Abstract
The article deals with one of the effects of health inequalities and gaps in access to treatments for rare diseases, namely health-driven emigration. The purpose of the paper is to systematize knowledge about the phenomenon of health emigration observed among families affected by rare diseases, for which reimbursed treatment is available, but only in selected countries. The topic proved to be niche; the issue of “health emigration in rare diseases” is an area for exploration. Therefore, the further analysis used text mining and machine learning methods based on a database selected based on keywords related to this issue. The results made it possible to systematize the guesses made by researchers in management and economic fields, to identify the most common keywords and thematic clusters around the perspective of the patient, drug manufacturer and treatment reimbursement decision-maker, and the perspective integrating all the others. Since the topic of health emigration was not directly addressed in the selected sources, the authors attempted to define the related concepts and discussed the importance of this phenomenon in managing the support system in rare diseases. Thus, they indicated directions for further research in this area.
1. Introduction
1.1. Presentation of Research Problems
The problem addressed in the article concerns one of the effects of unequal opportunities for families affected by rare diseases to access treatment for a narrow range of conditions for which there is a therapeutic option in the form of an orphan drug, and reimbursed treatment is only possible in selected countries around the world. As a result, some families choose to relocate and emigrate to a country where the patient’s health needs are likely to be met.
The starting point for the article was a pre-diagnosed research gap in the field of health emigration in rare diseases after a literature review according to the keywords (a) “health emigration” and (b) “emigration” and “rare disease*”. Thus, the authors attempted to systematize socioeconomic and management knowledge around the issue of rare diseases in the context of unequal access to treatment, to capture potential gaps for further research directions, and to define concepts related to the research problem presented.
The phenomenon of “health emigration” (named by the authors as emigration for a cure) is observed among patients affected by rare diseases, i.e., diseases that individually affect a small population. The definition of a rare disease, adopted in European Union countries, specifies the incidence of the disease at less than one person per 2000 inhabitants []. With a prevalence of 1 person per 50,000 population, the disease is defined as ultra-rare. The definition of rare (or ultra-rare) disease is not standardized globally (e.g., USA, Japan, Canada). Nevertheless, in each case, the rarity of the condition and the challenges it presents, especially in terms of the unmet health needs of patients, are emphasized. This thread is because rare diseases generally have a severe course and chronic nature and, in many cases, lead to the patient’s premature death. They are, therefore, particularly physically and psychologically burdensome not only for him, but also for his immediate family [].
The scale of the problem is significant, as at least 6000 types of rare diseases have been defined so far, and it is estimated that they affect as much as 6–8% of the world’s population []. That is about 300 million people, among whom only 6% of medicine can offer a therapeutic option []. At the same time, it should be emphasized that a drug invented and approved for marketing does not always have a chance to reach the patient due to the countries’ different policies regarding reimbursement of drugs dedicated to rare diseases. Moreover, since these drugs—being innovative and produced on a small scale, usually with orphan drug status—most often tend to be horrendously expensive, their financing is problematic for limited public budgets and unattainable for the patient alone. As a result, because rare diseases mainly affect children, families choose to emigrate, with all its consequences [].
For the sake of completeness, an orphan drug is the only or significantly more good product than an existing one for the diagnosis, prevention or treatment of a rare disease or other condition, for which marketing a medicinal product will not generate a sufficient return on investment. In Europe, this status, in principle, provides the drug manufacturer with ten years of market exclusivity in marketing medicinal products for a given indication [,].
Defining the phenomenon of health emigration in the literature is not apparent; hence the authors search and attempt to highlight this problem.
According to the Merriam-Webster online dictionary, emigration means leaving one’s residence, home or country to live or stay in another []. Investopedia, on the other hand, narrows the meaning of “emigration” to the relocation or process of people leaving one country to live in another []. The Cambridge English Dictionary gives a similar definition to the previous one but emphasizes the process of leaving a country “permanently” or living “permanently” in another country []. In turn, the process of people moving (usually en masse) to a new place of residence is called migration, according to the same dictionary []. It also represents the daily population movements associated with commuting to work or school []. Migration also has other meanings, not always related to humans. According to the Cambridge English Dictionary, it can mean, first, the movement of animals to another place—usually with a change of season. Second, migration can start using a new computer system and moving information, software or hardware from one computer system to another. Third, migration is also called the process of existing customers changing services or companies []. Two types of customer migration can be voluntary churn, i.e., the customer abandons the product, and involuntary churn, i.e., the supplier, discontinues the relationship with the customer. Forced migration often occurs due to a customer’s failure to pay fees or breach of contract [].
Migration is either the result of environmental changes or the cause of their occurrence. According to this division, migration is either a dependent variable, determined by specific characteristics, or an independent variable focusing on its occurrence’s economic, social, political and natural consequences []. According to economic theories, one can primarily determine migration by economic variables. Sociological theories, on the other hand, focus on the motives behind migration decisions and are based on behaviourist research. Finally, geographic theories analyze the pull factors and barriers to migration in spatially diverse environments []. According to Wojciech Janicki, none of the migration theories developed so far can comprehensively and convincingly explain the variability of complex migration processes. This author argues that the universalization of migration theory would require coordinating interdisciplinary research conducted by specialists from different scientific disciplines [].
Another concept related to movement is immigration. According to the PWN Dictionary of the Polish Language, immigration means an influx of foreign people into a country to settle there, and a foreign population settled in some country []. A more expansive definition of immigration is leaving one’s own country and moving to another country of which one is not an indigenous resident or citizen in order to []:
- Settle or live there (as a permanent resident or naturalized citizen);
- Take up employment as a migrant worker;
- Live temporarily as a foreign worker.
To summarise, emigration and immigration are types of external migration, i.e., foreign migration associated with crossing national borders. Within the framework of external migration, other types of migration can also be distinguished []:
- Re-emigration, or the return of emigrants to the country after a permanent stay abroad;
- Repatriation, i.e., the mass return to the country, organized by state authorities, of prisoners of war, internees, people who left the country, e.g., for political reasons;
- Deportation, i.e., forced expulsion from the national territory, most often having to do with illegal immigrants;
- Expatriation is the voluntary or forced departure from one’s national territory, signifying a break from the country.
In addition to external migration, it is necessary to mention the second—internal migration, i.e., taking place within a country. Here, there is a change of residence within a country—inter-regional and intra-regional migration can occur [].
A concept also related to the process of movement is refugeeism. Refugees are people who flee their country to another country to avoid some conflict/problem. The term refugee also includes asylum seekers, i.e., people forced to leave their country to avoid persecution—often because of political or religious beliefs. Internally displaced persons forced to leave their homes and move to a new place in their country are also considered refugees [].
The strongest determinants of migration processes at present, in the context of the sizeable interregional variation in the economic situation, are motives of an economic nature and the propensity to improve living conditions []. Other determinants of migration processes are family, education, religion, politics, nationality, health, leisure, and climate [,,,]. According to Nicole B. Simpson, the key predictors of migration are income disparity, migrant networks and demographic factors (age, education, marital status, and language) []. Studies show that public spending by home country governments on free public education, access to health care and unemployment benefits reduces international emigration [].
The phenomenon of “health emigration” refers to a situation in which a family seeks the most advanced treatment abroad for their sick child []. Health emigration is believed to be getting rid of citizens, both parents who have a sick child and that child, but also healthy siblings. In doing so, the author draws attention to the disturbing profile of these emigrant parents in the context of abandoning their country of origin: they are educated, working-age persons who will eventually work for the economy and development of another country []. Health emigration is an effect of desperation. If one receives information about the lack of funding for treatment in a particular country and the diagnosis of an illness, attachment to the home country goes down the drain [].
Machine learning is being applied to studying health emigration in the context of rare diseases. It is used, for example, for literature review. In addition, machine learning will shortly support medical professionals in diagnosing diseases [].
Machine learning (ML) is the scientific study of algorithms and statistical models used by computer systems to perform a specific task without explicit programming []. It represents a growing branch of computational algorithms in emulating human intelligence by learning from the surrounding environment []. Machine learning helps decision-making through prediction and classification mechanisms based on historical data []. In general, ML algorithms are designed to perform two main tasks: supervised and unsupervised learning. In supervised learning, the sample contains both input and output indicators. In this case, ML algorithms build a rule, and the input indicator is mapped to the output using the rule. In unsupervised learning, on the other hand, the samples have no original labels, which should be understood as the absence of output indicators [].
In conclusion, machine learning has potential and offers excellent opportunities in the social sciences. In addition, technological advances are making machine learning tools an attractive alternative to classical methods, and the technical barriers to using ML are decreasing thanks to available open-source software [].
1.2. Organization of the Paper
The article aims to systematize knowledge on health emigration among families affected by rare diseases through a literature review using machine learning and an analysis of available industry reports.
In order to achieve the goal, the following research questions were formulated:
- Define the basic concepts related to the topics of the article: emigration and rare diseases.
- What socioeconomic and management issues dominate scientific analysis in the area of unequal access to the treatment of rare diseases?
- To what extent does machine learning help identify the state of knowledge and research on health emigration in rare diseases and its limitations?
- For what reasons is the phenomenon of health emigration relevant to the management strategy of the support system in rare diseases?
The structure of the article corresponds to the objectives set. The introduction describes the genesis, research problem, and basic definitions related to the phenomenon of emigration, including that of health emigration observed in rare diseases. A literature review of socioeconomic issues related to rare diseases, orphan drugs, treatment and access to treatment using a machine learning tool was conducted. Unequal access to treatment with orphan drugs is a direct cause of health emigration in rare diseases, hence this choice of keywords. The discussion develops emigration’s theme from the point of view of its relevance to rare disease management strategies. The article closes with conclusions and an indication of the limitations of the analyzes conducted and potential research directions.
2. Materials and Methods
Implementing the article’s aim required a specific research procedure regarding sources and methodology. The authors have based the study on a literature review on access to treatment for rare diseases. The bibliography includes 343 works, among them scientific articles, books, specialist literature, and electronic sources from 1989–2022, and among these works, the most were those from 2017–2021. During desk research analysis, the authors have used the following professional scientific databases: Scopus, the Web of Science, and PubMed. The authors used these scientific databases due to their possible access by having an account. Secondly, these databases made it possible to complete the literature for this article. The authors wanted to show scientists’ contributions to social and health sciences development through these databases.
All publications were searched on the Web of Science [], Scopus [], and PubMed [] databases. The keywords sequence used for article selection was: orphan* and “rare disease*” and treatment* and access*.
In the next phase of literature selection, the duplicate was removed, and the content was checked to match the topic. Many valuable publications in other languages or indexed in other databases, or those not indexed, were not included in the study. Due to the necessity of tokenization and transformation of texts (including lemmatization), similar restrictions had to be adopted. Ultimately, 343 publications in English were selected for the analysis of text mining and machine learning.
The analysis used techniques in the field of machine learning and mining text such as word cloud [,], mapping latent variables with network connections [,], a bag of words [,], topic modeling by Latent Semantic Index (LSI) [,], emotion recognition (profiler by Plutchik emotions) [,] and geomapping [].
The research procedure consisted of 10 stages (Figure 1). The preprocessed text was performed in stage zero. This stage focused on the transformation (lowercase, remove URLs) and tokenization of the text (regexp) as well as its normalization (Lemmagen Lematizer) and filtering (stopwords, lexicon, regexp). The Bag of Words (term frequency—count, regularization—L2 (Euclidean)) was used in the first stage. Step 2 identifies latent variables in the term relationship network map. Stages 3–6 were carried out in parallel. In step 3, a word cloud was created for all publications. Then, in stage 4, these publications were geomapped based on abstracts. In step 5, a word cloud was created for geographic locations. In step 6, the Latent Semantic Index was used for topic modeling. Based on the collected information, the topic modeling (LSI) results were geomapped with a selection of two cases: Poland and Brazil (stage 7). These results were enriched with emotion profiling (recognition of emotions) in publications using (Plutchik’s profile) for the case of Poland and Brazil (stage 8). The last stage (9th) is an extension of stage 8 by analyzing the range of emotions for two keywords: access and immigration.
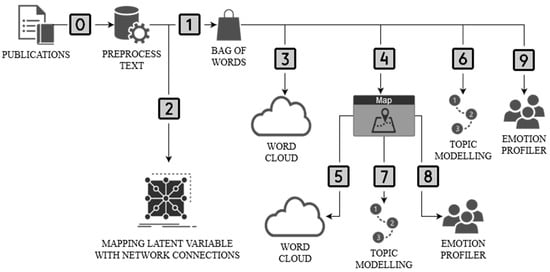
Figure 1.
The framework of the authors’ research procedure (methodology). Source: own elaboration.
3. Results
Figure 2 shows word clouds based on the examined corpus, which included the title and keywords. It illustrates the most common terms around the studied issue. In the initial examination, it could be noticed that the most important words were: disease (weight 406), rare (weight 351), an orphan (weight 283), drug (weight 281), patient (weight 112), access (weight 102), health (weight 98). In a tag cloud, the weight is the same as the number of occurrences.
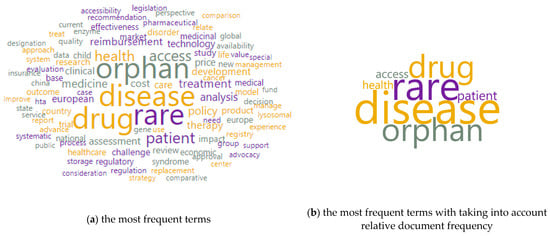
Figure 2.
Word clouds of terms. Limitations: the authors did not take into account many valuable publications in the study, instead taking into account the need to narrow the thematic scope of the described issues. Source: own elaboration based on 343 references [,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,].
While exploring the studied publications in-depth, particular attention was paid to mapping latent variables in the network connections map (Figure 3 and Figure 4). The larger the ball, the more often the latent variable in the analyzed texts appeared. The shorter the bond line, the stronger the bond, and the longer, the weaker the interdependence of the latent variables.
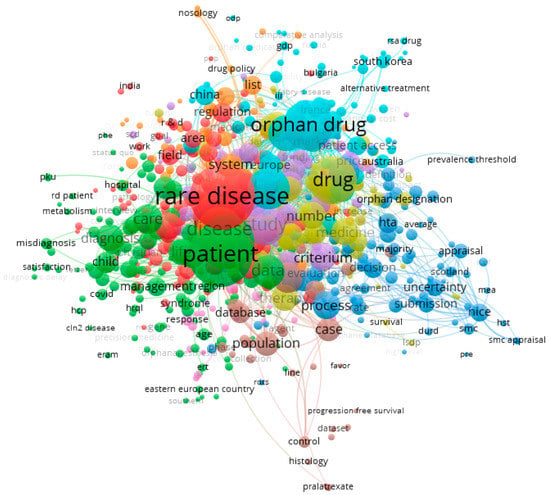
Figure 3.
Mapping latent variables from titles and abstracts of the publications on issues connected with rare diseases. Note: Technical publication on mapping and clustering using VOSviewer [,]. Limitations: the authors did not take into account many valuable publications in the study, instead taking into account the need to narrow the thematic scope of the described issues. Source: own elaboration based on 343 references [,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,].
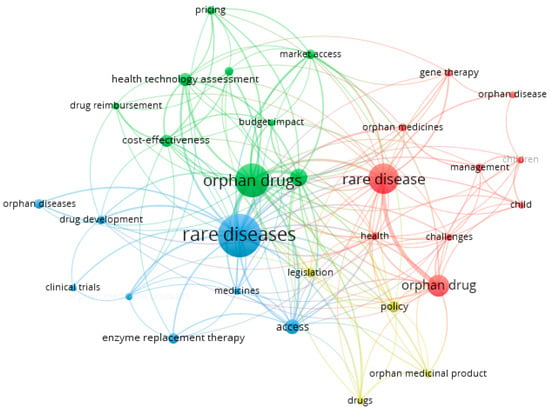
Figure 4.
Mapping latent variables from keywords of the publications on issues connected with rare diseases. Note: Technical publication on mapping and clustering using VOSviewer [,]. Limitations: the authors did not take into account many valuable publications in the study, instead taking into account the need to narrow the thematic scope of the described issues. Source: own elaboration based on 343 references [,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,].
Based on the titles and abstracts of the analyzed publications, 9189 terms (latent variables) were found, 746 of which appeared five times in the text. On their basis, using the “association length” method, nine clusters were found in the connection network, with a total number of 96,778 connections (Figure 3):
- Cluster 1—red (149 items);
- Cluster 2—green (138 items);
- Cluster 3—blue (104 items);
- Cluster 4—yellow (92 items);
- Cluster 5—purple (82 items);
- Cluster 6—celeste (74 items);
- Cluster 7—orange (47 items);
- Cluster 8—brown (34 items);
- Cluster 9—pink (26 items).
