. Value Health 2010, 13, A533. [Google Scholar] [CrossRef]
- Postma, M.J.; Noone, D.; Rozenbaum, M.H.; Carter, J.A.; Botteman, M.F.; Fenwick, E.; Garrison, L.P. Assessing the value of orphan drugs using conventional cost-effectiveness analysis: Is it fit for purpose? Orphanet J. Rare Dis. 2022, 17, 157. [Google Scholar] [CrossRef]
- Rollet, P.; Lemoine, A.; Dunoyer, M. Sustainable Rare Diseases Business and Drug Access: No Time for Misconceptions. Orphanet J. Rare Dis. 2013, 8, 109. [Google Scholar] [CrossRef]
- Stolk, P.; Willemen, M.J.; Leufkens, H.G.M. “Rare Essentials”: Drugs for Rare Diseases as Essential Medicines. Bull. World Health Organ 2006, 84, 745–751. [Google Scholar] [CrossRef]
- Stolk, P.; Heemstra, H.E.; Leufkens, H.G.; Bloechl-Daum, B.; Heerdink, E.R. No Difference in Between-Country Variability in Use of Newly Approved Orphan and Non-Orphan Medicinal Products-a Pilot Study. Orphanet J. Rare Dis. 2009, 4, 27. [Google Scholar] [CrossRef]
- Verma, I.C.; El-Beshlawy, A.; Tylki-Szymańska, A.; Martins, A.; Duan, Y.-L.; Collin-Histed, T.; Schoneveld Van Der Linde, M.; Mansour, R.; Dũng, V.C.; Mistry, P.K. Transformative effect of a Humanitarian Program for individuals affected by rare diseases: Building support systems and creating local expertise. Orphanet J. Rare Dis. 2022, 17, 87. [Google Scholar] [CrossRef] [PubMed]
- Schlander, M.; Dintsios, C.M.; Gandjour, A. Budgetary Impact and Cost Drivers of Drugs for Rare and Ultrarare Diseases. Value Health 2018, 21, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Kessel, M.; Hannemann-Weber, H.; Kratzer, J. Innovative Work Behavior in Healthcare: The Benefit of Operational Guidelines in the Treatment of Rare Diseases. Health Policy 2012, 105, 146–153. [Google Scholar] [CrossRef]
- Winquist, E.; Bell, C.M.; Clarke, J.T.R.; Evans, G.; Martin, J.; Sabharwal, M.; Gadhok, A.; Stevenson, H.; Coyle, D. An Evaluation Framework for Funding Drugs for Rare Diseases. Value Health 2012, 15, 982–986. [Google Scholar] [CrossRef]
- Faulkner, E.; Spinner, D.S.; Ringo, M.; Carroll, M. Are Global Health Systems Ready for Transformative Therapies? Value Health 2019, 22, 627–641. [Google Scholar] [CrossRef] [PubMed]
- Clarke, J.T.R.; Coyle, D.; Evans, G.; Martin, J.; Winquist, E. Toward a Functional Definition of a “Rare Disease” for Regulatory Authorities and Funding Agencies. Value Health 2014, 17, 757–761. [Google Scholar] [CrossRef]
- Ollendorf, D.A.; Chapman, R.H.; Pearson, S.D. Evaluating and Valuing Drugs for Rare Conditions: No Easy Answers. Value Health 2018, 21, 547–552. [Google Scholar] [CrossRef]
- Rodwell, C.; Aymé, S. Rare Disease Policies to Improve Care for Patients in Europe. Biochim. Et Biophys. Acta (BBA)—Mol. Basis Dis. 2015, 1852, 2329–2335. [Google Scholar] [CrossRef] [PubMed]
- Richter, T.; Nestler-Parr, S.; Babela, R.; Khan, Z.M.; Tesoro, T.; Molsen, E.; Hughes, D.A. Rare Disease Terminology and Definitions—A Systematic Global Review: Report of the ISPOR Rare Disease Special Interest Group. Value Health 2015, 18, 906–914. [Google Scholar] [CrossRef]
- Aranda-Reneo, I.; Rodríguez-Sánchez, B.; Peña-Longobardo, L.M.; Oliva-Moreno, J.; López-Bastida, J. Can the Consideration of Societal Costs Change the Recommendation of Economic Evaluations in the Field of Rare Diseases? An Empirical Analysis. Value Health 2021, 24, 431–442. [Google Scholar] [CrossRef]
- Belousova, O.A.; Groen, A.J.; Ouendag, A.M. Opportunities and Barriers for Innovation and Entrepreneurship in Orphan Drug Development. Technol. Forecast. Soc. Change 2020, 161, 120333. [Google Scholar] [CrossRef]