Using bibliographic data and analyzing only “keywords”, 31 keywords were selected (out of 1724) that appeared at least five times in the text (full counting). Four clusters with 174 connections were obtained (Figure 4):
- Cluster 1 (red) includes 10 latent variables: challenges, child, children, gene therapy, health, management, orphan disease, orphan drug, orphan medicines, and rare disease.
- In cluster 2 (green), there are nine latent variables: budget impact, cost-effectiveness, drug reimbursement, health policy, health technology assessment, market access, orphan drug, pricing, and reimbursement.
- Cluster 3 (blue) includes seven latent variables: access, clinical trials, drug development, enzyme replacement therapy, medicines, orphan diseases, and rare disease.
- Cluster 4 (yellow) includes four latent variables: drugs, legislation, orphan medicine product, and policy.
Individual clusters can be assigned a perspective that seems to bind together the keywords extracted within it. Thus, cluster 1 is the perspective of the patient; cluster 2 is the perspective of the policy maker/payer responsible for reimbursement in a given country; cluster 3 is the perspective of the researcher/inventor/manufacturer of the drug, while cluster 4 may bring together all stakeholders in international agreements and solutions to better meet the needs of rare disease patients. All perspectives naturally intersect, which only underscores the need for inclusive and acceptable solutions for all parties.
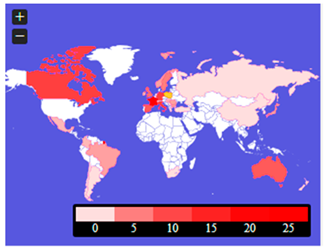
Figure 5 is the publications’ content maps. They include geographical locations (probably country cases, country clinical trials, or country system solutions) mentioned in the abstracts and keywords of the analyzed publications. These threads require further in-depth analysis, set in the context of the genetic determinants of the area on the one hand and the health policies of individual countries on the other.
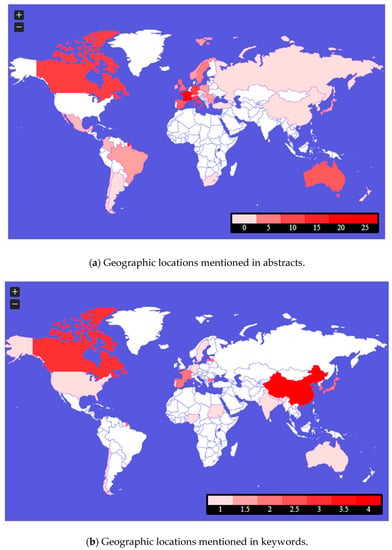
Figure 5.
Publications’ content maps from abstracts and keywords. Note: the more intense the color in the legend, the more often the geographic location is mentioned. Source: own elaboration based on 343 references [,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,].
Figure 5 shows which locations were most often referred to by the authors in the context of rare diseases and medical availability. The abstracts show that such countries were: France, Germany, Canada, Italy, Australia, and Great Britain. There were also countries such as Mexico, Columbia, Brazil, Argentina, Chile, South Africa, Spain, Ireland, Switzerland, the Netherlands, Poland, Sweden, Norway, Slovakia, Hungary, Serbia, Romania, Bulgaria, Greece, Turkey, Russia, China, Korea, Japan, and New Zealand. However, in keywords, they most often referred to countries such as China and Canada, Japan, Korea, Bulgaria, Latvia, France, and Spain. There were also countries such as the United States, Chile, Nigeria, Sudan, Portugal, Germany, Sweden, India, Nepal, and Australia.
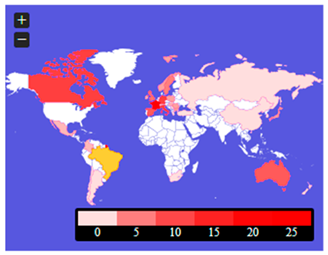
Figure 6 presents word clouds again, but in the context of spatial differentiation. The case of Poland and Brazil, which had the same number of presentations in the abstracts, was taken at random.
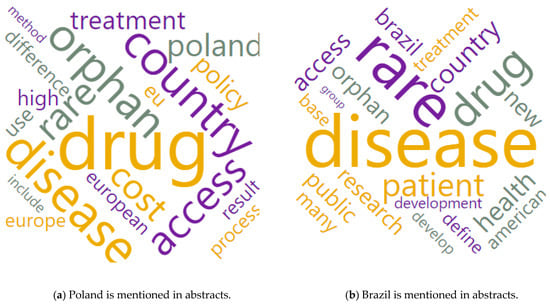
Figure 6.
Word clouds of terms from abstracts connected with geographic locations. Limitations: the authors did not take into account many valuable publications in the study, instead taking into account the need to narrow the thematic scope of the described issues. Source: own elaboration based on 343 references [,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,].
Figure 6 shows that the case of Poland was characterized by an orientation to the European market, medical availability in terms of costs, differences in treatment methods, results, treatment process, and the sector policy itself. On the other hand, in the case of Brazil, the focus was mainly on the American market, development, the role of the patient, access to public goods, and research development. The economic difference between these cases may lead to the supposition that in the case of Brazil, the threads focus on the availability of public health care in the case of rare diseases and the case of Poland—on the cost (e.g., from private funds) of such care. Two different approaches may be the aftermath of the policy of financing the treatment of rare diseases (or even the lack thereof), the level of economic development of the country, and the wealth of the society (measured by GDP/capita purchasing power parity (PPP)) and the necessity of health emigration.
Based on Table 1, five topics were identified, which are shaped by keywords. Topic modeling allows the discovery of research issues based on clusters of words from each publication (document). One document can contain many subjects with different relationships and proportions. Among them, keywords have been distinguished that strongly represent the topic and those that correspond to it to a lesser extent (links are not transparent). For example, if the text contains many references to China, HTA, policy, rare, insurance, and country, and no reference to a product, designation, FDA, or approval, it falls under topic 4. If the text only refers to rare diseases, care, patient, and R&D sphere, but does not refer to costs, price, drugs, HTA, or orphan, it belongs to topic 2. Based on the topic consistency score = 0.3841, it can be concluded that it is good but moderate topic consistency in 343 publications. This conclusion is consistent with the conclusion from the latent variable mapping (Figure 3 and Figure 4). It was also the basis for narrowing down the topic modeling criteria.

Table 1.
Topic modeling using latent semantic indexing (LSI).
Topic modeling, taking into account an additional criterion, i.e., referring to the country in the abstract, is presented in Table 2.

Table 2.
Topic modeling using latent semantic indexing (LSI) for countries.
Table 2 shows 3 thematic groups for selected cases: Poland and Brazil. For example, if the text frequently refers to such terms as drug, country, disease, orphan, requirement, rare, access, GDP, MCDA, and HTA, it represents the first thematic group (the case of Poland). However, if in the text there are many references to GDP per capita on an annual basis to costs but without the words, MCDA, OD, Russia, criterion, or world, the publications represent the 2nd thematic group (the Polish case). In the case of frequent reference to the words: drug, regard, LA, market, Latin, orphan, and EU, but excluding the words: policy, patient, and company, the topic represents the 3rd thematic group for the Brazil case. The simultaneous appearance of the words: disease, rare, policy, country, patient, legislation, Latin, orphan, and clinical is characteristic of the modeling of the first group of the topic for the case of Brazil. Narrowing the reconnaissance by considering an additional (geographical) criterion increases topic consistency, which allows us to conclude that geographic location changes the optics of the approach to access to treatment in rare diseases. In publications where Brazil was mentioned, there is greater topic coherence in this area than in Poland (slight difference).
An extension of the above analysis is the recognition (profiling) of emotions according to the Plutchik classification (Table 3 and Figure 7).

Table 3.
Emotion recognition in publications with a selection of country.
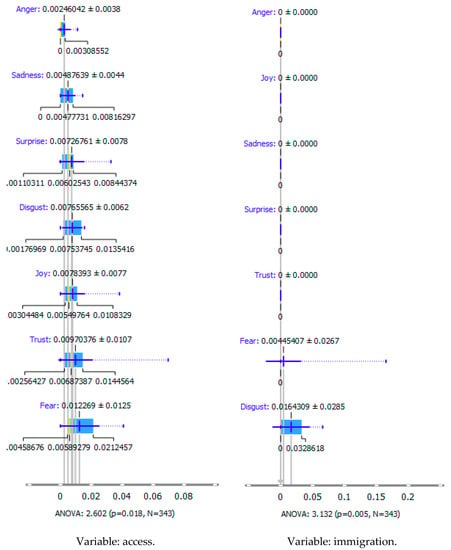
Figure 7.
Emotion recognition for health migration in rare diseases with access and immigration. Source: own elaboration based on 343 references [,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,].
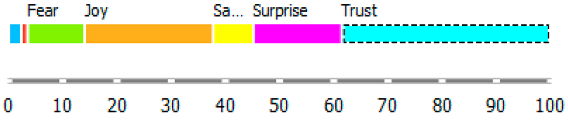
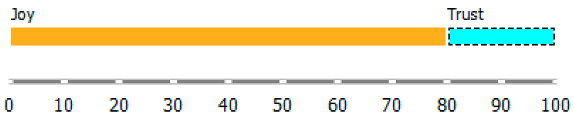
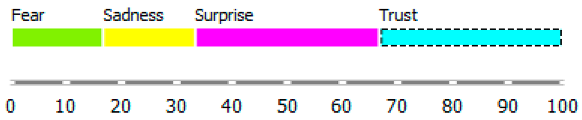
As shown in Table 3, the content of publications on migration due to rare diseases has a dynamic character and carries an emotional charge with different markings (positive and negative). For the most part, however, the positive dominate over the negative. When profiling emotions in publications where Poland was mentioned in the abstract, joy dominates over trust in a 4: 1 ratio, but these are positive emotions. However, where Brazil has been mentioned, there are more emotions: trust, surprise, sadness, and fear. Positive emotions versus negative emotions are 2:1. In the case of the analysis of all publications, where the abstract refers to any geographical location, the profile of emotions is more diverse, with the ratio of positive to negative emotions being approximately 4:1. By digging into the data, it can be identified the words that are the creators of these emotions in the message of content and define the scope.
During reading publications, it can be concluded that the term “access” in this issue evokes seven emotions, from positive to negative, which statistically differs from each other. “Access” carries a load of emotions, such as fear, trust, joy, disgust, surprise, sadness, and anger. Another term that evokes emotions and is statistically significant from the point of view of profiling emotions is “immigration”. This term evokes negative emotions: fear and disgust. The emotion of the word “emigration” could not be recognized. The issue of immigration has appeared in publications in the context of studying the regularities characteristic of migrant communities and their impact on the solutions offered/postulated in the host country.
Summing up, it can be said that the most significant ranges of emotions were recorded for the word access, such as fear, trust, and disgust. The most significant deviation (and the coefficient of variation) is that of trust. In the case of immigration, the more fantastic range of the scale of emotions is “disgust”, and the greater the coefficient of variation is “fear”. The word immigration does not evoke positive emotions: trust, surprise, joy, but also does not carry negative feelings such as sadness and anger in these publications.
4. Discussion
The literature has turned out to be scarce in the threads devoted to health emigration in the area of rare diseases. The scientific and expert communities discuss health inequalities, gaps in access to treatment, problems with reimbursement for orphan drugs and the need for change. According to the articles’ authors, it is worth filling this research gap. After all, emigration for medicine, dubbed “health emigration” by the authors, raises several micro and macro consequences—for the family in question and the system [,]. Moreover, they cite these arcane arguments as part of the discussion.
Although the problem still affects a narrow range of people, its importance is significant since a possible solution will stimulate the development of other much-needed orphan therapies. However, the process requires cross-state and cross-sectoral agreements [,,,]. The efforts of the last two decades, made internationally and by individual countries to eliminate restrictions and fight the exclusion of this group of societies, have brought many positive changes []. However, disparities in the implementation of pro-patient solutions and funding for rare disease treatment will exacerbate the phenomenon of health emigration []. Emigration, while paradoxically solving the individual patient’s problem, distorts the pattern of systemic solutions. If the patient (or his or her family) finds a solution in another country, the other stakeholders (the drug manufacturer and the reimbursement decision maker in the country of origin) have no incentive to seek a reimbursement compromise for the original outlet.
The incentive system for the science and technology sector has significantly increased the number of new therapies. Between 2010 and 2021, six hundred and sixty new molecules previously approved by the EMA were launched on the EU market, 19% of which were orphan products []. Orphan products are no longer a niche; they are one of the fastest-growing areas of pharmaceutical manufacturing. Experts estimate that by 2026 orphan drugs will account for 20% of all prescription drug sales and 30% of the global drug pipeline’s value []. The enormous potential does not translate into tangible health benefits due to gaps in access to therapies. This problem is raised in the European pharmaceutical strategy, which emphasizes the need to analyze the reasons for the deferred marketing of centrally authorized products []. August 2022 marked the end of the pilot program “Market launch of Centrally Authorized Medicinal Products,” initiated by the EC in cooperation with the EMA, EU countries and potential marketing authorization holders to analyze the problem []. In the Visegrad countries, the highest aggregate level of restrictions on access to modern treatment was found for rare diseases. To illustrate, Poland’s average time from registration to reimbursement for all drugs included in the study was 940 days, 3.4 years. The time between registration for an indication and reimbursement decision was longest for new therapies for mucoviscidosis (7.4 years) and diabetes (5.2 years) and shortest for NSCLC (2.3 years) and SMA (1.6 years) []. An example outside the list of diseases shown in the report can also be an ultra-rare metabolic disease—ceroid lipofuscinosis neuronal type 2, the treatment of which is financed in selected European countries but not in Poland, even under the procedure of emergency access to drug technology []. This situation results in the emigration of the vast majority of newly diagnosed patients to neighbouring Germany [].
The challenges are many, but awareness is definitely growing, and so is the stature of rare diseases. The last decade has brought several ideas, solutions and recommendations for positive change for patients. Many are under the auspices of EURORDIS (European Organization for Rare Diseases), which aims to increase the number of new therapies for rare diseases annually by 3 to 5 times by 2025 while lowering their cost by 3 to 5 times []. The UN, the WHO, and IRDiRC are moving in the same direction—rare diseases are an essential link in the chain of sustainable development [,,,]. A recent Rare 2030 trends study shows that increasing strains on healthcare budgets will force the emergence of new models of care delivery while inequalities in access to treatment and care continue to grow across Europe among people with rare diseases []. It is essential to eliminate gaps in access to orphan therapies and increase their supply to develop a consensus that benefits patients, manufacturers and payers. Restricting markets to a narrow customer base limits drug pricing and risk-sharing mechanisms. Under current circumstances, with deadly diseases and often the only treatment options, health emigration seems to have a forced character. This situation prompts intensified discussion of creating supra-state governance rules for rare diseases, including therapy financing. The issue is complex and challenging but has its grounding in the context of rare diseases and will be taken up by the article’s authors in future research.
5. Conclusions
The goal of the study, which was to systematize knowledge on health emigration in rare diseases, mainly using machine learning, was achieved. The main conclusions (the context is described further) are as follows:
- ML allowed us to objectively diagnose a research gap in the area of rare disease management—the issue of health emigration is not directly studied;
- Analysis of grey literature (including industry reports) confirmed the gap;
- The need to define the term “health emigration”;
- Health emigration as a derivative of health inequalities should be included on the social cost side of the rare disease management model at the national and supranational levels;
- The topic of health emigration in rare diseases needs further research at several levels.
The analysis of scientific literature, as well as industry literature, provided answers to the research questions posed. Namely, the application of machine learning in terms of article titles and keywords resulted in extracting the most critical words, among which the following dominated: disease, rare, orphan, drug, patient, access, and health. An in-depth analysis of the latent words made it possible to see the researchers’ concentration around four complementary thematic clusters—the perspectives of the patient, the decision-maker, the manufacturer, and the perspective integrating all stakeholders. The authors found the indicated theme of geographic differentiation inspiring, although requiring further in-depth analysis taking into account the specifics of the areas in question to draw firm conclusions. The review identified researchers’ research interests in rare diseases within the framework of socioeconomic issues but lacked direct threads related to health emigration. Available industry reports point to the problem of ever-increasing inequalities in access to treatment and care in European countries but does not directly address the derivative of these inequalities, i.e., health emigration and the effects it generates.