- Bai, J.P.F.; Barrett, J.S.; Burckart, G.J.; Meibohm, B.; Sachs, H.C.; Yao, L. Strategic Biomarkers for Drug Development in Treating Rare Diseases and Diseases in Neonates and Infants. AAPS J. 2013, 15, 447–454. [Google Scholar] [CrossRef]
- Putzeist, M.; Mantel-Teeuwisse, A.K.; Gispen-De Wied, C.C.; Hoes, A.W.; Leufkens, H.G.; La De Vrueh, R. Drug Development for Exceptionally Rare Metabolic Diseases: Challenging but Not Impossible. Orphanet J. Rare Dis. 2013, 8, 179. [Google Scholar] [CrossRef] [PubMed]
- Harari, S. Why We Should Care about Ultra-Rare Disease. Eur. Respir. Rev. 2016, 25, 101–103. [Google Scholar] [CrossRef]
- Janoudi, G.; Amegatse, W.; Mcintosh, B.; Sehgal, C.; Richter, T. Health Technology Assessment of Drugs for Rare Diseases: Insights, Trends, and Reasons for Negative Recommendations from the CADTH Common Drug Review. Orphanet J. Rare Dis. 2016, 11, 164. [Google Scholar] [CrossRef]
- Annemans, L.; Aymé, S.; Le Cam, Y.; Facey, K.; Gunther, P.; Nicod, E.; Reni, M.; Roux, J.-L.; Schlander, M.; Taylor, D.; et al. Recommendations from the European Working Group for Value Assessment and Funding Processes in Rare Diseases (ORPH-VAL). Orphanet J. Rare Dis. 2017, 12, 50. [Google Scholar] [CrossRef] [PubMed]
- Volmar, C.-H.; Brothers, S. Orphan Diseases: State of the Drug Discovery Art. Wien. Med. Wochenschr. 2017, 167, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Southall, N.T.; Natarajan, M.; Pek, L.; Lau, L.; Jonker, A.H.; Deprez, B.; Guilliams, T.; Hunter, L.; Rademaker, C.M.; Hivert, V.; et al. The Use or Generation of Biomedical Data and Existing Medicines to Discover and Establish New Treatments for Patients with Rare Diseases-Recommendations of the IRDiRC Data Mining and Repurposing Task Force on Behalf of the IRDiRC Data Mining and Repurposing Task Force. Orphanet J. Rare Dis. 2019, 14, 1–8. [Google Scholar] [CrossRef]
- Pires De Mello, C.P.; Rumsey, J.; Slaughter, V.; Hickman, J.J. A Human-on-a-Chip Approach to Tackling Rare Diseases. Drug Discov. Today 2019, 24, 2139–2151. [Google Scholar] [CrossRef] [PubMed]
- Lanar, S.; Acquadro, C.; Seaton, J.; Savre, I.; Arnould, B. To What Degree Are Orphan Drugs Patient-Centered? A Review of the Current State of Clinical Research in Rare Diseases. Lanaret Al. Orphanet J. Rare Dis. 2020. [Google Scholar] [CrossRef]
- Armeni, P.; Cavazza, M.; Xoxi, E.; Taruscio, D.; Kodra, Y. Reflections on the Importance of Cost of Illness Analysis in Rare Diseases: A Proposal. J. Environ. Res. Public Health 2021, 18, 1101. [Google Scholar] [CrossRef] [PubMed]
- Aartsma-Rus, A.; Dooms, M.; Le Cam, Y.; Economics, C. Orphan Medicine Incentives: How to Address the Unmet Needs of Rare Disease Patients by Optimizing the European Orphan Medicinal Product Landscape Guiding Principles and Policy Proposals by the European Expert Group for Orphan Drug Incentives (OD Expert Gr. Front. Pharmacol. 2021, 12, 3666. [Google Scholar] [CrossRef]
- Gorini, F.; Santoro, M.; Pierini, A.; Mezzasalma, L.; Baldacci, S.; Bargagli, E.; Boncristiano, A.; Rossana Brunetto, M.; Cameli, P.; Cappelli, F.; et al. Orphan Drug Use in Patients With Rare Diseases: A Population-Based Cohort Study. Front. Pharmacol. 2022, 13, 1. [Google Scholar] [CrossRef]
- Directive 2011/24/EU of the European Parliament and of the Council of 9 March 2011 on the Application of Patients’ Rights in Cross-Border Healthcare. 2011, Vol. 088. L 88/45. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32011L0024 (accessed on 17 September 2022).
- Władysiuk, M.; Skweres-Kuchta, M. Rodziny Dzieci Dotkniętych Chorobą Rzadką Muszą Wyjeżdżać z Polski o. Available online: https://politykazdrowotna.com/artykul/rodziny-dzieci-dotknietych-choroba-rzadka-musza-wyjezdzac-z-polski/831472 (accessed on 10 November 2022).