Given the diagnosed research gap in the definition, characteristics, and effects of “health emigration”, the authors offer their definition. Health emigration is a trip to another country to live permanently or for a temporary stay caused by the desire to obtain more effective health care not available in the country of origin. This situation is not health tourism, but a permanent/long-term trip since rare diseases tend to be chronic and require long-term treatment. Significantly, the problem affects the whole family, as rare diseases mainly manifest during childhood. Although the decision to emigrate is at the family’s discretion, it often bears the hallmarks of a forced one since remaining in the country would mean death for the patient in the short or long term. It mainly affected families who emigrate, for whom the first therapeutic option, possibly another far more effective one, has appeared on the market. The social and economic costs that emigration entails should be considered in the analysis process for reimbursement decisions.
Despite increasing awareness in public about rare diseases and their growing prominence in health policies, disparities between countries in implementing pro-patient solutions and funding for treating these diseases are very pronounced. With simultaneous access to information, awareness and knowledge of the patient community, decisions to leave the country to obtain treatment are made frequently. Assuming that the number of orphan therapies implemented will continue to grow without innovative solutions for equal access to reimbursed treatment, the phenomenon of health emigration in rare diseases will increase.
This study has several limitations in terms of the following:
- The selection of the base of the analyzed literature;
- Tthe supplementation of the analyzes with selected industry reports;
- The lack of references to domestic migration;
The article helped identify a gap in researcher activity on the topic of health emigration in rare diseases; therefore, the article in question only provides suggestions for further research to improve health policy for rare diseases in the future.
Only articles indexed in databases were used in the analysis: Scopus, the Web of Science and PubMed, which may have caused the authors to omit valuable literature items. To complete the picture, they used an analysis of selected industry reports where, to the best of the authors’ knowledge, issues of health inequality and the gap in access to therapy were addressed. At the same time, these reports are geographically piecemeal, dealing with selected markets and do not analyze the problem of health emigration. A related issue, nevertheless not addressed in this work, is migration and travel within a country. Access to medical services, especially specialized ones, is limited to one/some specialized centres in a country, which reduces the comfort of the chronically treated patient. Managing access to treatment within a country to increase patient comfort is an area for consideration in addressing health needs in rare diseases. Therefore, the authors of the article propose and, in their further research, will address these issues in terms of the scale and effects of emigration on several levels: for the family, the country of origin, the destination country, and the rare disease management system in general. In the future, it is essential to add other languages and sources to the database, such as medical organizations, regional and global health reports, and other publications that are not focused on business and science.
Author Contributions
Conceptualization, M.S.-K., I.C. and E.S.; methodology, M.S.-K., I.C. and E.S.; validation, M.S.-K., I.C. and E.S.; formal analysis, E.S.; investigation, M.S.-K., I.C. and E.S.; resources, M.S.-K. and I.C.; writing—original draft preparation, M.S.-K., I.C. and E.S.; writing—review and editing, M.S.-K., I.C. and E.S.; visualization, M.S.-K., I.C. and E.S.; supervision, M.S.-K., I.C. and E.S.; project administration, M.S.-K., I.C. and E.S.; funding acquisition, M.S.-K., I.C. and E.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- The European Parliament and the Council. Regulation (EC) No 141/2000 of the European Parliament and of the Council of 16 December 1999 on Orphan Medicinal Products. Off. J. Eur. Communities 2000, L18/1. [Google Scholar]
- Libura, M.; Władusiuk, M.; Małowicka, M.; Grabowska, E.; Gałązka-Sobotka, M.; Gryglewicz, J. Choroby Rzadkie w Polsce: Stan Obecny i Perspektywy; Uczelnia Łazarskiego: Warszawa, Poland, 2016; ISBN 9788364054730. [Google Scholar]
- Wakap, N.S.; Lambert, D.M.; Olry, A.; Rodwell, C.; Gueydan, C.; Lanneau, V.; Murphy, D.; Le Cam, Y.; Rath, A. Estimating cumulative point prevalence of rare diseases: Analysis of the Orphanet database. Eur. J. Hum. Genet. 2020, 28, 165–173. [Google Scholar] [CrossRef]
- EURORDIS-Rare Diseases Europe. High-Level Ministerial Conference: Care and Innovation Pathways for a European Rare Diseases Policy; EURORDIS-Rare Diseases Europe: Paris, France, 2022. [Google Scholar]
- Juchniewicz, M.; Rzempała, J.; Skweres-Kuchta, M. Initiating and Defining a Sustainable Project on the Example of Rare Disease Therapy Initiating and Defining a Sustainable Project on the Example of Rare Disease Therapy. Eur. Res. Stud. J. 2021, XXIV, 663–681. [Google Scholar] [CrossRef]
- Emigration. Definition & Meaning. Available online: https://www.merriam-webster.com/dictionary/emigration (accessed on 17 September 2022).
- Kenton, W. What Is Emigration? Available online: https://www.investopedia.com/terms/e/emigration.asp (accessed on 17 September 2022).
- Emigration—Meaning, Definition. Available online: https://dictionary.cambridge.org/dictionary/english/emigration (accessed on 17 September 2022).
- Migration—Meaning, Definition. Available online: https://dictionary.cambridge.org/dictionary/english/migration (accessed on 17 September 2022).
- Ministerstwo Edukacji i Nauki. Zintegrowana Platforma Edukacyjna. Available online: https://zpe.gov.pl/a/przeczytaj/D10e2ylE1?fbclid=IwAR2lqsn4bhaOt_-7XG-fNh3lv_tPTwXn2qXGwqsenIVQQCkPEwZNt6xVpzM (accessed on 19 September 2022).
- Analiza Migracji Klientów (Churn)—StatSoft Polska. Available online: https://www.statsoft.pl/Rozwiazania/Zastosowania-biznesowe/Analiza-migracji-klientow-churn/ (accessed on 17 September 2022).
- Janicki, W. Migration Theories—a Review. Ann. Univ. Mariae Curie-Skłodowska Sect. B Geogr. Geol. Mineral. Et Petrogr. 2007, 62, 285–304. [Google Scholar]
- Imigracja—Definicja, Synonimy, Przykłady Użycia. Available online: https://sjp.pwn.pl/slowniki/imigracja.html (accessed on 19 September 2022).
- Immigration Definition—US Immigration Glossary. Available online: https://www.usimmigration.org/glossary/immigration (accessed on 17 September 2022).
- Zakład Demografii i Gerontologii Społecznej UŁ Migracje Ludności. Materiały Dydaktyczne. Available online: http://www.demografia.uni.lodz.pl/dlastud/migracje.pdf?fbclid=IwAR21hmPCRMo_IS1iERNB8Wmb-v37EyPagtYOpsSQYM6xa8T_psZcCg5Ox_k (accessed on 19 September 2022).
- Ringold, S.; Burke, A.; Glass, R.M. Refugee Mental Health. JAMA 2005, 294, 646. [Google Scholar] [CrossRef]
- Yorimitsu, M. A Review on the Determinants of Migration. Hitotsubashi J. Soc. Stud. 1985, 17, 17–27. [Google Scholar]
- Patnaik, B.C.M.; Satpathy, I.; Mandal, A. Determinants of Migration—A Review of Literature. Online Int. Interdiscip. Res. J. 2014, 4, 349–357. [Google Scholar]
- De Haas, H. The Determinants of International Migration. Conceptualising Policy, Origin and Destination Effects. IMI Work. Pap. Ser. 2011, 32. [Google Scholar]
- Simpson, N.B. Demographic and Economic Determinants of Migration. Push and Pull Factors Drive the Decision to Stay or Move. IZA World Labor 2022, 373. [Google Scholar] [CrossRef]
- De Haas, H.; Czaika, M.; Flahaux, M.-L.; Mahendra, E.; Natter, K.; Vezzoli, S.; Villares-Varela, M. Notes and Commentary International Migration: Trends, Determinants, and Policy Effects. Popul. Dev. Rev. 2019, 45, 885–922. [Google Scholar] [CrossRef]
- Massimo, L.M.; Wiley, T.J.; Caprino, D. Health Emigration: A Challenge in Paediatric Oncology. J. Child Health Care 2008, 12, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Skweres-Kuchta, M. Choroby Rzadkie Wśród Dzieci—Zarządzanie Systemem z Perspektywy Rodziny Pacjenta. In Zdrowie i style życia. Wyzwania Ekonomiczne i Społeczne; Nowak, W., Szalonka, K., Eds.; E-Wydawnictwo; Prawnicza i Ekonomiczna Biblioteka Cyfrowa. Wydział Prawa, Administracji i Ekonomii Uniwersytetu Wrocławskiego: Wrocław, Poland, 2019; pp. 299–310. [Google Scholar]
- Czy Emigracja Zdrowotna to Jedyny Lek Na CLN2? Available online: https://wpolityce.pl/spoleczenstwo/467935-czy-emigracja-zdrowotna-to-jedyny-lek-na-cln2 (accessed on 29 September 2022).
- Krawitz, P.M. Artificial Intelligence in the Diagnosis of Rare Disorders: The Development of Phenotype Analysis. Bundesgesundheitsblatt Gesundh. Gesundh. 2022, 65, 1159–1163. [Google Scholar] [CrossRef]
- Mahesh, B. Machine Learning Algorithms—A Review. Int. J. Sci. Res. 2018, 9, 381–386. [Google Scholar] [CrossRef]
- el Naqa, I.; Murphy, M.J. What Is Machine Learning? In Machine Learning in Radiation Oncology; el Naqa, I., Li, R.M.M., Eds.; Springer: Cham, Switzerland, 2015; pp. 3–11. [Google Scholar]
- Kamila, N.K.; Fonda, J.; Pani, S.K.; Das, R.; Islam, S.M.N.; Bharti, P.K.; Muduli, K. Machine Learning Model Design for High Performance Cloud Computing & Load Balancing Resiliency: An Innovative Approach. J. King Saud Univ. —Comput. Inf. Sci. 2022, 34, 9991–10009. [Google Scholar] [CrossRef]
- Ren, Y.-S.; Ma, C.-Q.; Kong, X.-L.; Baltas, K.; Zureigat, Q. Past, Present, and Future of the Application of Machine Learning in Cryptocurrency Research. Res. Int. Bus. Finance 2022, 63, 101799. [Google Scholar] [CrossRef]
- Lundberg, I.; Brand, J.E.; Jeon, N. Researcher Reasoning Meets Computational Capacity: Machine Learning for Social Science. Soc. Sci. Res. 2022, 108, 102807. [Google Scholar] [CrossRef]
- Document Search—Web of Science Core Collection. Available online: https://www.webofscience.com/wos/woscc/basic-search (accessed on 1 August 2022).
- Scopus—Document Search. Available online: https://www.scopus.com/search/form.uri?display=basic#basic (accessed on 1 August 2022).
- PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov (accessed on 1 August 2022).
- Ning, W.; Lin, S.; Zhou, J.; Guo, Y.; Zhang, Y.; Peng, D.; Deng, W.; Xue, Y. WocEA: The Visualization of Functional Enrichment Results in Word Clouds. J. Genet. Genom. 2018, 45, 415–417. [Google Scholar] [CrossRef]
- Jin, Y. Development of Word Cloud Generator Software Based on Python. Procedia Eng. 2017, 174, 788–792. [Google Scholar] [CrossRef]
- van Eck, N.J.L.; Waltman, L. Visualizing Bibliometric Networks. In Measuring Scholarly Impact; Ding, Y., Rousseau, R., Wolfram, D., Eds.; Springer International Publishing: Cham, Switzerland, 2014; pp. 285–320. ISBN 19103778_13. [Google Scholar]
- Szaruga, E.; Załoga, E. Qualitative-Quantitative Warning Modeling of Energy Consumption Processes in Inland Waterway Freight Transport on River Sections for Environmental Management. Energies 2022, 15, 4660. [Google Scholar] [CrossRef]
- Ishihara, S. Score-Based Likelihood Ratios for Linguistic Text Evidence with a Bag-of-Words Model. Forensic Sci. Int. 2021, 327, 110980. [Google Scholar] [CrossRef]
- Crain, S.P.; Zhou, K.; Yang, S.H.; Zha, H. Dimensionality Reduction and Topic Modeling: From Latent Semantic Indexing to Latent Dirichlet Allocation and Beyond. Mining Text Data 2012, 9781461432234, 129–161. [Google Scholar] [CrossRef]
- Dewangan, J.K.; Sharaff, A.; Pandey, S. Improving Topic Coherence Using Parsimonious Language Model and Latent Semantic Indexing. Lect. Notes Electr. Eng. 2020, 601, 823–830. [Google Scholar] [CrossRef]
- Colneric, N.; Demsar, J. Emotion Recognition on Twitter: Comparative Study and Training a Unison Model. IEEE Trans. Affect. Comput. 2020, 11, 433–446. [Google Scholar] [CrossRef]
- Szaruga, E.; Załoga, E. Machine Learning in Exploration the Decoupling Paradigm in Transport. Procedia Comput. Sci. 2022, 207, 3904–3914. [Google Scholar] [CrossRef]
- Sesana, M.M.; Cuca, B.; Iannaccone, G.; Brumana, R.; Caccavelli, D.; Gay, C. Geomapping Methodology for the GeoCluster Mapping Tool to Assess Deployment Potential of Technologies for Energy Efficiency in Buildings. Sustain. Cities Soc. 2015, 17, 22–34. [Google Scholar] [CrossRef]
- Reinhard, C.; Bachoud-Lévi, A.C.; Bäumer, T.; Bertini, E.; Brunelle, A.; Buizer, A.I.; Federico, A.; Gasser, T.; Groeschel, S.; Hermanns, S.; et al. The European Reference Network for Rare Neurological Diseases. Front. Neurol. 2021, 11, 616569. [Google Scholar] [CrossRef]
- Forman, J.; Taruscio, D.; Llera, V.A.; Barrera, L.A.; Coté, T.R.; Edfjäll, C.; Gavhed, D.; Haffner, M.E.; Nishimura, Y.; Posada, M.; et al. The Need for Worldwide Policy and Action Plans for Rare Diseases. Acta Paediatr. Int. J. Paediatr. 2012, 101, 805–807. [Google Scholar] [CrossRef]
- Schey, C.; Postma, M.J.; Krabbe, P.F.M.; Topachevskyi, O.; Volovyk, A.; Connolly, M. Assessing the Preferences for Criteria in Multi-Criteria Decision Analysis in Treatments for Rare Diseases. Front. Public Health 2020, 8, 162. [Google Scholar] [CrossRef]
- Hyry, H.I.; Roos, J.C.; Manuel, J.; Cox, T.M. The Legal Imperative for Treating Rare Disorders. Orphanet J. Rare Dis. 2013, 8, 1. [Google Scholar] [CrossRef]
- Münster, T.; Richards, E.; Dillow, J.; Percesepe, A. OrphanAnesthesia—A Common Project of the Scientific Working Group of Paediatric Anaesthesia of the German Society of Anaesthesiology and Intensive Care Medicine. Anasthesiol. Und Intensivmed. 2019, 60, S82–S94. [Google Scholar] [CrossRef]
- Detiček, A.; Locatelli, I.; Kos, M. Patient Access to Medicines for Rare Diseases in European Countries. Value Health 2018, 21, 553–560. [Google Scholar] [CrossRef]
- Stakisaitis, D.; Spokiene, I.; Jukevicius, J.; Valuckas, K.P.; Baiardi, P. Access to Information Supporting Availability of Medicines for Patients Suffering from Rare Diseases Looking for Possible Treatments: The EuOrphan Service. Medicina 2007, 43, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Martín, J.; Díaz-Rodríguez, L.; Piqueras-Sola, B.; Rodríguez-Blanque, R.; Bermejo-Fernández, A.; Sánchez-García, J.C. Hajdu-Cheney Syndrome: A Systematic Review of the Literature. Int. J. Environ. Res. Public Health 2020, 17, 6174. [Google Scholar] [CrossRef]
- Spokiene, I. Legal Assessment of Current Situation on Orphan Patients in Lithuania. Medicina 2008, 44, 571–576. [Google Scholar] [CrossRef]
- de Andres-Nogales, F.; Cruz, E.; Calleja, M.Á.A.; Delgado, O.; Gorgas, M.Q.; Espin, J.; Mestre-Ferrandiz, J.; Palau, F.; Ancochea, A.; Arce, R.; et al. A Multi-Stakeholder Multicriteria Decision Analysis for the Reimbursement of Orphan Drugs (FinMHU-MCDA Study). Orphanet J. Rare Dis. 2021, 16, 1–12. [Google Scholar] [CrossRef]
- Karpman, D.; Höglund, P. Orphan Drug Policies and Use in Pediatric Nephrology. Pediatr. Nephrol. 2017, 32, 1–6. [Google Scholar] [CrossRef]
- Wilson, C. Policies and Research Funding. Orphan Drugs: Underst. Rare Dis. Mark. Its Dyn. 2013, 145–186. [Google Scholar] [CrossRef]
- Pavan, S.; Rommel, K.; Marquina, M.E.M.; Höhn, S.; Lanneau, V.; Rath, A. Clinical Practice Guidelines for Rare Diseases: The Orphanet Database. PLoS ONE 2017, 12, e0170365. [Google Scholar] [CrossRef] [PubMed]
- Heard, J.M.; Vrinten, C.; Schlander, M.; Bellettato, C.M.; Van Lingen, C.; Scarpa, M.; Matthijs, G.; Nassogne, M.C.; Debray, F.G.; Roland, D.; et al. Availability, Accessibility and Delivery to Patients of the 28 Orphan Medicines Approved by the European Medicine Agency for Hereditary Metabolic Diseases in the MetabERN Network. Orphanet J. Rare Dis. 2020, 15, 1–10. [Google Scholar] [CrossRef]
- Almalki, Z.S.; Alahmari, A.K.; Guo, J.J.; Kelton, C.M.L. Access to Orphan Drugs in the Middle East: Challenge and Perspective. Intractable Rare Dis. Res. 2012, 1, 139–143. [Google Scholar] [CrossRef]
- Szegedi, M.; Molnár, M.J.; Boncz, I.; Kosztolányi, G. Focus shifts in the Hungarian reimbursement system: Funding of orphan medicinal products for rare disease patients in Hungary: Financing of orphan medicines. Orv Hetil. 2014, 155, 1735–1741. [Google Scholar] [CrossRef]
- Hanisch, M.; Hanisch, L.; Benz, K.; Kleinheinz, J.; Jackowski, J. Development of a Database to Record Orofacial Manifestations in Patients with Rare Diseases: A Status Report from the ROMSE (Recording of Orofacial Manifestations in People with Rare Diseases) Database. Br. J. Oral Maxillofac. Surg. 2017, 55, 500–503. [Google Scholar] [CrossRef] [PubMed]
- Mayrides, M.; Ruiz De Castilla, E.M.; Szelepski, S.; de Castilla, E.M.R.; Szelepski, S.; Ruiz De Castilla, E.M.; Szelepski, S.; de Castilla, E.M.R.; Szelepski, S.; Ruiz De Castilla, E.M.; et al. A Civil Society View of Rare Disease Public Policy in Six Latin American Countries. Orphanet J. Rare Dis. 2020, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hernández, A.M.B.; Almela, J.A.S. The Debate on Rare Diseases: A Look at Media Response. Metode 2016, 6, 209–213. [Google Scholar] [CrossRef]
- Kole, A.; Faurisson, F. Rare Diseases Social Epidemiology: Analysis of Inequalities. Adv. Exp. Med. Biol. 2010, 686, 223–250. [Google Scholar] [CrossRef] [PubMed]
- Zelei, T.; Molnár, M.J.; Szegedi, M.; Kaló, Z.; Molnar, M.J.; Szegedi, M.; Kalo, Z. Systematic Review on the Evaluation Criteria of Orphan Medicines in Central and Eastern European Countries. Orphanet J. Rare Dis. 2016, 11, 72. [Google Scholar] [CrossRef]
- Bogart, K.; Hemmesch, A.; Barnes, E.; Blissenbach, T.; Beisang, A.; Engel, P. Chloe Barnes Advisory Council on Rare Diseases Healthcare Access, Satisfaction, and Health-Related Quality of Life among Children and Adults with Rare Diseases. Orphanet J. Rare Dis. 2022, 17, 196. [Google Scholar] [CrossRef]
- Dunkle, M.; Pines, W.; Saltonstall, P.L. Advocacy Groups and Their Role in Rare Diseases Research. Adv. Exp. Med. Biol. 2010, 686, 515–525. [Google Scholar] [CrossRef]
- Kerpel-Fronius, S.; Baroutsou, V.; Becker, S.; Carlesi, R.; Collia, L.; Franke-Bray, B.; Kleist, P.; Kurihara, C.; Laranjeira, L.F.; Matsuyama, K.; et al. Development and Use of Gene Therapy Orphan Drugs—Ethical Needs for a Broader Cooperation Between the Pharmaceutical Industry and Society. Front. Med. 2020, 7, 608249. [Google Scholar] [CrossRef]
- Pearson, I.; Rothwell, B.; Olaye, A.; Knight, C. Economic Modeling Considerations for Rare Diseases. Value Health 2018, 21, 515–524. [Google Scholar] [CrossRef]
- Gupta, R.; Bollyky, T.J.; Cohen, M.; Ross, J.S.; Kesselheim, A.S. Affordability and Availability of Off-Patent Drugs in the United States—The Case for Importing from Abroad: Observational Study. BMJ 2018, 360, k381. [Google Scholar] [CrossRef]
- Martins, P. Orphan Anesthesia. Anasthesiol. Und Intensivmed. 2020, 61, S108–S118. [Google Scholar] [CrossRef]
- Pavlović, N.; Stanimirov, B.; Stojančević, M.; Paut-Kusturica, M.; Stoimenova, A.; Goločorbin-Kon, S.; Mikov, M. An Insight on Differences in Availability and Reimbursement of Orphan Medicines among Serbia, Bulgaria and Sweden. Biotechnol. Biotechnol. Equip. 2012, 26, 26–3236. [Google Scholar] [CrossRef]
- Bavisetty, S.; Grody, W.W.; Yazdani, S. Emergence of Pediatric Rare Diseases Review of Present Policies and Opportunities for Improvement. Rare Dis. 2013, 1, 1–5. [Google Scholar] [CrossRef]
- Kanters, T.A.; Hakkaart, L.; Rutten-Van Mölken, M.P.M.H.; Redekop, W.K. Access to Orphan Drugs in Western Europe: Can More Systematic Policymaking Really Help to Avoid Different Decisions about the Same Drug? Expert Rev. Pharmacoecon Outcomes Res. 2015, 15, 557–559. [Google Scholar] [CrossRef] [PubMed]
- Soon, S.S.; Lopes, G.; Lim, H.Y.; Wong-Rieger, D.; Bahri, S.; Hickinbotham, L.; Jha, A.; Ko, B.S.; MacDonell, D.; Pwu, J.R.F.; et al. A Call for Action to Improve Access to Care and Treatment for Patients with Rare Diseases in the Asia-Pacific Region. Orphanet J. Rare Dis. 2014, 9, 137. [Google Scholar] [CrossRef]
- Chalamon, I. Consumer Resistance between Conflict and Cooperation: The Extreme Case of Orphan Drugs. Eur. J. Mark 2011, 45, 1736–1745. [Google Scholar] [CrossRef]
- Robinson, S.W.; Brantley, K.; Liow, C.; Russell Teagarden, J. An Early Examination of Access to Select Orphan Drugs Treating Rare Diseases in Health Insurance Exchange Plans. J. Manag. Care Spec. Pharm. 2014, 20, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Derham, R. Rare Disease and Orphan Drug Leadership—CBI’s Seventh Annual Conference, Philadelphia, Pennsylvania, USA, 18–19 July 2012. Drugs Future 2012, 37, 829–831. [Google Scholar] [CrossRef]
- Gong, S.W.; Jin, S. Current Progress in the Management of Rare Diseases and Orphan Drugs in China. Intractable Rare Dis. Res. 2012, 1, 45–52. [Google Scholar] [CrossRef]
- Godeau, B. Objectives and organization of a reference center for adults. Bull. Acad. Natl. Med. 2009, 193, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Kamusheva, M.; Dimitrova, M.; Tachkov, K.; Petrova, G.; Mitkova, Z. Pharmacotherapeutic Patterns and Patients’ Access to Pharmacotherapy for Some Rare Diseases in Bulgaria—A Pilot Comparative Study. Front. Pharmacol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Hughes-Wilson, W.; Palma, A.; Schuurman, A.; Simoens, S. Paying for the Orphan Drug System: Break or Bend? Is It Time for a New Evaluation System for Payers in Europe to Take Account of New Rare Disease Treatments? Orphanet J. Rare Dis. 2012, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Pasquini, T.L.S.; Goff, S.L.; Whitehill, J.M. Navigating the U.S. Health Insurance Landscape for Children with Rare Diseases: A Qualitative Study of Parents’ Experiences. Orphanet J. Rare Dis. 2021, 16, 1–14. [Google Scholar] [CrossRef]
- Cannizzo, S.; Lorenzoni, V.; Palla, I.; Pirri, S.; Trieste, L.; Triulzi, I.; Turchetti, G. Rare Diseases under Different Levels of Economic Analysis: Current Activities, Challenges and Perspectives. RMD Open 2018, 4, 794. [Google Scholar] [CrossRef] [PubMed]
- Rixen, S. Rare diseases—A problem of healthcare-related social security law. Z Evid Qual Gesundhwes 2008, 102, 31–36. [Google Scholar] [CrossRef]
- Boudes, P.F. Clinical Studies in Lysosomal Storage Diseases Past, Present, and Future. Rare Dis. 2013, 1, e26690. [Google Scholar] [CrossRef]
- Blankart, C.R.; Stargardt, T.; Schreyögg, J. Availability of and Access to Orphan Drugs: An International Comparison of Pharmaceutical Treatments for Pulmonary Arterial Hypertension, Fabry Disease, Hereditary Angioedema and Chronic Myeloid Leukaemia. Pharmacoeconomics 2011, 29, 63–82. [Google Scholar] [CrossRef]
- Orofino, J.; Soto, J.; Casado, M.A.; Oyagez, I. Global Spending on Orphan Drugs in France, Germany, the UK, Italy and Spain during 2007. Appl. Health Econ. Health Policy 2010, 8, 301–315. [Google Scholar] [CrossRef]
- Winstone, J.; Chadda, S.; Ralston, S.; Sajosi, P. Review and Comparison of Clinical Evidence Submitted to Support European Medicines Agency Market Authorization of Orphan-Designated Oncological Treatments. Orphanet J. Rare Dis. 2015, 10, 1–7. [Google Scholar] [CrossRef]
- Bellgard, M.I.; Napier, K.R.; Bittles, A.H.; Szer, J.; Fletcher, S.; Zeps, N.; Hunter, A.A.; Goldblatt, J. Design of a Framework for the Deployment of Collaborative Independent Rare Disease-Centric Registries: Gaucher Disease Registry Model. Blood Cells Mol. Dis. 2018, 68, 232–238. [Google Scholar] [CrossRef]
- Ghedira, K.; Kouidhi, S.; Hamdi, Y.; Othman, H.; Kechaou, S.; Znaidi, S.; Haïtham, S.; Rabhi, I. Pathway Maps of Orphan and Complex Diseases Using an Integrative Computational Approach. Biomed Res. Int. 2020, 2020, 1–11. [Google Scholar] [CrossRef]
- Witkowska, J. Corporate Social Responsibility (CSR) of Innovative Pharmaceutical Corporations. The Case of BIOGEN. Comp. Econ. Res. 2018, 21, 45–62. [Google Scholar] [CrossRef]
- Thompson, R.; Abicht, A.; Beeson, D.; Engel, A.G.; Eymard, B.; Maxime, E.; Lochmüller, H. A Nomenclature and Classification for the Congenital Myasthenic Syndromes: Preparing for FAIR Data in the Genomic Era. Orphanet J. Rare Dis. 2018, 13, 211. [Google Scholar] [CrossRef] [PubMed]
- Drummond, M.; Towse, A. Orphan Drugs Policies: A Suitable Case for Treatment. Eur. J. Health Econ. 2014, 15, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Nestler-Parr, S.; Korchagina, D.; Toumi, M.; Pashos, C.L.; Blanchette, C.; Molsen, E.; Morel, T.; Simoens, S.; Kaló, Z.; Gatermann, R.; et al. Challenges in Research and Health Technology Assessment of Rare Disease Technologies: Report of the ISPOR Rare Disease Special Interest Group. Value Health 2018, 21, 493–500. [Google Scholar] [CrossRef]
- Annemans, L.; Makady, A. TRUST4RD: Tool for Reducing Uncertainties in the Evidence Generation for Specialised Treatments for Rare Diseases. Orphanet J. Rare Dis. 2020, 15, 1–9. [Google Scholar] [CrossRef]
- Sachs-Barrable, K.; Conway, J.; Gershkovich, P.; Ibrahim, F.; Wasan, K.M. The Use of the United States FDA Programs as a Strategy to Advance the Development of Drug Products for Neglected Tropical Diseases. Drug Dev. Ind. Pharm. 2014, 40, 1429–1434. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Chen, L. Orphan Drug Development in China—Turning Challenges into Opportunities. Intractable Rare Dis. Res. 2016, 5, 308–313. [Google Scholar] [CrossRef]
- Logviss, K.; Krievins, D.; Purvina, S. Rare Diseases and Orphan Drugs: Latvian Story. Orphanet J. Rare Dis. 2014, 9, 147. [Google Scholar] [CrossRef]
- Giugliani, L.; Vanzella, C.; Zambrano, M.B.; Donis, K.C.; Wallau, T.K.W.; Da Costa, F.M.; Giugliani, R. Clinical Research Challenges in Rare Genetic Diseases in Brazil. Genet. Mol. Biol. 2019, 42, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Luisetti, M.; Balfour-Lynn, I.M.; Johnson, S.R.; Miravitlles, M.; Strange, C.; Trapnell, B.C.; Van Bronswijk, H.; Vogelmeier, C. Perspectives for Improving the Evaluation and Access of Therapies for Rare Lung Diseases in Europe. Respir. Med. 2012, 106, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Potter, B.K.; Chakraborty, P.; Kronick, J.B.; Wilson, K.; Coyle, D.; Feigenbaum, A.; Geraghty, M.T.; Karaceper, M.D.; Little, J.; Mhanni, A.; et al. Achieving the “Triple Aim” for Inborn Errors of Metabolism: A Review of Challenges to Outcomes Research and Presentation of a New Practice-Based Evidence Framework. Genet. Med. 2013, 15, 415–422. [Google Scholar] [CrossRef]
- Simoens, S.; Cassiman, D.; Dooms, M.; Picavet, E. Orphan Drugs for Rare Diseases: Is It Time to Revisit Their Special Market Access Status? Drugs 2012, 72, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- Park, T.; Griggs, S.K.; Suh, D.C. Cost Effectiveness of Monoclonal Antibody Therapy for Rare Diseases: A Systematic Review. BioDrugs 2015, 29, 259–274. [Google Scholar] [CrossRef]
- Czech, M.; Baran-Kooiker, A.; Holownia-Voloskova, M.; Kooiker, C.; Sykut-Cegielska, J. Bridging East with West of Europe—A Comparison of Orphan Drugs Policies in Poland, Russia and the Netherlands. Acta Pol. Pharm. —Drug Res. 2018, 75, 1409–1422. [Google Scholar] [CrossRef]