- Hivert, V.; Jonker, A.H.; O’Connor, D.; Ardigo, D. IRDiRC: 1000 New Rare Diseases Treatments by 2027, Identifying and Bringing Forward Strategic Actions. Rare Dis. Orphan Drugs J. 2021, 1, 3. [Google Scholar] [CrossRef]
- Rare 2030. Panel of Experts. Everything you would like to know about foresight but were afraid to ask. Available online: https://www.rare2030.eu/wp-content/uploads/2019/09/Explaining-Foresight-resource-for-the-Rare2030-project-panel-of-experts.pdf (accessed on 27 January 2023).
- United Nations. Transforming Our World: The 2030 Agenda for Sustainable Development; United Nations: New York, NY, USA, 2015. [Google Scholar]
- United Nations. Addressing the Challenges Of Persons Living With A Rare Disease And Their Families: Resolution / adopted by the General Assembly; United Nations: New York, NY, USA, 2021. [Google Scholar]
- Władysiuk, M.; Plisko, R.; Kostrzewska, K.; Tóth, A.; Molnar, M.G.A.P. Gearing up. Improving Time to Patient Access to Innovative Therapies in V4. Executive Summary; G.A.P.: Kraków, Poland; Warsaw, Poland; Budapest, Hungary, 2022. [Google Scholar]
- Senior, M.; Hadjivasiliou, A. Orphan Drug. Report 2022; Evaluate Pharma: London, UK, 2022. [Google Scholar]
- European Commission. Pharmaceutical Strategy for Europe; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- European Commission. European Medicines Agency Pilot Project “Market Launch of Centrally Authorised Products”; European Commission: Brussels, Belgium, 2021. [Google Scholar]
- Minister Zdrowia. Komunikat Ministra Zdrowia w Sprawie Produktów Leczniczych Niepodlegających Finansowaniu w Ramach Procedury Ratunkowego Dostępu Do Technologii Lekowych; Ministerstwo Zdrowia: Warsaw, Poland, 2022.
- EURORDIS-Rare Diseases Europe. Breaking the Access Deadlock to Leave No One Behind. A contribution by EURORDIS and its Members on possibilities for patients’ full and equitable access to rare disease therapies in Europe; EURORDIS-Rare Diseases Europe: Paris, France, 2018. [Google Scholar]
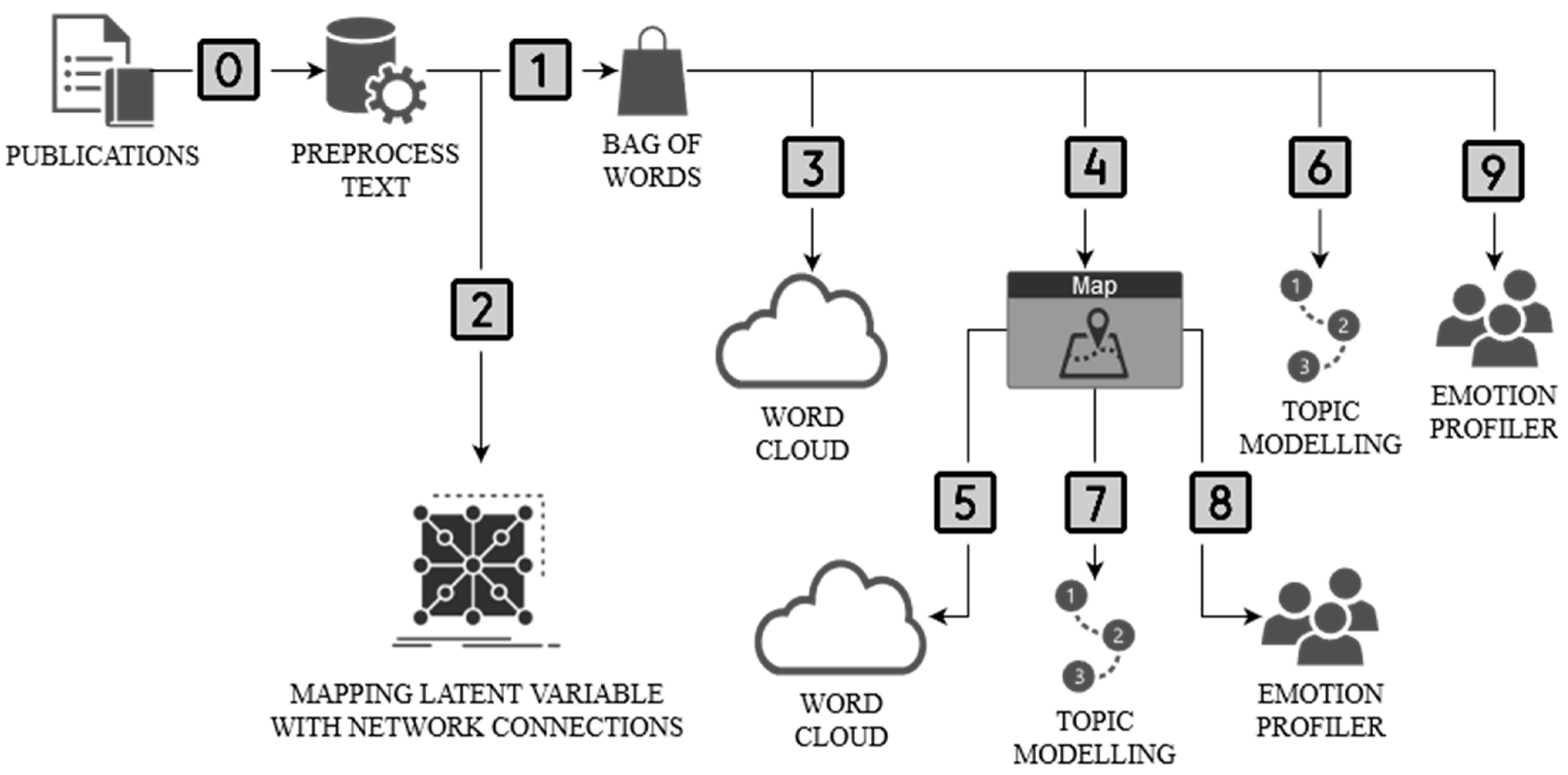
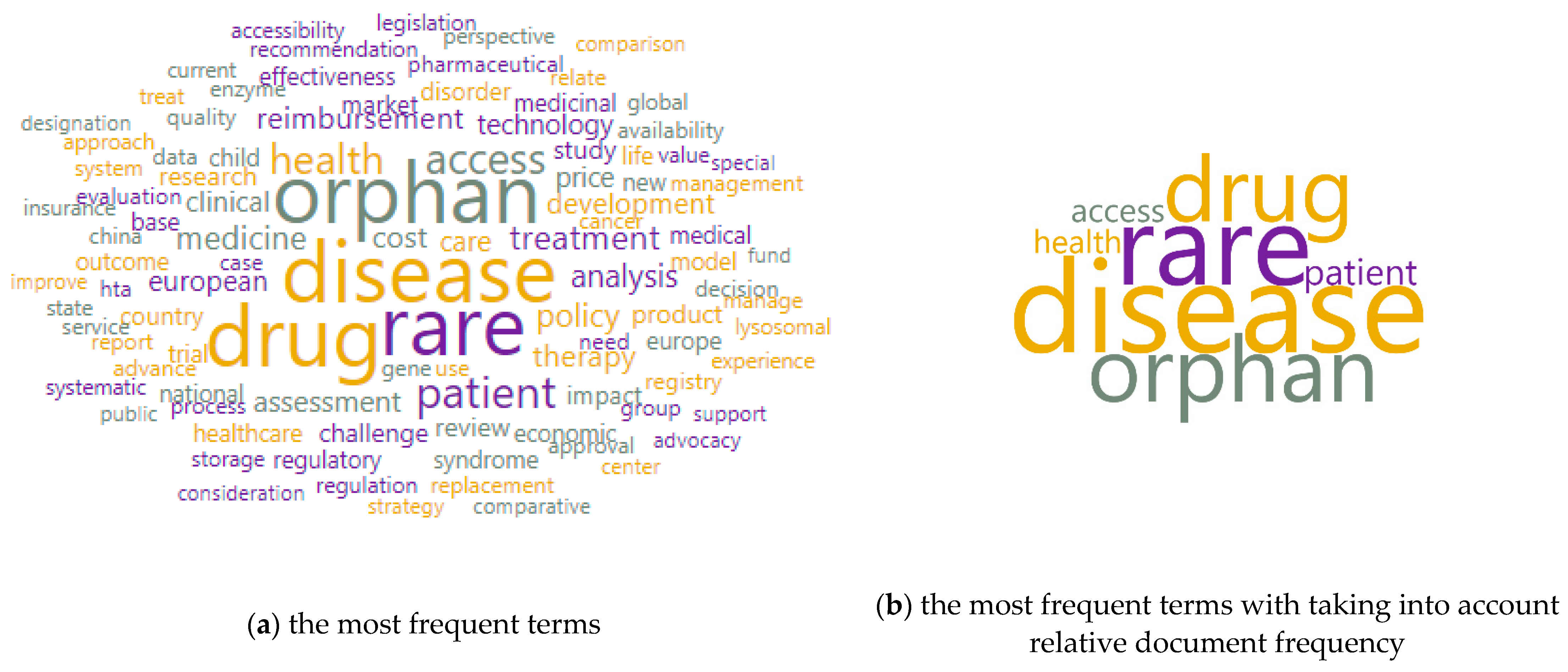
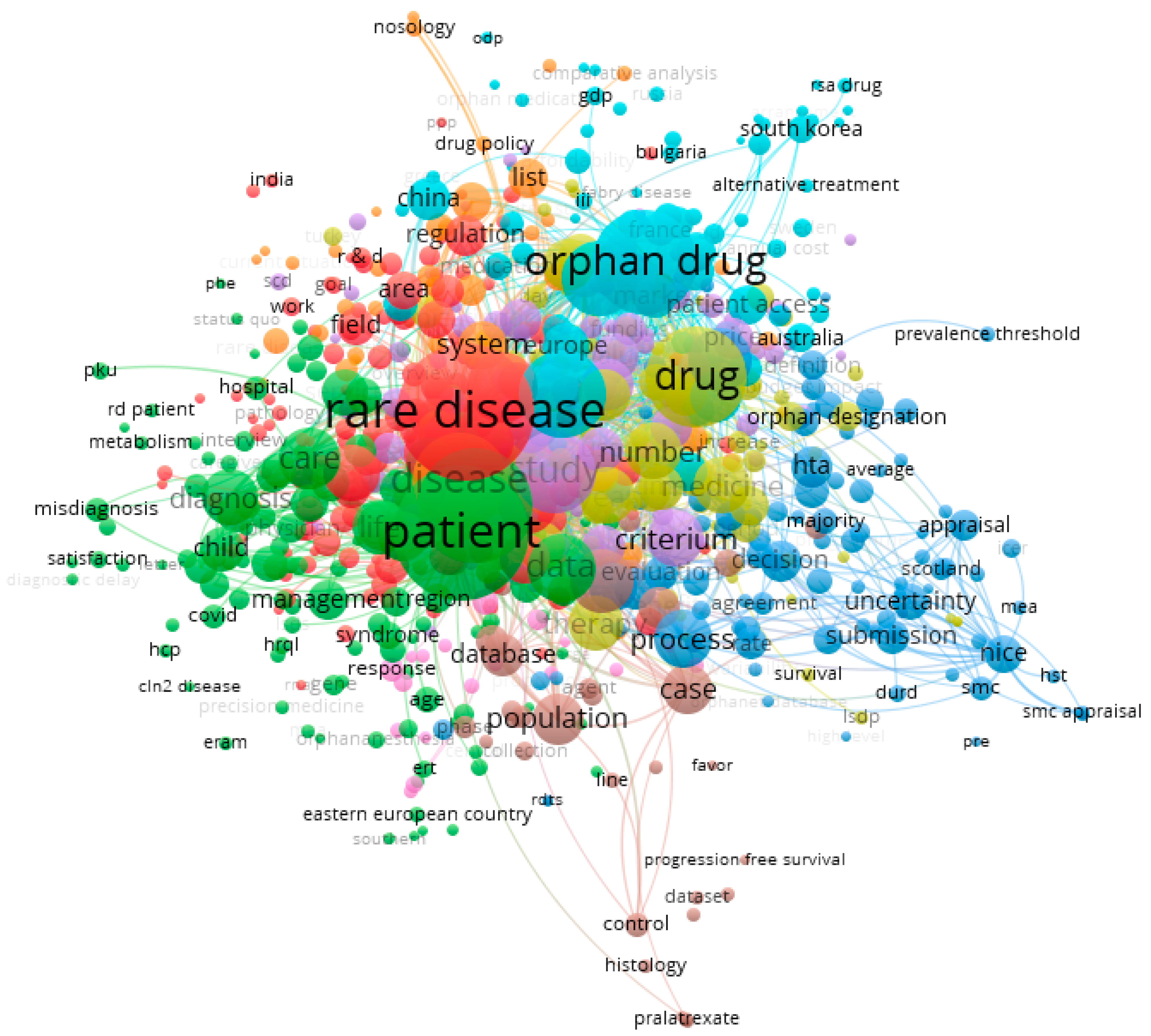
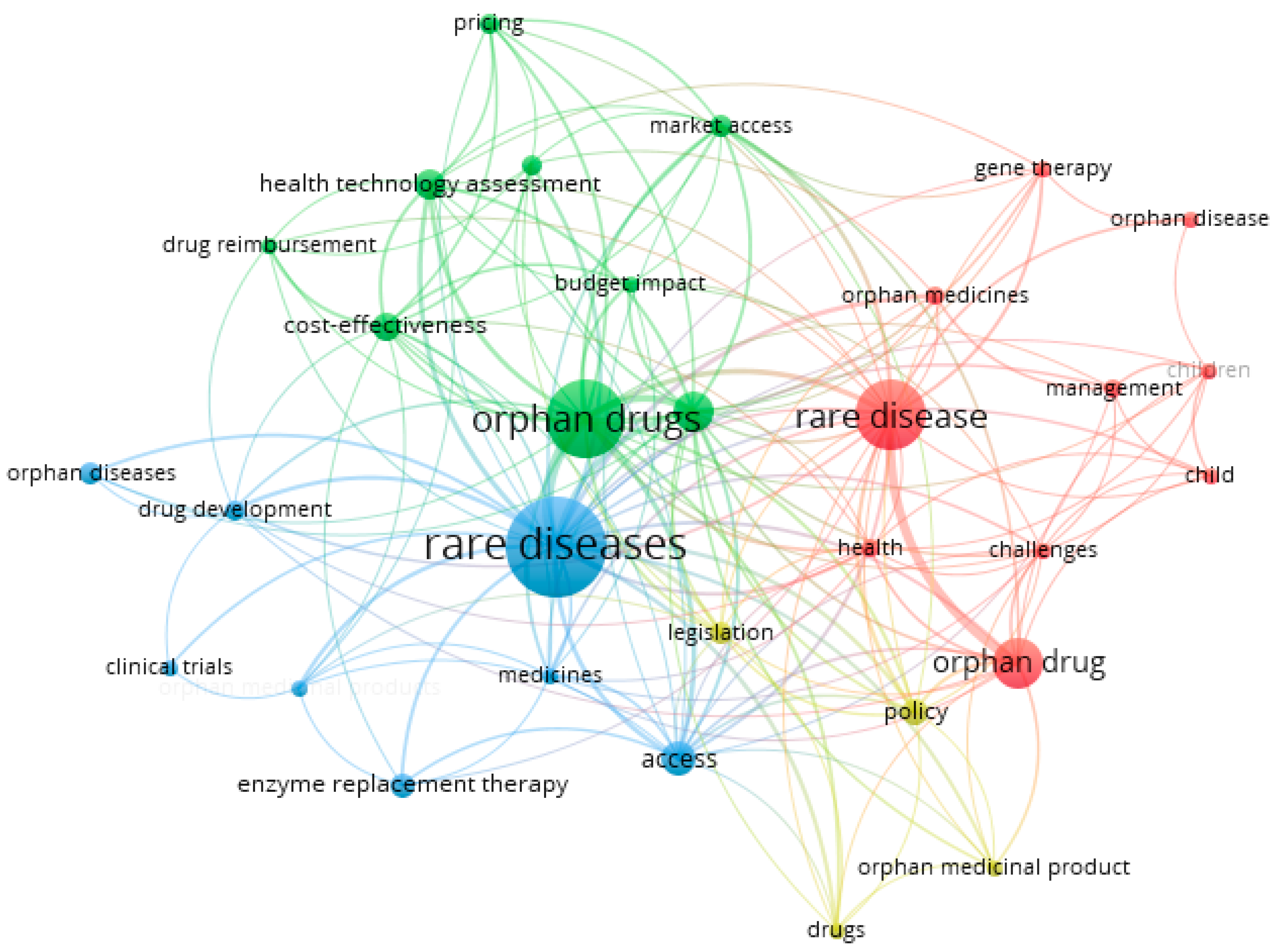
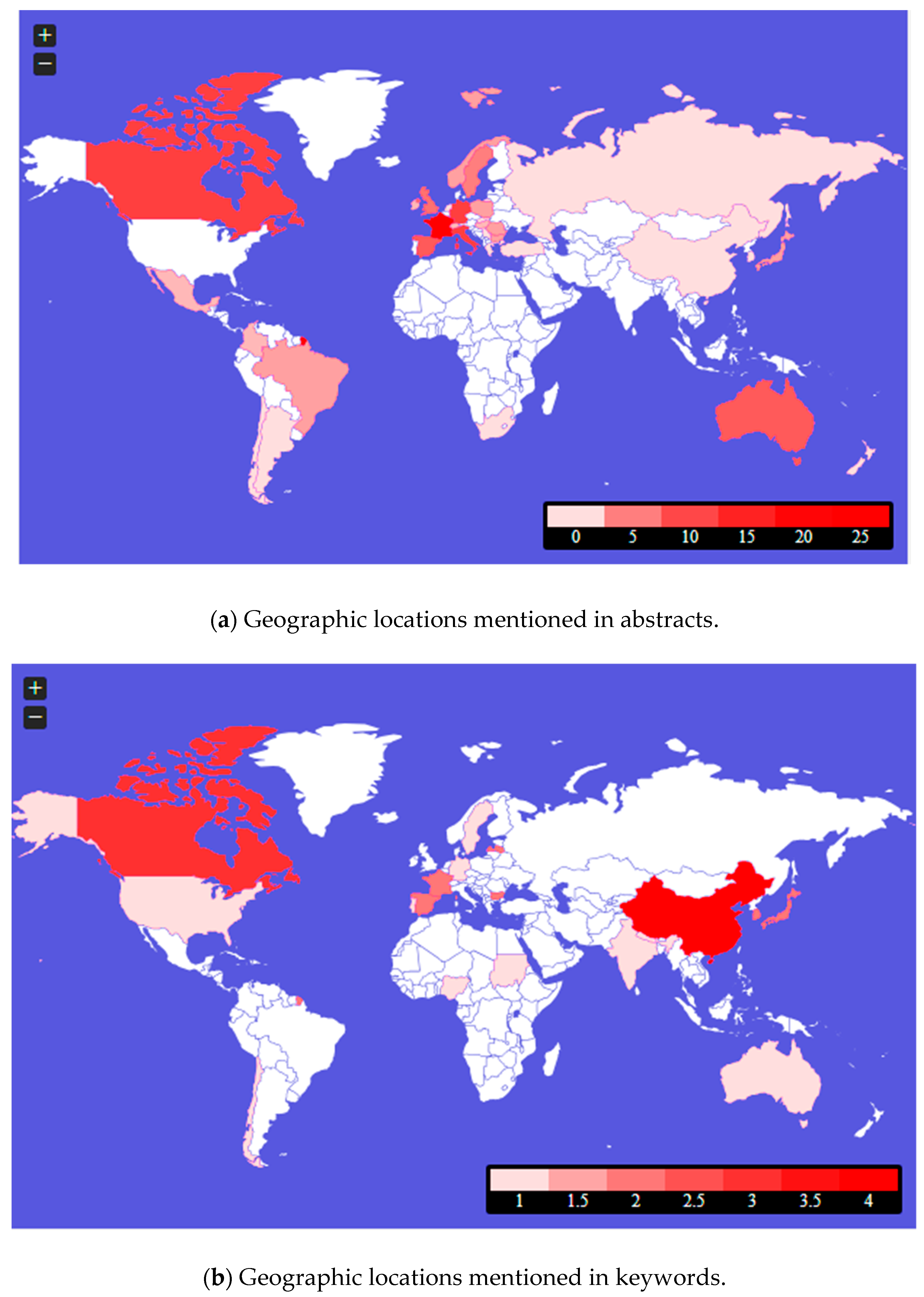

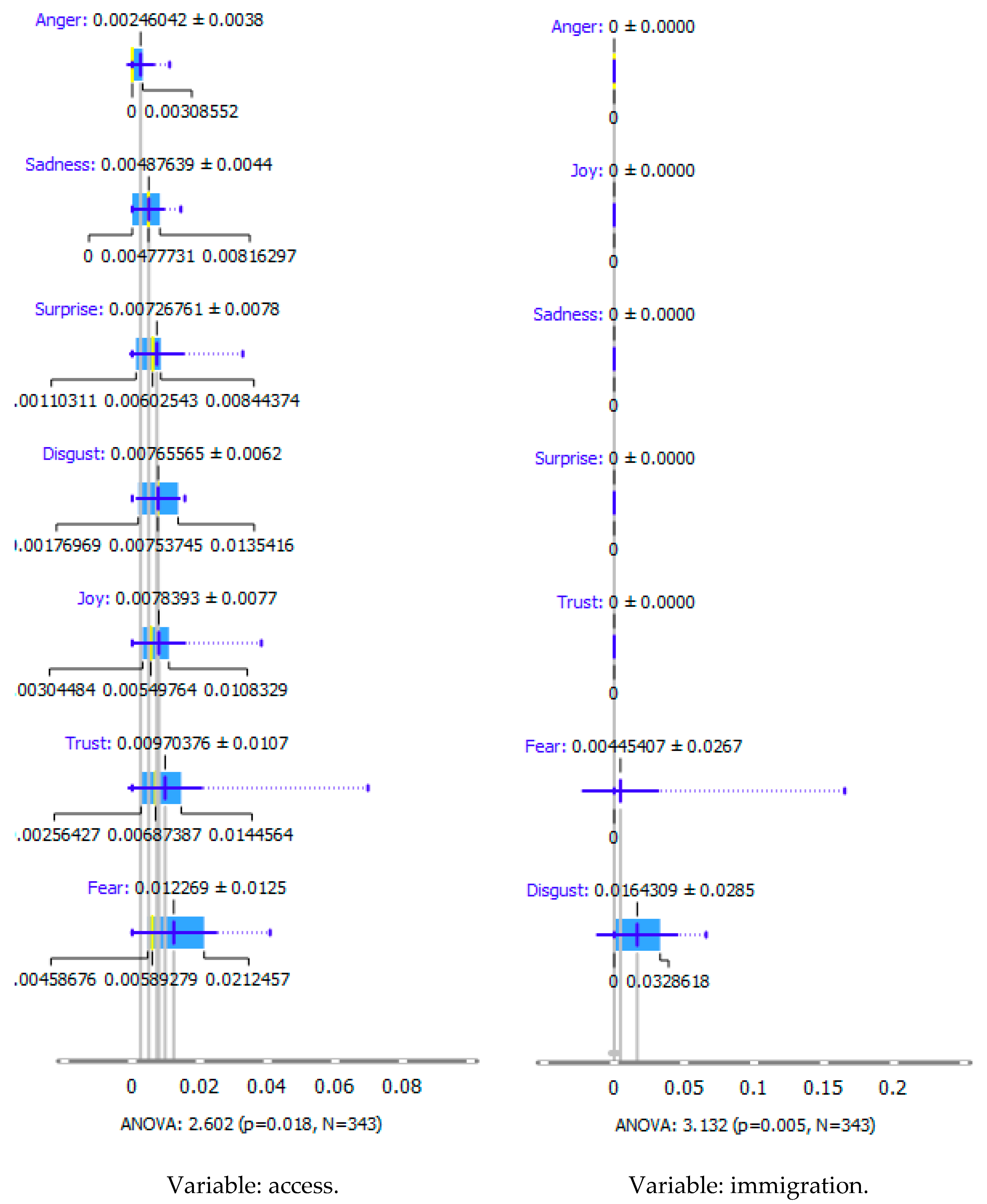
| Topic | Topic Keywords |
|---|---|
| 1 | drug, disease, orphan, rare, patient, treatment, cost, policy, access, medicine |
| 2 | drug, disease, rare, orphan, HTA, price, patient, cost, R&D, care |