- Yang, Y.; Kang, Q.; Hu, J.; Kong, F.; Tang, M.; He, J.; Jin, C. Accessibility of Drugs for Rare Diseases in China: Policies and Current Situation. Intractable Rare Dis. Res. 2019, 8, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Feltmate, K.; Janiszewski, P.M.; Gingerich, S.; Cloutier, M. Delayed Access to Treatments for Rare Diseases: Who’s to Blame? Respirology 2015, 20, 361–369. [Google Scholar] [CrossRef]
- Carr, D.R.; Bradshaw, S.E. Gene Therapies: The Challenge of Super-High-Cost Treatments and How to Pay for Them. Regen. Med. 2016, 11, 381–393. [Google Scholar] [CrossRef]
- Dunoyer, M. Accelerating Access to Treatments for Rare Diseases. Nat. Rev. Drug Discov. 2011, 10, 475–476. [Google Scholar] [CrossRef]
- Adkins, E.M.; Nicholson, L.; Floyd, D.; Ratcliffe, M.; Chevrou-Severac, H. Oncology Drugs for Orphan Indications: How Are HTA Processes Evolving for This Specific Drug Category? Clin. Outcomes Res. 2017, 9, 327–342. [Google Scholar] [CrossRef] [PubMed]
- Bogart, K.R.; Irvin, V.L. Health-Related Quality of Life among Adults with Diverse Rare Disorders. Orphanet J. Rare Dis. 2017, 12, 177. [Google Scholar] [CrossRef] [PubMed]
- Min, R.; Zhang, X.; Fang, P.; Wang, B.; Wang, H. Health Service Security of Patients with 8 Certain Rare Diseases: Evidence from China’s National System for Health Service Utilization of Patients with Healthcare Insurance. Orphanet J. Rare Dis. 2019, 14, 1–18. [Google Scholar] [CrossRef]
- Hyry, H.I.; Manuel, J.; Cox, T.M.; Roos, J.C.P. Compassionate Use of Orphan Drugs. Orphanet J. Rare Dis. 2015, 10, 100. [Google Scholar] [CrossRef] [PubMed]
- Medic, G.; Korchagina, D.; Young, K.E.; Toumi, M.; Postma, M.J.; Wille, M.; Hemels, M.; Postma, J.; Wille, M.; Hemels, M.; et al. Do Payers Value Rarity? An Analysis of the Relationship between Disease Rarity and Orphan Drug Prices in Europe. J. Mark. Access Health Policy 2017, 5, 1299665. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, L.; Patris, J.; Hutchings, A.; Cowell, W. Principles for Consistent Value Assessment and Sustainable Funding of Orphan Drugs in Europe. Orphanet J. Rare Dis. 2015, 10, 1–9. [Google Scholar] [CrossRef]
- Logviss, K.; Krievins, D.; Purvina, S. Impact of Orphan Drugs on Latvian Budget. Orphanet J. Rare Dis. 2016, 11, 1–13. [Google Scholar] [CrossRef]
- Hanisch, M.; Hanisch, L.; Kleinheinz, J.; Danesh, G.; Benz, K.; Jackowski, J. Orthodontically Relevant Manifestations in People with Rare Diseases. Med. Princ. Pract. 2019, 28, 216–221. [Google Scholar] [CrossRef]
- Alfaro, T.M.; Wijsenbeek, M.S.; Powell, P.; Stolz, D.; Hurst, J.R.; Kreuter, M.; Moor, C.C. Educational Aspects of Rare and Orphan Lung Diseases. Respir Res. 2021, 22, 1–8. [Google Scholar] [CrossRef]
- Vanhoorne, V.; Peeters, E.; Van Tongelen, I.; Boussery, K.; Wynendaele, E.; De Spiegeleer, B.; Remon, J.P.; Vervaet, C. Pharmaceutical Compounding of Orphan Active Ingredients in Belgium: How Community and Hospital Pharmacists Can Address the Needs of Patients with Rare Diseases. Orphanet J. Rare Dis. 2019, 14, 1–9. [Google Scholar] [CrossRef]
- Hughes, D.A.; Tunnage, B.; Yeo, S.T. Drugs for Exceptionally Rare Diseases: Do They Deserve Special Status for Funding? QJM 2005, 98, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.Y.L.; Chan, V.K.Y.; Olsson, S.; Fan, M.; Jit, M.; Gong, M.; Zhang, S.; Ge, M.; Pathadka, S.; Chung, C.C.Y.; et al. Access and Unmet Needs of Orphan Drugs in 194 Countries and 6 Areas: A Global Policy Review With Content Analysis. Value Health 2020, 23, 1580–1591. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Song, P.; Kang, Q.; Zhang, X.; Hu, J.; Yang, Y.; Tang, M.; Chen, D.; Hu, S.; Jin, C. Overview on Social Security System of Rare Diseases in China. Biosci. Trends 2019, 13, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Teagarden, J.R.; Unger, T.F.; Hirsch, G. Access and Availability of Orphan Drugs in the United States: Advances or Cruel Hoaxes? Expert Opin. Orphan Drugs 2014, 2, 1147–1150. [Google Scholar] [CrossRef]
- Gong, S.; Wang, Y.; Pan, X.; Zhang, L.; Huang, R.; Chen, X.; Hu, J.; Xu, Y.; Jin, S. The Availability and Affordability of Orphan Drugs for Rare Diseases in China. Orphanet J. Rare Dis. 2016, 11, 1–12. [Google Scholar] [CrossRef]
- Korchagina, D.; Millier, A.; Vataire, A.L.; Aballea, S.; Falissard, B.; Toumi, M. Determinants of Orphan Drugs Prices in France: A Regression Analysis. Orphanet J. Rare Dis. 2017, 12, 75. [Google Scholar] [CrossRef]
- Choudhury, M.C.; Saberwal, G. The Work, Goals, Challenges, Achievements, and Recommendations of Orphan Medicinal Product Organizations in India: An Interview-Based Study. Orphanet J. Rare Dis. 2019, 14, 241. [Google Scholar] [CrossRef]
- Li, X.; Liu, M.; Lin, J.; Li, B.; Zhang, X.; Zhang, S.; Lu, Z.; Zhang, J.; Zhou, J.; Ou, L. A Questionnaire-Based Study to Comprehensively Assess the Status Quo of Rare Disease Patients and Care-Givers in China. Orphanet J. Rare Dis. 2021, 16, 327. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.E.; Neidle, S.; Taylor, D.G. Developing and Paying for Medicines for Orphan Indications in Oncology: Utilitarian Regulation vs Equitable Care? Br. J. Cancer 2012, 106, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Yong, J.; Moffett, M.; Lucas, S. Implementing a Global Expanded Access Program (EAP) for Infantile-Onset Spinal Muscular Atrophy (Type I): Understanding the Imperative, Impact and Challenges. J. Neuromuscul. Dis. 2019, 6, 227–231. [Google Scholar] [CrossRef]
- Nicod, E.; Whittal, A.; Drummond, M.; Facey, K. Are Supplemental Appraisal/Reimbursement Processes Needed for Rare Disease Treatments? An International Comparison of Country Approaches. Orphanet J. Rare Dis. 2020, 15, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Dharssi, S.; Wong-Rieger, D.; Harold, M.; Terry, S. Review of 11 National Policies for Rare Diseases in the Context of Key Patient Needs. Orphanet J. Rare Dis. 2017, 12, 1303–1304. [Google Scholar] [CrossRef] [PubMed]
- Watson, M.; Directors, A.B. Insuring Patient Access and Affordability for Treatments for Rare and Ultrarare Diseases: A Policy Statement of the American College of Medical Genetics and Genomics. Genet. Med. 2018, 20, 1303–1304. [Google Scholar] [CrossRef]
- So, D.; Joly, Y.; Knoppers, B.M. Clinical Trial Transparency and Orphan Drug Development: Recent Trends in Data Sharing by the Pharmaceutical Industry. Public Health Genom. 2013, 16, 322–335. [Google Scholar] [CrossRef]
- Stein, S.; Bogard, E.; Boice, N.; Fernandez, V.; Field, T.; Gilstrap, A.; Kahn, S.R.; Larkindale, J.; Mathieson, T. Principles for Interactions with Biopharmaceutical Companies: The Development of Guidelines for Patient Advocacy Organizations in the Field of Rare Diseases. Orphanet J. Rare Dis. 2018, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Monguio, R.; Spargo, T.; Seoane-Vazquez, E. Ethical Imperatives of Timely Access to Orphan Drugs: Is Possible to Reconcile Economic Incentives and Patients’ Health Needs? Orphanet J. Rare Dis. 2017, 12, 1–8. [Google Scholar] [CrossRef]
- Blonda, A.; Barcina Lacosta, T.; Toumi, M.; Simoens, S. Assessing the Value of Nusinersen for Spinal Muscular Atrophy: A Comparative Analysis of Reimbursement Submission and Appraisal in European Countries. Front. Pharmacol. 2022, 12, 750742. [Google Scholar] [CrossRef]
- Hsiao, E.C.; Di Rocco, M.; Cali, A.; Zasloff, M.; Al Mukaddam, M.; Pignolo, R.J.; Grunwald, Z.; Netelenbos, C.; Keen, R.; Baujat, G.; et al. Special Considerations for Clinical Trials in Fibrodysplasia Ossificans Progressiva (FOP). Br. J. Clin. Pharmacol. 2019, 85, 1199–1207. [Google Scholar] [CrossRef]
- Liu, X.; Tang, Y.; Zhang, B.; Li, J.T.; Mei, D. Off-Label Drug Use in the T88888reatment of Rare Diseases:The Current Situation. J. Int. Pharm. Res. 2019, 46, 685–690. [Google Scholar] [CrossRef]
- Tafuri, G.; Bracco, A.; Grueger, J. Access and Pricing of Medicines for Patients with Rare Diseases in the European Union: An Industry Perspective. Expert Rev. Pharm. Outcomes Res. 2022, 22, 381–389. [Google Scholar] [CrossRef]
- Weerasooriya, S.U. The Impact of Orphan Drug Policies in Treating Rare Diseases. Health Inf. Libr. J. 2019, 36, 179–184. [Google Scholar] [CrossRef] [PubMed]
- McCormack, P.; Woods, S.; Aartsma-Rus, A.; Hagger, L.; Herczegfalvi, A.; Heslop, E.; Irwin, J.; Kirschner, J.; Moeschen, P.; Muntoni, F.; et al. Guidance in Social and Ethical Issues Related to Clinical, Diagnostic Care and Novel Therapies for Hereditary Neuromuscular Rare Diseases: “Translating” the Translational. PLoS Curr. 2013, 5, 259–276. [Google Scholar] [CrossRef] [PubMed]
- Fontrier, A.-M. Market Access for Medicines Treating Rare Diseases: Association between Specialised Processes for Orphan Medicines and Funding Recommendations. Soc. Sci. Med. 2022, 306, 115119. [Google Scholar] [CrossRef] [PubMed]
- Calleri, G.; Angheben, A.; Albonico, M. Neglected Tropical Diseases in Europe: Rare Diseases and Orphan Drugs? Infection 2019, 47, 3–5. [Google Scholar] [CrossRef]
- Zurynski, Y.; Deverell, M.; Dalkeith, T.; Johnson, S.; Christodoulou, J.; Leonard, H.; Elliott, E.J. Australian Children Living with Rare Diseases: Experiences of Diagnosis and Perceived Consequences of Diagnostic Delays. Orphanet J. Rare Dis. 2017, 12, 68. [Google Scholar] [CrossRef]
- Rawson, N.S.B. Alignment of Health Technology Assessments and Price Negotiations for New Drugs for Rare Disorders in Canada: Does It Lead to Improved Patient Access? J. Popul. Ther. Clin. Pharmacol. 2020, 27, e48–e64. [Google Scholar] [CrossRef]
- Benson, M.; Albanese, A.; Bhatia, K.P.; Cavillon, P.; Cuffe, L.; König, K.; Reinhard, C.; Graessner, H. Development of a Patient Journey Map for People Living with Cervical Dystonia. Orphanet J. Rare Dis. 2022, 17, 1–9. [Google Scholar] [CrossRef]
- Bienstock, R.J. Data Sharing Advances Rare and Neglected Disease Clinical Research and Treatments. ACS Pharmacol. Transl. Sci. 2019, 2, 491–496. [Google Scholar] [CrossRef]
- Foltanova, T.; Majernik, A.; Malikova, E.; Kosirova, S. Availability and Accessibility of Orphan Medicinal Products to Patients in Slovakia in the Years 2010–2019. Front. Pharmacol. 2022, 13, 768325. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, G.; de Andrade, P.V.; Dos Santos, J.M.; do Amaral, J.L.G.; da Silva, H.C.A. Impact of a Digital Manual for Guidance on Malignant Hyperthermia: Patient Education. Orphanet J. Rare Dis. 2022, 17, 265. [Google Scholar] [CrossRef]
- Sardella, M.; Lungu, C. Evaluation of Quantitative Signal Detection in EudraVigilance for Orphan Drugs: Possible Risk of False Negatives. Ther. Adv. Drug Saf. 2019, 10, 2042098619882819. [Google Scholar] [CrossRef] [PubMed]
- Schroader, B.; Kong, S.; Anderson, S.; Williamson, T.; Sireci, A.; Shields, K. Current Status of Biomarker Testing in Historically Rare, High-Unmet-Need Tumors: Soft Tissue Sarcomas and Thyroid Cancers. Expert Rev. Anticancer. Ther. 2019, 19, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Sisk, B.A.; Kerr, A.; King, K.A. Factors Affecting Pathways to Care for Children and Adolescents with Complex Vascular Malformations: Parental Perspectives. Orphanet J. Rare Dis. 2022, 17, 271. [Google Scholar] [CrossRef] [PubMed]
- Koçkaya, G.; Atalay, S.; Oğuzhan, G.; Kurnaz, M.; Ökçün, S.; Sar Gedik, Ç.; Şaylan, M.; Şencan, N. Analysis of Patient Access to Orphan Drugs in Turkey. Orphanet J. Rare Dis. 2021, 16, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Richter, T.; Janoudi, G.; Amegatse, W.; Nester-Parr, S. Characteristics of Drugs for Ultra-Rare Diseases versus Drugs for Other Rare Diseases in HTA Submissions Made to the CADTH CDR. Orphanet J. Rare Dis. 2018, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tomeo, F.; Mariz, S.; Brunetta, A.L.; Stoyanova-Beninska, V.; Penttila, K.; Magrelli, A. Haemophilia, State of the Art and New Therapeutic Opportunities, a Regulatory Perspective. Br. J. Clin. Pharmacol. 2021, 87, 4183–4196. [Google Scholar] [CrossRef]
- Lei, X.; Dai, G.; Chen, Q.; Hu, H. Design of the Knowledge Database and Model Database for Rare Diseases. Chin. Gen. Pract. 2021, 24, 3634. [Google Scholar] [CrossRef]
- Bouwman, M.L.; Sousa, J.J.S.; Pina, M.E.T. Regulatory Issues for Orphan Medicines: A Review. Health Policy Technol. 2020, 9, 115–121. [Google Scholar] [CrossRef]
- Menon, D.; Clark, D.; Stafinski, T. Reimbursement of Drugs for Rare Diseases through the Public Healthcare System in Canada: Where Are We Now? Healthcare Policy 2015, 11, 15–32. [Google Scholar] [CrossRef]
- Lumry, W.R. Pharmacoeconomics of Orphan Disease Treatment with a Focus on Hereditary Angioedema. Immunol. Allergy Clin. N. Am. 2017, 37, 617–628. [Google Scholar] [CrossRef]
- Lam, B.L.; Leroy, B.P.; Black, G.; Ong, T.; Yoon, D.; Trzupek, K. Genetic Testing and Diagnosis of Inherited Retinal Diseases. Orphanet J. Rare Dis. 2021, 16, 1–9. [Google Scholar] [CrossRef]
- Nuijten, M.; Capri, S. Pricing of Orphan Drugs in Oncology and Rare Diseases. J. Mark. Access Health Policy 2020, 8, 1838191. [Google Scholar] [CrossRef]
- Low, Z.Y.; Farouk, I.A.; Lal, S.K. Drug Repositioning: New Approaches and Future Prospects for Life-Debilitating Diseases and the COVID-19 Pandemic Outbreak. Viruses 2020, 12, 1058. [Google Scholar] [CrossRef]
- Kakkis, E.D.; O’Donovan, M.; Cox, G.; Hayes, M.; Goodsaid, F.; Tandon, P.K.; Furlong, P.; Boynton, S.; Bozic, M.; Orfali, M.; et al. Recommendations for the Development of Rare Disease Drugs Using the Accelerated Approval Pathway and for Qualifying Biomarkers as Primary Endpoints. Orphanet J. Rare Dis. 2015, 10, 16. [Google Scholar] [CrossRef]
- Chorostowska-Wynimko, J.; Barrecheguren, M.; Ferrarotti, I.; Greulich, T.; Sandhaus, R.A.; Campos, M. New Patient-Centric Approaches to the Management of Alpha-1 Antitrypsin Deficiency. Int. J. COPD 2020, 15, 345–355. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.Y.; Yue, X.M.; Wu, J.H. Considerations on the Health Techology Assessment for Rare Diseases and Orphan Drugs in China. J. Int. Pharm. Res. 2019, 46, 666–672. [Google Scholar] [CrossRef]
- Arnold, R.J.G.; Bighash, L.; Bryón Nieto, A.; Tannus Branco de Araújo, G.; Gay-Molina, J.G.; Augustovski, F. The Role of Globalization in Drug Development and Access to Orphan Drugs: Orphan Drug Legislation in the US/EU and in Latin America. F1000Res 2015, 4, 57. [Google Scholar] [CrossRef]
- Abdallah, K.; De Boeck, K.; Dooms, M.; Simoens, S. A Comparative Analysis of Pricing and Reimbursement of Cystic Fibrosis Transmembrane Conductance Regulator Modulators in Europe. Front. Pharmacol. 2021, 12, 746710. [Google Scholar] [CrossRef] [PubMed]
- Tylki-Szymańska, A.; Almássy, Z.; Christophidou-Anastasiadou, V.; Avdjieva-Tzavella, D.; Barisic, I.; Cerkauskiene, R.; Cuturilo, G.; Djiordjevic, M.; Gucev, Z.; Hlavata, A.; et al. The Landscape of Mucopolysaccharidosis in Southern and Eastern European Countries: A Survey from 19 Specialistic Centers. Orphanet J. Rare Dis. 2022, 17, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Oro-Ayude, M.; Batalla, A.; Dávila-Pousa, C.; González-Freire, L.; Flórez, Á. Effect of Drug Compounding on Quality of Life in Patients With Genodermatoses: A Cross-Sectional Study. Actas Dermosifiliogr. 2022, 113, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Dahir, K.M.; Seefried, L.; Kishnani, P.S.; Petryk, A.; Högler, W.; Linglart, A.; Martos-Moreno, G.Á.; Ozono, K.; Fang, S.; Rockman-Greenberg, C. Clinical Profiles of Treated and Untreated Adults with Hypophosphatasia in the Global HPP Registry. Orphanet J. Rare Dis. 2022, 17, 277. [Google Scholar] [CrossRef] [PubMed]
- Magner, M.; Almássy, Z.; Gucev, Z.; Kieć-Wilk, B.; Plaiasu, V.; Tylki-Szymańska, A.; Zafeiriou, D.; Zaganas, I.; Lampe, C. Consensus Statement on Enzyme Replacement Therapy for Mucopolysaccharidosis IVA in Central and South-Eastern European Countries. Orphanet J. Rare Dis. 2022, 17, 1–10. [Google Scholar] [CrossRef]
- Laimer, M.; Pohla-Gubo, G.; Diem, A.; Prodinger, C.; Bauer, J.W.; Hintner, H. Epidermolysis Bullosa House Austria and Epidermolysis Bullosa Clinical Network: Example of a Centre of Expertise Implemented in a European Reference Network to Face the Burden of a Rare Disease. Wien. Klin. Wochenschr. 2017, 129, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Di Paolo, A.; Arrigoni, E. Generic Substitution of Orphan Drugs for the Treatment of Rare Diseases: Exploring the Potential Challenges. Drugs 2018, 78, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Jyothi, M.S.; Balamuralidhara, V.; Nagaraju, S. Regulatory Overview on Rare Diseases and Orphan Drugs in India. Int. J. Pharm. Res. 2020, 12, 1957–1965. [Google Scholar] [CrossRef]
- Mortensen, A.; Raebel, E.M.; Wiseman, S. Impact of the COVID-19 Pandemic on Access to the Cerliponase Alfa Managed Access Agreement in England for CLN2 Treatment. Orphanet J. Rare Dis. 2022, 17, 1–15. [Google Scholar] [CrossRef]
- Bang, J.S.; Lee, J.H. The National Drug Formulary Listing Process for Orphan Drugs in South Korea: Narrative Review Focused on Pricing and Reimbursement Pathways. Expert Opin. Orphan Drugs 2021, 9, 105–112. [Google Scholar] [CrossRef]
- Varghese, M.S.; Liu, C.L.; Kazi, D.S. The Price of Progress: Cost, Access, and Adoption of Novel Cardiovascular Drugs in Clinical Practice. Curr. Cardiol. Rep. 2021, 23, 1–7. [Google Scholar] [CrossRef]
- Solís, L.; Nordin, J.; Prevot, J.; Mahlaoui, N.; Sánchez-Ramón, S.; Ali, A.; Cassignol, E.; Seymour, J.W.; Pergent, M. The PID Life Index: An Interactive Tool to Measure the Status of the PID Healthcare Environment in Any given Country. Orphanet J. Rare Dis. 2022, 17, 1–8. [Google Scholar] [CrossRef]
- Jagadeesan, M.; Harini, R.R.; Chinnasami, B. Rational Prescribing Challenges in Paediatrics: A Evidence Based Review. Res. J. Pharm. Technol. 2020, 13, 5551–5556. [Google Scholar] [CrossRef]
- Kamusheva, M.; Milushewa, P. Rare Disease Patients’ Needs: An up-to-Date Analysis and Future Directions. Pharmacia 2021, 68, 763–770. [Google Scholar] [CrossRef]
- Groft, S.C.; Posada, M.; Taruscio, D. Progress, Challenges and Global Approaches to Rare Diseases. Acta Paediatr. Int. J. Paediatr. 2021, 110, 2711–2716. [Google Scholar] [CrossRef] [PubMed]
- Leyens, J.; Bender, T.T.A.; Mücke, M.; Stieber, C.; Kravchenko, D.; Dernbach, C.; Seidel, M.F. The Combined Prevalence of Classified Rare Rheumatic Diseases Is Almost Double That of Ankylosing Spondylitis. Orphanet J. Rare Dis. 2021, 16, 1–43. [Google Scholar] [CrossRef] [PubMed]
- Reardon, G. Pharmacoeconomics of Biologic Medicines and Biosimilars. Biol. Biosimilars Biobetters Introd. Pharm. Physicians Other Health Pract. 2021, 47–69. [Google Scholar] [CrossRef]
- Schey, C.; Postma, M.; Krabbe, P.; Medic, G.; Connolly, M. The Application of Multi-Criteria Decision Analysis to Inform in Resource Allocation. F1000Res 2020, 9, 445. [Google Scholar] [CrossRef]
- Campa, C.; Gallenga, C.E.; Bolletta, E.; Perri, P. The Role of Gene Therapy in the Treatment of Retinal Diseases: A Review. Curr. Gene Ther. 2017, 17, 194–213. [Google Scholar] [CrossRef]
- Guien, C.; Blandin, G.; Lahaut, P.; Sanson, B.; Nehal, K.; Rabarimeriarijaona, S.; Bernard, R.; Lévy, N.; Sacconi, S.; Béroud, C. The French National Registry of Patients with Facioscapulohumeral Muscular Dystrophy. Orphanet J. Rare Dis. 2018, 13, 318. [Google Scholar] [CrossRef]
- Encina, G.; Castillo-Laborde, C.; Lecaros, J.A.; Dubois-Camacho, K.; Calderón, J.F.; Aguilera, X.; Klein, A.D.; Repetto, G.M. Rare Diseases in Chile: Challenges and Recommendations in Universal Health Coverage Context. Orphanet J. Rare Dis. 2019, 14, 1–8. [Google Scholar] [CrossRef]
- Mulberg, A.E.; Bucci-Rechtweg, C.; Giuliano, J.; Jacoby, D.; Johnson, F.K.; Liu, Q.; Marsden, D.; McGoohan, S.; Nelson, R.; Patel, N.; et al. Regulatory Strategies for Rare Diseases under Current Global Regulatory Statutes: A Discussion with Stakeholders. Orphanet J. Rare Dis. 2019, 14, 1–10. [Google Scholar] [CrossRef]
- Al-Attar, M. TRAPPED—An Insight into Two Sisters’ Struggle to Access Treatment for a Rare Genetic Disease. Orphanet J. Rare Dis. 2018, 13, 1–3. [Google Scholar] [CrossRef]
- Barak, A.; Shankar Nandi, J. Orphan Drugs: Pricing, Reimbursement and Patient Access. Int. J. Pharm. Healthc Mark. 2011, 5, 299–317. [Google Scholar] [CrossRef]
- Giannetta, N.; Cianciulli, A.; Dionisi, S.; Figura, M.; Di Simone, E.; Di Muzio, M. Orphan Drugs: An European Production, Research and Development Policies. G. Ital. Di Farm. Clin. 2019, 33, 29–34. [Google Scholar] [CrossRef]
- Weinstein, N.; Martin, M.; Campbell, R. Orphan Drugs In The Uk, Do They Meet The Nice Highly Specialised Technology Threshold? Value Health 2017, 20, A660. [Google Scholar] [CrossRef]
- Friedlander, L.; Berdal, A.; Boizeau, P.; Licht, B.A.; Manière, M.C.; Picard, A.; Azzis, O.; Vazquez, M.P.; Alberti, C.; Molla, M.D.L.D. Oral Health Related Quality of Life of Children and Adolescents Affected by Rare Orofacial Diseases: A Questionnaire-Based Cohort Study. Orphanet J. Rare Dis. 2019, 14, 124. [Google Scholar] [CrossRef]
- Shih, D.Y.; Jarrett, J. Orphan Drug Policy: Approaches To Market Access In Multiple Countries. Value Health 2014, 17, A539. [Google Scholar] [CrossRef]
- Ozbek, U. Molecular Analysis of Consanguineous Rare Syndromes in Turkey. J. Biotechnol. 2014, 185, S16. [Google Scholar] [CrossRef]
- Painous, C.; van Os, N.J.H.; Delamarre, A.; Michailoviene, I.; Marti, M.J.; van de Warrenburg, B.P.; Meissner, W.G.; Utkus, A.; Reinhard, C.; Graessner, H.; et al. Management of Rare Movement Disorders in Europe: Outcome of Surveys of the European Reference Network for Rare Neurological Diseases. Eur. J. Neurol. 2020, 27, 1493–1500. [Google Scholar] [CrossRef]
- Bax, B.E. Mitochondrial Neurogastrointestinal Encephalomyopathy: Approaches to Diagnosis and Treatment. J. Transl. Genet. Genom. 2020, 4, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Margaretos, N.M.; Bawa, K.; Engmann, N.J.; Chambers, J.D. Patients’ Access to Rare Neuromuscular Disease Therapies Varies across US Private Insurers. Orphanet J. Rare Dis. 2022, 17, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mikami, K.; Sturdy, S. Patient Organization Involvement and the Challenge of Securing Access to Treatments for Rare Diseases: Report of a Policy Engagement Workshop. Res. Involv Engagem. 2017, 3, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Macaulay, R.; Liu, L.W.; Turkstra, E. PRO112 THE HST TEST: GOOD, BETTER, BEST? Value Health 2019, 22, S861–S862. [Google Scholar] [CrossRef]
- Boncheva, E.; Benisheva, T.; Cherneva, D. PRO74 Is IT Possible to Accelerate the Access of Patients with RARE Diseases to New Therapy—the Case with IPF Medicines. Value Health 2020, 23, S702. [Google Scholar] [CrossRef]
- Kennedy, C.; Janwar, J.; McBride, M.; Connolly, E.; Tilson, L.; Barry, M. Pns164 Pharmacoeconomic Assessment And Drug Costs of Treatments for Cancer and Rare Diseases in Ireland. Value Health 2019, 22, S788–S789. [Google Scholar] [CrossRef]
- Godinas, L.; Iyer, K.; Meszaros, G.; Quarck, R.; Escribano-Subias, P.; Vonk Noordegraaf, A.; Jansa, P.; D’Alto, M.; Luknar, M.; Milutinov Ilic, S.; et al. PH CARE COVID Survey: An International Patient Survey on the Care for Pulmonary Hypertension Patients during the Early Phase of the COVID-19 Pandemic. Orphanet J. Rare Dis. 2021, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.Y.L.; Chan, V.K.Y.; Olsson, S.; Fan, M.; Jit, M.; Gong, M.; Zhang, S.; Ge, M.; Pathadka, S.; Chui, C.S.L.; et al. Orphan Drugs—Access and Unmet Needs in 194 Countries and Six Regions: A Comprehensive Policy Review with Content Analysis. Lancet 2019, 394, S72. [Google Scholar] [CrossRef]
- Aldosari, M.H.; den Hartog, M.; Ganizada, H.; Evers, M.J.W.; Mastrobattista, E.; Schellekens, H. Feasibility Study for Bedside Production of Recombinant Human Acid α-Glucosidase: Technical and Financial Considerations. Curr. Pharm. Biotechnol. 2020, 21, 467–479. [Google Scholar] [CrossRef]
- Hernberg-Ståhl, E.; Reljanovic, M. Orphan Drugs: Understanding the Rare Disease Market and Its Dynamics; Elsevier: Amsterdam, The Netherlands, 2013; pp. 1–317. [Google Scholar] [CrossRef]
- Gahl, W.A.; Wong-Rieger, D.; Hivert, V.; Yang, R.; Zanello, G.; Groft, S. Essential List of Medicinal Products for Rare Diseases: Recommendations from the IRDiRC Rare Disease Treatment Access Working Group. Orphanet J. Rare Dis. 2021, 16, 1–11. [Google Scholar] [CrossRef]
- Stevens, B.; Kenny, T.; Thomas, S.; Morrison, A.; Jarrett, J.; Jain, M. Elosulfase Alfa in the Treatment of Mucopolysaccharidosis Type IVA: Insights from the First Managed Access Agreement. Orphanet J. Rare Dis. 2021, 16, 1–8. [Google Scholar] [CrossRef]
- McCabe, C.; Edlin, R.; Round, J. Economic Considerations in the Provision of Treatments for Rare Diseases. Adv. Exp. Med. Biol. 2010, 686, 211–222. [Google Scholar] [CrossRef]
- Costa, E.; Schieppati, A.; Luzzatto, L.; Remuzzi, G. Drugs for Rare Diseases: The Blessing of Being Orphans. Recenti Prog. Med. 2019, 110, 221–229. [Google Scholar] [CrossRef]
- Qiu, T.; Wang, Y.; Dabbous, M.; Hanna, E.; Han, R.; Liang, S.; Toumi, M. Current State of Developing Advanced Therapies for Rare Diseases in the European Union. Expert Opin. Orphan Drugs 2020, 8, 417–429. [Google Scholar] [CrossRef]
- Jarosławski, S.; Auquier, P.; Borissov, B.; Dussart, C.; Toumi, M. Low Rates of Patient-Reported Outcome Claims for Orphan Drugs Approved by the Us Food and Drug Administration. J. Mark. Access Health Policy 2018, 6, 1433426. [Google Scholar] [CrossRef] [PubMed]
- Cleary, M.; Davison, J.; Gould, R.; Geberhiwot, T.; Hughes, D.; Mercer, J.; Morrison, A.; Murphy, E.; Santra, S.; Jarrett, J.; et al. Impact of Long-Term Elosulfase Alfa Treatment on Clinical and Patient-Reported Outcomes in Patients with Mucopolysaccharidosis Type IVA: Results from a Managed Access Agreement in England. Orphanet J. Rare Dis. 2021, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Ramaswami, U.; Muenzer, J.; Giugliani, R.; Ullrich, K.; Collin-Histed, T.; Panahloo, Z.; Wellhoefer, H.; Frader, J. A Charitable Access Program for Patients with Lysosomal Storage Disorders in Underserved Communities Worldwide. Orphanet J. Rare Dis. 2021, 16, 8. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.; Schlander, M. Health Technology Assessment (HTA) and Economic Evaluation: Efficiency or Fairness First. J. Mark. Access Health Policy 2019, 7, 1557981. [Google Scholar] [CrossRef]
- Café, A.; Carvalho, M.; Crato, M.; Faria, M.; Kjollerstrom, P.; Oliveira, C.; Pinto, P.R.; Salvado, R.; Dos Santos, A.A.; Silva, C. Haemophilia A: Health and Economic Burden of a Rare Disease in Portugal. Orphanet J. Rare Dis. 2019, 14, 1–11. [Google Scholar] [CrossRef]
- Petrongonas, M.; Rinaki, E.; Fragiadaki, M.; Tzimis, L. GM-005 Orphan Drugs’ Pharmacoeconomic Data and the Impact on a County Hospital’s Budget. Eur. J. Hosp. Pharm. 2017, 24 (Suppl. S1), A159.2–A161.2. [Google Scholar] [CrossRef]
- Kaufmann, P.; Pariser, A.R.; Austin, C. From Scientific Discovery to Treatments for Rare Diseases—The View from the National Center for Advancing Translational Sciences—Office of Rare Diseases Research. Orphanet J. Rare Dis. 2018, 13, 196. [Google Scholar] [CrossRef]
- Kaiser, K.; Yount, S.E.; Martens, C.E.; Webster, K.A.; Shaunfield, S.; Sparling, A.; Peipert, J.D.; Cella, D.; Rottinghaus, S.T.; Donato, B.M.K.; et al. Assessing Preferences for Rare Disease Treatment: Qualitative Development of the Paroxysmal Nocturnal Hemoglobinuria Patient Preference Questionnaire (PNH-PPQ©). Patient Prefer. Adherence 2020, 14, 705–715. [Google Scholar] [CrossRef]