| 3 | drug, HTA, orphan, China, OD, R&D, nice, technology, development, OMP |
| 4 | China, HTA, policy, product, designation, rare, insurance, country, FDA, approval |
| 5 | medicine, cost, HTA, OD, drug, R&D, orphananesthesia, China, recommendation, nice |
| Topic Keywords Map | Topic | Topic Keywords |
|---|---|---|
 | # | case: Poland |
| 1 | drug, country, disease, orphan, requirement, rare, access, GDP, MCDA, HTA | |
| 2 | GDP, per, MCDA, cost, OD, capita, annual, Russia, criterion, world | |
| 3 | MCDA, GDP, criterion, world, real, Russia, per, Netherlands, framework, search | |
 | # | case: Brazil |
| 1 | disease, rare, policy, country, patient, legislation, Latin, orphan, clinical | |
| 2 | plan, need, law, adoption, analysis, six, strengthen, patient, policy, implementation | |
| 3 | drug, policy, regard, LA, market, Latin, orphan, patient, EU, company |
| Emotion Profile | Structure of Emotions |
|---|---|
| All selected countries from Figure 5a | |
 | In descending order: turquoise bar means trust (38.52%); orange bar—joy (23.74%); pink—surprise (16.34%); green—fear (10.51%); yellow—sadness (7.39%); blue—anger (2.33%); red—disgust (1.17%) |
| Poland | |
 | In descending order: orange bar means joy (80%); turquoise—trust (20%) |
| Brazil | |
 | In descending order: turquoise bar means trust (33.33%); pink—surprise (33.33%); yellow—sadness (16.67%); green—fear (16.67%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skweres-Kuchta, M.; Czerska, I.; Szaruga, E. Literature Review on Health Emigration in Rare Diseases—A Machine Learning Perspective. Int. J. Environ. Res. Public Health 2023, 20, 2483. https://doi.org/10.3390/ijerph20032483
Skweres-Kuchta M, Czerska I, Szaruga E. Literature Review on Health Emigration in Rare Diseases—A Machine Learning Perspective. International Journal of Environmental Research and Public Health. 2023; 20(3):2483. https://doi.org/10.3390/ijerph20032483
Chicago/Turabian StyleSkweres-Kuchta, Małgorzata, Iwona Czerska, and Elżbieta Szaruga. 2023. "Literature Review on Health Emigration in Rare Diseases—A Machine Learning Perspective" International Journal of Environmental Research and Public Health 20, no. 3: 2483. https://doi.org/10.3390/ijerph20032483
APA StyleSkweres-Kuchta, M., Czerska, I., & Szaruga, E. (2023). Literature Review on Health Emigration in Rare Diseases—A Machine Learning Perspective. International Journal of Environmental Research and Public Health, 20(3), 2483. https://doi.org/10.3390/ijerph20032483