- Tosi, L.L.; Floor, M.K.; Dollar, C.M.; Gillies, A.P.; Lee, B.; Nagamani, S.C.S.; Rauch, F.; Glorieux, F.; Retrouvey, J.M.; Esposito, P.; et al. Assessing Disease Experience across the Life Span for Individuals with Osteogenesis Imperfecta: Challenges and Opportunities for Patient-Reported Outcomes (PROs) Measurement: A Pilot Study. Orphanet J. Rare Dis. 2019, 14, 1–12. [Google Scholar] [CrossRef]
- Badia, X.; Gil, A.; Poveda-Andrés, J.L.; Shepherd, J.; Tort, M. Analysing Criteria for Price and Reimbursement of Orphan Drugs in Spain. Farm. Hosp. 2019, 43, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Atalaia, A.; Thompson, R.; Corvo, A.; Carmody, L.; Piscia, D.; Matalonga, L.; Macaya, A.; Lochmuller, A.; Fontaine, B.; Zurek, B.; et al. A Guide to Writing Systematic Reviews of Rare Disease Treatments to Generate FAIR-Compliant Datasets: Building a Treatabolome. Orphanet J. Rare Dis. 2020, 15, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Krajnović, D.; Arsić, J.; Tasić, L.; Petrova, G.; Milijić, S. Access To Orphan Drugs: A Cross Country Comparison Of Legislative Approach Among Serbia, Croati A And Macedonia. Acta Med. Median. 2018, 57, 43–51. [Google Scholar] [CrossRef]
- Young, K.E.; Soussi, I.; Hemels, M.; Toumi, M. A Comparative Study of Orphan Drug Prices in Europe. J. Mark. Access Health Policy 2017, 5, 1297886. [Google Scholar] [CrossRef] [PubMed]
- Horgan, D.; Moss, B.; Boccia, S.; Genuardi, M.; Gajewski, M.; Capurso, G.; Fenaux, P.; Gulbis, B.; Pellegrini, M.; Mañú Pereira, M.d.M.; et al. Time for Change? The Why, What and How of Promoting Innovation to Tackle Rare Diseases—Is It Time to Update the EU’s Orphan Regulation? And If so, What Should Be Changed? Biomed Hub. 2020, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.; Redmond, S. To HTA or Not to HTA: Identifying the Factors Influencing the Rapid Review Outcome in Ireland. Value Health 2019, 22, 385–390. [Google Scholar] [CrossRef]
- Miyamoto, B.E.; Kakkis, E.D. The Potential Investment Impact of Improved Access to Accelerated Approval on the Development of Treatments for Low Prevalence Rare Diseases. Orphanet J. Rare Dis. 2011, 6, 49. [Google Scholar] [CrossRef]
- Foteeva, A.V.; Rostova, N.B.; Isupova, A.D.; Gerbergagen, E.S. The Orphan Preparations: International Approaches and National Regulation. Probl. Sotsialnoi Gig Zdravookhranenniiai Istor Med. 2021, 29, 1490–1497. [Google Scholar] [CrossRef]
- Bojakowski, S.; Spoors, J. The Funding of Orphan Medicines in the UK. Br. J. Health Care Manag. 2014, 20, 384–391. [Google Scholar] [CrossRef]
- Young, K.E.; Soussi, I.; Toumi, M. The Perverse Impact of External Reference Pricing (ERP): A Comparison of Orphan Drugs Affordability in 12 European Countries. A Call for Policy Change. J. Mark. Access Health Policy 2017, 5, 1369817. [Google Scholar] [CrossRef]
- Lampe, C.; Dionisi-Vici, C.; Bellettato, C.M.; Paneghetti, L.; van Lingen, C.; Bond, S.; Brown, C.; Finglas, A.; Francisco, R.; Sestini, S.; et al. The Impact of COVID-19 on Rare Metabolic Patients and Healthcare Providers: Results from Two MetabERN Surveys. Orphanet J. Rare Dis. 2020, 15, 341. [Google Scholar] [CrossRef] [PubMed]
- Kanavos, P.; Nicod, E. What Is Wrong with Orphan Drug Policies? Suggestions for Ways Forward. Value Health 2012, 15, 1182–1184. [Google Scholar] [CrossRef]
- Murphy, S.M.; Puwanant, A.; Griggs, R.C. Unintended Effects of Orphan Product Designation for Rare Neurological Diseases. Ann. Neurol. 2012, 72, 481–490. [Google Scholar] [CrossRef] [PubMed]
- de Dios García-Díaz, J.; López-Rodríguez, M.; Morales-Conejo, M.; Riera-Mestre, A. Understanding the Ecosystem of Patients with Lysosomal Storage Diseases in Spain: A Qualitative Research with Patients and Health Care Professionals. Orphanet J. Rare Dis. 2022, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Berdud, M.; Drummond, M.; Towse, A. Establishing a Reasonable Price for an Orphan Drug. Cost Eff. Resour. Alloc. 2020, 18, 31. [Google Scholar] [CrossRef]
- Luzzatto, L.; Makani, J. Treating Rare Diseases in Africa: The Drugs Exist but the Need Is Unmet. Front. Pharmacol. 2022, 12, 770640. [Google Scholar] [CrossRef]
- Cousyn, C.; Bouchard, K.; Gaboury, S.; Bouchard, B. Towards Using Scientific Publications to Automatically Extract Information on Rare Diseases. Mob. Netw. Appl. 2020, 25, 953–960. [Google Scholar] [CrossRef]
- Jarosławski, S.; Auquier, P.; Borissov, B.; Dussart, C.; Toumi, M. Patient-Reported Outcome Claims in European and United States Orphan Drug Approvals. J. Mark. Access Health Policy 2018, 6, 1542920. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, K.; Vernon, M.K.; Patrick, D.L.; Perfetto, E.; Nestler-Parr, S.; Burke, L. Patient-Reported Outcome and Observer-Reported Outcome Assessment in Rare Disease Clinical Trials: An ISPOR COA Emerging Good Practices Task Force Report. Value Health 2017, 20, 838–855. [Google Scholar] [CrossRef] [PubMed]
- Groft, S.C.; Posada De La Paz, M. Rare Diseases: Joining Mainstream Research and Treatment Based on Reliable Epidemiological Data. Adv. Exp. Med. Biol. 2017, 1031, 3–21. [Google Scholar] [CrossRef]
- Hu, S.L. Characteristics of Drug Policy and Pharmacoeconomics Study on Rare Diseases. J. Int. Pharm. Res. 2019, 46, 652–658. [Google Scholar] [CrossRef]
- Tingley, K.; Coyle, D.; Graham, I.D.; Chakraborty, P.; Wilson, K.; Potter, B.K. Stakeholder Perspectives on Clinical Research Related to Therapies for Rare Diseases: Therapeutic Misconception and the Value of Research. Orphanet J. Rare Dis. 2021, 16, 1–11. [Google Scholar] [CrossRef]
- Gulliver, W.; Landells, I.D.R.; Morgan, D.; Pirzada, S. Hidradenitis Suppurativa: A Novel Model of Care and an Integrative Strategy to Adopt an Orphan Disease. J. Cutan. Med. Surg. 2018, 22, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Chung, R.Y.N.; Chan, R.H.W.; Gong, S.; Xu, R.H. Why Is Misdiagnosis More Likely among Some People with Rare Diseases than Others? Insights from a Population-Based Cross-Sectional Study in China. Orphanet J. Rare Dis. 2020, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; An, Z.; Ming, Y.; Guo, Y.; Li, W.; Liang, Y.; Guo, D.; Li, X.; Tai, J.; Chen, G.; et al. ERAM: Encyclopedia of Rare Disease Annotations for Precision Medicine. Nucleic Acids Res. 2018, 46, D937–D943. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.; Goodson, N.; Wicks, P.; Reites, J. What Role Can Decentralized Trial Designs Play to Improve Rare Disease Studies? Orphanet J. Rare Dis. 2022, 17, 240. [Google Scholar] [CrossRef]
- Balasopoulou, A.; Κokkinos, P.; Pagoulatos, D.; Plotas, P.; Makri, O.E.; Georgakopoulos, C.D.; Vantarakis, A.; Li, Y.; Liu, J.J.; Qi, P.; et al. Symposium Recent Advances and Challenges in the Management of Retinoblastoma Globe—Saving Treatments. BMC Ophthalmol. 2017, 17, 1. [Google Scholar] [CrossRef]
- O’Connor, O.A.; Marchi, E.; Volinn, W.; Kim, W.S. Case Match Control Analysis of Propel Reveals Survival Advantage for Patients with Relapsed/Refractory (R/R) Peripheral T-Cell Lymphoma (PTCL) Treated with Pralatrexate. Blood 2016, 128, 4149. [Google Scholar] [CrossRef]
- Fidanci, B.; Ozen, S.; Acikel, C.; Fidanci, K.; Yildiz, D.; Basbozkurt, G.; Ravelli, A.; Demirkaya, E. THU0473-HPR Living with Childhood Vasculitis; a Qualitative Study. Ann. Rheum. Dis. 2013, 71, 738. [Google Scholar] [CrossRef]
- Reddy, D.S. Clinical Overview of Innovative New Drug Approvals in 2017. Int. J. Pharm. Sci. Nanotechnol. 2018, 11, 4225–4230. [Google Scholar] [CrossRef]
- Martínez-Rodríguez, I.; Uría, E.; Pomares, E.; Cuesta, M. PHP34—TIME ANALYSIS OF THE DRUG APPROVAL PROCESS IN EUROPE. Value Health 2018, 21, S156. [Google Scholar] [CrossRef]
- Ogden, K.; Thompson, J.; Halfpenny, N.; Scott, D. A Conceptual Search Filter to Identify Real-World Evidence. Value Health 2015, 18, A728. [Google Scholar] [CrossRef]
- O’Connor, O.A.; Marchi, E.; Kim, W.S. Case match control analysis of propel reveals a survival advantage for patients with relapsed PTCL receiving pralatrexate: A novel approach to benchmark drugs in rare diseases. Hematol. Oncol. 2017, 35, 250. [Google Scholar] [CrossRef]
- Valinotti, G.; Scaldaferri, M.; Tonelli, M.; Crosasso, P.; Chiappetta, M.; Cattel, F. 4CPS-215 Access to ‘Fondo AIFA 5%’ as an Instrument Supporting the Sustainability in a Shared Clinical Management of Rare and Difficult-to-Treat Diseases. Eur. J. Hosp. Pharm. 2019, 26, A169–A170. [Google Scholar] [CrossRef]
- Wang, G.; Macaulay, R. The Cost-Effectiveness of Manufacturer Fees for Htas: Are They Promoting or Hindering Innovation? Value Health 2017, 20, A696. [Google Scholar] [CrossRef]
- Nayroles, G.; Gabriel, S.; Tolley, K.; Espin, J.; Toumi, M.; Kornfeld, A.; Frybourg, S. Extension of Indication With Mature Products: Toward More Incentives Rewarding Innovation? Value Health 2015, 18, A570. [Google Scholar] [CrossRef]
- Zhuravleva, M.V.; Lebedeva, A.Y. Organization of Pharmacological Support of Patients with Rare Diseases in Moscow as Exemplified by Pulmonary Arterial Hypertension. Med. Counc. 2018, 24–31. [Google Scholar] [CrossRef]
- Flostrand, S.; Rodriguez, I.; Maddox, B.; Finch, L.; Belulaj, S.; Gould, A. Is the Orphanage Filling Up? Projecting the Growth and Budget Impact of Orphan Drugs in Europe. Value Health 2016, 19, A599. [Google Scholar] [CrossRef]
- Mwamburi, M.; Nanavaty, M.; Gala, S.; Nyandege, A.; Ramesh, V. Special Considerations and Patient Access Schemes for Oncology by Health Technology Assessment (HTA) Bodies: Systematic Evaluation in 25 Countries. Value Health 2016, 19, A761. [Google Scholar] [CrossRef]
- Mardiguian, S.; Stefanidou, M.; Sheppard, F. Trends and Key Decision Drivers For Rejecting An Orphan Drug Submission Across Five Different HTA Agencies. Value Health 2014, 17, A438. [Google Scholar] [CrossRef]
- Prada, M.; Bertozzi, C.; Proietti, B.; Urbinati, D. Italian Law 326/2003 for The Reimbursement of Orphan and Life Saving Drugs Awaiting Market Entry: Approvals, Rejections And Methods In Aifa’s Evaluation Process Between January 2013 And May 2015. Value Health 2015, 18, A544. [Google Scholar] [CrossRef]
- Almutairi, R.D.; Alghamdi, A.A.; Felemban, D.F.; Seoane-Vazquez, E.; Rodriguez-Monguio, R.; Szeinbach, S.L. Analysis of Orphan Drug Designations and Approvals in the United States and the European Union. Value Health 2013, 16, A124. [Google Scholar] [CrossRef]
- Zhang, A.; Weisse, S.; Chen, X. Health Technology Assessment (HTA) for Orphan Drugs in Cost-Effectiveness (CE) Markets: Current Development and Future Trends. Value Health 2016, 19, A601. [Google Scholar] [CrossRef]
- Allen, G.; Hall, A.; Hanman, K.; Le Fevre, R.; Griffiths, A. Do EU5 Countries with Favourable Healthcare Expenditure and Reimbursement Indicators Have Better Patient-Reported Access to Treatments for Rare Diseases? Value Health 2017, 20, A409–A410. [Google Scholar] [CrossRef]
- Marshall, J.; Clayton, J. Regional Variations In Appraisal And Uptake Of New Treatments For Ultra Rare Diseases In The UK: A Case Study Of Ataluren For Nonsense Mutation Duchenne Muscular Dystrophy (NMDMD). Value Health 2017, 20, A562–A563. [Google Scholar] [CrossRef]
- Nicolodi, C. Orphan Drug Designation in Europe-Procedural Guidance and Challenges. Int. J. Drug Regul. Aff. 2019, 7, 1–7. [Google Scholar] [CrossRef]
- Skeldon, G.; Adkins, E.; Ayre, S.; Langham, S.; Nicholson, L. PRO95 Patient Engagement Process in Rare Disease: How Does It Differ between Countries? Value Health 2020, 23, S706. [Google Scholar] [CrossRef]
- Macaulay, R. Encouraging Orphan Designation for New Ebola Treatments—Could This Do More Harm than Good? Value Health 2015, 18, A243–A244. [Google Scholar] [CrossRef]
- Dastan, S.; Kalieva, S.; Yesbatyrova, L.; Myasnikova, Z.; Kayupova, G. Comparative Analysis of the Availability of Medicines for Patients with Orphan Diseases in the Republic of Kazakhstan for 2015 and 2020. J. Health Dev. 2021, 1, 54–60. [Google Scholar] [CrossRef]
- Nicholson, L.; Fountain, D.; Longworth, L.; Adkins, E. How Will Proposed Changes To The Nice Highly Specialised Technology Evaluation Process Impact Patient Access To Innovative Drugs for Rare Diseases? Value Health 2017, 20, A694. [Google Scholar] [CrossRef]
- Palmer, M.; Hughes, D.A. Orphan Drug Legislations: Heyday or Had Their Day? Value Health 2013, 16, A491. [Google Scholar] [CrossRef]
- Office, E. Letter of Editor. J. Rare Dis. Orphan Drugs 2020, 1. [Google Scholar] [CrossRef]
- Koury, C.D.N.; Silva, M.T. Rapid Economic Evaluation Review for Rare Diseases Treatments—The Case of Pegvisomant for Acromegaly. Value Health 2013, 16, A119. [Google Scholar] [CrossRef]
- Tsiantou, V.; Mylona, K.; Karampli, E.; Boubouchairopoulou, N.; Kyriopoulos, I.I.; Athanasakis, K.; Gabriel, E.; Makridaki, D.; Kyriopoulos, J. Access To Orphan Drugs In Greece During Economic Crisis. Value Health 2014, 17, A541. [Google Scholar] [CrossRef] [PubMed]
- Macaulay, R.; Roibu, C.; Ivanova, H.; Campbell, J. PSY136—A comparison of p&r requirements for orphan drugs in 25 markets. Value Health 2018, 21, S459. [Google Scholar] [CrossRef]
- Franceschini, M.; Soon, J.; Han, Y. PRO99 Real-World Application of Multiple Criteria Decision Analysis (MCDA) for Evaluating Orphan Drugs (ODS) in Europe. Value Health 2020, 23, S707. [Google Scholar] [CrossRef]
- Pavlović, N.; Stanimirov, B.; Stojančević, M.; Paut Kusturica, M.; Goloc orbin-Kon, S.; Mikov, M. Low Availability of Orphan Medicines in Serbia. Value Health 2013, 16, A123–A124. [Google Scholar] [CrossRef]
- Montilva, J.; Xue, Y.; Degun, R. Impact of National Orphan Drug Policy and Reimbursement Mechanisms Over the Implementation of Managed Entry Agreements in Select Asia-Pacific Countries. Value Health 2016, 19, A884. [Google Scholar] [CrossRef]
- Sburlan, E.A.; Voinea, L.-M.; Alexandrescu, C.; Istrate, S.; Iancu, R.; Pirvulescu, R.; Geamanu, A.; Ghita, M.; Ungureanu, E.; Radu, C. Rare Ophthalmology Diseases. Rom. J. Ophthalmol. 2019, 63, 10–14. [Google Scholar] [CrossRef]
- Mwamburi, M.; Nanavaty, M.; Gala, S.; Nyandege, A. Ramesh Provisions and Special Considerations for Rare Diseases / Orphan Drugs by Health Technology Assessment (HTA) Bodies: Systematic Evaluation in 25 Countries. Value Health 2016, 19, A602. [Google Scholar] [CrossRef]
- Hutchings, A.; Palaska, C.; Schey, C.; Pericleous, L.; Petersen, J. Payer Assessment and Reimbursement Policy for Rare Diseases: A Review of the Literature. Value Health 2013, 16, A391. [Google Scholar] [CrossRef]
- Macaulay, R.; Wang, G.; Fernandez Dacosta, R. PRO41 The Omar Era—Fate Of Orphan Designations In Europe. Value Health 2019, 22, S342–S343. [Google Scholar] [CrossRef]
- Paz, S.; Torrent, J.; Poveda, J.; Perez, J.; Moreno, J.; Martin, A.; Gonzalez, L.; Cruz, J.; Comellas, M.; Abaitua, I.; et al. Experts Consensus on The Future of Rare Diseases Care and Orphan Drugs Access In Spain: A Delphi Study. Value Health 2015, 18, A679. [Google Scholar] [CrossRef]
- Wang, G.; Macaulay, R. Orphan Legislation—Leave No One Behind? Value Health 2018, 21, S260. [Google Scholar] [CrossRef]
- Lise Aagaard, L.; Kristensen, K. Access to Cross-Border Health Care Services for Patients with Rare Diseases in the European Union. Orphan Drugs Res. Rev. 2014, 4, 39. [Google Scholar] [CrossRef]
- Lee, C.; Proudfoot, E.; O’Leary, B. Access To Medicines For Rare Diseases In Australia: The Current Climate For Reimbursement. Value Health 2017, 20, A567–A568. [Google Scholar] [CrossRef]
- Dodman, S.; Iheanacho, I.; Koufopoulou, M. PRO67 Does Society Support The Prioritisation Of High-Cost Treatments For Rare Diseases? A Systematic Literature Review. Value Health 2020, 23, S701–S702. [Google Scholar] [CrossRef]
- Schey, C.; Irwin, J.; Teneishvili, M.; Krabbe, P.F.M.; Connolly, M. Assessing The Relationship Between Individual Attributes Identified In Review Of Multi-Criteria Decision Analysis (MCDA) Of Rare Diseases And Annual Treatment Costs In Rare Endocrine Disorders. Value Health 2014, 17, A562. [Google Scholar] [CrossRef] [PubMed]
- Mennezein, L.; Avot, D.; Laigle, V. Orphan Drugs In France: Key Market Access Incentives. Value Health 2017, 20, A565. [Google Scholar] [CrossRef]
- Garcia Sanchez, J.; Sattar, S.; Hill, C. Review of Health Technology Assessment (HTA) Requirements for Rare Diseases Across European Countries. Value Health 2016, 19, A491. [Google Scholar] [CrossRef]
- Shah, R.R. Regulatory Framework for the Treatment of Orphan Diseases. In Fabry Disease: Perspectives from 5 Years of FOS; Oxford PharmaGenesis: Oxford, UK, 2006. [Google Scholar]
- Nagore Induráin, C.; Lacalle, E.; Arteche, L. El Farmacéutico En El Contexto de Las Enfermedades Raras y Los Medicamentos Huérfanos. An. Sist. Sanit. Navar. 2008, 31, 127–143. [Google Scholar] [CrossRef] [PubMed]
- Deegan, P.B.; Cox, T.M. Imiglucerase in the Treatment of Gaucher Disease: A History and Perspective. Drug Des Devel Ther 2012, 6, 81–106. [Google Scholar] [CrossRef] [PubMed]
- Coles, S.; Haire, K.; Kenny, T.; Jessop, E.G. Monitoring Access to Nationally Commissioned Services in England. Orphanet J. Rare Dis. 2012, 7, 85. [Google Scholar] [CrossRef] [PubMed]
- Colombatti, R.; Perrotta, S.; Samperi, P.; Casale, M.; Masera, N.; Palazzi, G.; Sainati, L.; Russo, G. Organizing National Responses for Rare Blood Disorders: The Italian Experience with Sickle Cell Disease in Childhood. Orphanet J. Rare Dis. 2013, 8, 169. [Google Scholar] [CrossRef]
- Žnidar, I.; Collin-Histed, T.; Niemeyer, P.; Parkkinen, J.; Lauridsen, A.G.; Zariņa, S.; Cohen, Y.; Manuel, J. The European Gaucher Alliance: A Survey of Member Patient Organisations’ Activities, Healthcare Environments and Concerns. Orphanet J. Rare Dis. 2014, 9, 1–14. [Google Scholar] [CrossRef]
- Schreiber-Katz, O.; Klug, C.; Thiele, S.; Schorling, E.; Zowe, J.; Reilich, P.; Nagels, K.H.; Walter, M.C. Comparative Cost of Illness Analysis and Assessment of Health Care Burden of Duchenne and Becker Muscular Dystrophies in Germany. Orphanet J. Rare Dis. 2014, 9, 210. [Google Scholar] [CrossRef]
- Bosch, A.M.; Burlina, A.; Cunningham, A.; Bettiol, E.; Moreau-Stucker, F.; Koledova, E.; Benmedjahed, K.; Regnault, A. Assessment of the Impact of Phenylketonuria and Its Treatment on Quality of Life of Patients and Parents from Seven European Countries. Orphanet J. Rare Dis. 2015, 10, 80. [Google Scholar] [CrossRef]
- Van Groenendael, S.; Giacovazzi, L.; Davison, F.; Holtkemper, O.; Huang, Z.; Wang, Q.; Parkinson, K.; Barrett, T.; Geberhiwot, T. High Quality, Patient Centred and Coordinated Care for Alstrom Syndrome: A Model of Care for an Ultra-Rare Disease Rare Systemic Diseases. Orphanet J. Rare Dis. 2015, 10, 149. [Google Scholar] [CrossRef] [PubMed]
- Garau, R. The Medical Experience of a Patient with a Rare Disease and Her Family. Orphanet J. Rare Dis. 2016, 11, 19. [Google Scholar] [CrossRef]
- Dalal, P.G. The project OrphanAnesthesia is internationally oriented. Thus all recommendations will be published in English. Anasthesiol. Intensivmed. 2019, 60, S26–S34. [Google Scholar]
- Wong-Rieger, D.; Rieger, F. Health Policies for Orphan Diseases: International Comparison of Regulatory, Reimbursement and Health Services Policies. Rare Dis. Age Health 2.0 2014, 4, 267–277. [Google Scholar] [CrossRef]
- Maresova, P.; Mohelska, H.; Kuca, K. Cooperation Policy of Rare Diseases in the European Union. 5th Iceepsy Int. Conf. Educ. Educ. Psychol. 2015, 171, 1302–1308. [Google Scholar] [CrossRef]
- Kogushi, K.; Ogawa, T.; Ikeda, S. An Impact Analysis of the Implementation of Health Technology Assessment for New Treatment of Orphan Diseases in Japan. Expert Rev. Pharm. Outcomes Res. 2020, 20, 455–471. [Google Scholar] [CrossRef] [PubMed]
- Pasceri, E. Analyzing Rare Diseases Terms in Biomedical Terminologies. Ital. J. Libr. Inf. Sci. 2012, 3, 1–15. [Google Scholar] [CrossRef]
- Bannister, J.B. Regulating rare disease: Safely facilitating access to orphan drugs. Fordham Law Rev. 2018, 86, 1889–1921. [Google Scholar]
- Xoxi, E.; Facey, K.M.; Cicchetti, A. The Evolution of AIFA Registries to Support Managed Entry Agreements for Orphan Medicinal Products in Italy. Front. Pharmacol. 2021, 12, 699466. [Google Scholar] [CrossRef]
- Mattingly, T.J.; Simoni-Wastila, L. Patient-Centered Drug Approval: The Role of Patient Advocacy in the Drug Approval Process. J. Manag. Care Spec. Pharm. 2017, 23, 1078–1082. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, I.A. Medical care to help improve the quality of life for children with rare diseases and their families. Rev. De Educ. Inclusiva 2014, 7, 14–32. [Google Scholar]
- Künnemann, K. 10th World Orphan Drug Congress (WODC) (November 12-14, 2019-Barcelona, Spain). Drugs Today 2019, 55, 753–758. [Google Scholar] [CrossRef]
- Picavet, E.; Annemans, L.; Cleemput, I.; Cassiman, D.; Simoens, S. Market Uptake of Orphan Drugs—A European Analysis. J. Clin. Pharm. Ther. 2012, 37, 664–667. [Google Scholar] [CrossRef]
- Iskrov, G.; Miteva-Katrandzhieva, T.; Stefanov, R. Health Technology Assessment and Appraisal of Therapies for Rare Diseases. Rare Dis. Epidemiol. Update Overv. 2nd Ed. 2017, 1031, 221–231. [Google Scholar] [CrossRef]
- Nagore, C.; Lacalle, E.; Arteche, L. The pharmacist, rare diseases and orphan medicines. An. Sist. Sanit. Navar. 2008, 31, 127–143. [Google Scholar]
- Ho, L.S.; Zhang, T.L.; Kwok, T.C.T.; Wat, K.P.; Lai, F.T.T.; Li, S.C. Financing Orphan Drugs Through a Blockchain-Supported Insurance Model. Front. Blockchain 2022, 5, 818807. [Google Scholar] [CrossRef]
- Ankit, T.; Shrikalp, D.; Maitreyi, Z.; Kumar, J.P.; Kiran, K. Transition of Pharmaceutical Regulations: The New Regulatory Era after Brexit. J. Pharm. Res.Int. 2021, 33, 804–817. [Google Scholar] [CrossRef]
- Sarpatwari, A.; Beall, R.F.; Abdurrob, A.; He, M.D.; Kesselheim, A.S. Evaluating The Impact Of The Orphan Drug Act’s Seven-Year Market Exclusivity Period. Health Aff. 2018, 37, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Yamoah, L.; Dragojlovic, N.; Smith, A.; Lynd, L.D.; Marra, C.A. Evaluating New Zealanders’ Values for Drug Coverage Decision Making: Trade-Offs between Treatments for Rare and Common Conditions. Pharmacoeconomics 2021, 39, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Dupont, A.G.; Van Wilder, P.B. Access to Orphan Drugs despite Poor Quality of Clinical Evidence. Br. J. Clin. Pharmacol. 2011, 71, 488–496. [Google Scholar] [CrossRef]
- Lee, B.; Bae, E.Y.; Bae, S.; Choi, H.J.; Son, K.B.; Lee, Y.S.; Jang, S.; Lee, T.J. How Can We Improve Patients’ Access to New Drugs under Uncertainties? South Korea’s Experience with Risk Sharing Arrangements. BMC Health Serv. Res. 2021, 21, 967. [Google Scholar] [CrossRef]
- Huber, J.O.; Schmidt, M. The contribution of the pharmaceutical industry to the diagnosis and treatment of children and adolescents, in particular those with rare and complex diseases. An overview on the economic and regulatory challenges to examples of successful implementation. Padiatr. Padol. 2015, 50, S103–S110. [Google Scholar] [CrossRef]
- Shafie, A.A.; Supian, A.; Hassali, M.A.A.; Ngu, L.H.; Thong, M.K.; Ayob, H.; Chaiyakunapruk, N. Rare Disease in Malaysia: Challenges and Solutions. PLoS ONE 2020, 15, e0230850. [Google Scholar] [CrossRef]
- Thaker, Z.; Jethva, K.; Bhatt, D.; Zaveri, M.; Deshpande, S. Orphan drugs: Overview and regulatory review process. Int. J. Pharm. Sci. Res. 2019, 10, 505–518. [Google Scholar] [CrossRef]
- Pogany, G. Rare diseases and their patient organization: The Hungarian Federation of People with Rare and Congenital Diseases. Orv Hetil 2014, 155, 329–333. [Google Scholar] [CrossRef]
- Pascarelli, D.B.N.; Pereira, E.L. Rare diseases in the Brazilian National Congress: Analysis of parliamentary action. Cad. Saude Publica 2022, 38, e00167721. [Google Scholar] [CrossRef]
- Spagnolo, P.; du Bois, R.M.; Cottin, V. Rare Lung Disease and Orphan Drug Development. Lancet Respir. Med. 2013, 1, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Enzmann, H.; Broich, K. Cancer: Is it really so different? Particularities of oncologic drugs from the perspective of the pharmaceutical regulatory agency. Z. Fur Evidenz Fortbild. Und Qual. Im Gesundh. 2013, 107, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Hogerzeil, H.V. Rare Diseases and Essential Medicines: A Global Perspective. Int. J. Pharm. Med. 2005, 19, 285–288. [Google Scholar] [CrossRef]
- Syrop, J. The World’s Costliest Drug: Conflict Overshadows Cure. P T 1994, 19, 286–287+290. [Google Scholar]
- Thielke, D.; Thyssen, J.P.; Hansen, B.J. Orphan Drugs: Medications for Patients with Rare Diseases. Ugeskr. Laeger 2006, 168, 2236–2238. [Google Scholar] [PubMed]
- Taruscio, D.; Trama, A.; Stefanov, R. Tackling Rare Diseases at European Level: Why Do We Need a Harmonized Framework? Folia Med. (Plovdiv) 2007, 49, 59–67. [Google Scholar]
- Malcolm, L.M. Information Service on Treatment for Rare Diseases: The National Information Center for Orphan Drugs and Rare Diseases. Am. Pharm. 1989, NS29, 32. [Google Scholar] [CrossRef]
- Vassal, G.; Méry-Mignard, D.; Caulin, C. Clinical Trials in Paediatric Oncology: Recommendations for the Development of New Anticancer Agents. Therapie 2003, 58, 239–246. [Google Scholar] [CrossRef]
- Drummond, M.F.; Wilson, D.A.; Kanavos, P.; Ubel, P.; Rovira, J. Assessing the Economic Challenges Posed by Orphan Drugs. Int. J. Technol. Assess Health Care 2007, 23, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liang, Y.Q.; Xu, H. Analysis of Orphan Drugs Approved by FDA in 2015. Chin. J. New Drugs 2017, 26, 841–844. [Google Scholar]
- Schlander, M.; Beck, M. Expensive Drugs for Rare Disorders: To Treat or Not to Treat? The Case of Enzyme Replacement Therapy for Mucopolysaccharidosis VI. Curr. Med. Res. Opin. 2009, 25, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Zaric, G.S. Optimal Drug Pricing, Limited Use Conditions and Stratified Net Benefits for Markov Models of Disease Progression. Health Econ. 2008, 17, 1277–1294. [Google Scholar] [CrossRef] [PubMed]
- Brecht, I.B.; Graf, N.; Von Schweinitz, D.; Frühwald, M.C.; Bielack, S.S.; Schneider, D.T. Networking for Children and Adolescents with Very Rare Tumors: Foundation of the Gpoh Pediatric Rare Tumor Group. Klin. Padiatr. 2009, 221, 181–185. [Google Scholar] [CrossRef] [PubMed]
- DeVincenzo, J.P. Harnessing RNA Interference to Develop Neonatal Therapies: From Nobel Prize Winning Discovery to Proof of Concept Clinical Trials. Early Hum. Dev. 2009, 85, S31–S35. [Google Scholar] [CrossRef] [PubMed]
- Beck, M. Emerging Drugs for Lysosomal Storage Diseases. Expert Opin. Emerg. Drugs 2010, 15, 495–507. [Google Scholar] [CrossRef]
- Butlen-Ducuing, F.; Rivière, F.; Aarum, S.; Llnares-Garcia, J. European Medicines Agency Support Mechanisms Fostering Orphan Drug Development. Drug News Perspect 2010, 23, 71–81. [Google Scholar] [CrossRef]
- McCabe, C.; Stafinski, T.; Menon, D. Is It Time to Revisit Orphan Drug Policies? BMJ 2010, 341, 614. [Google Scholar] [CrossRef]
- Liang, B.A.; Mackey, T. Reforming Off-Label Promotion to Enhance Orphan Disease Treatment. Science 2010, 327, 273–274. [Google Scholar] [CrossRef]
- Christensen, S.B.; Bygbjerg, I.C. Drugs for the Neglected Disease Malaria Based on Natural Products. Bioact. Compd. Nat. Sources: Nat. Prod. Lead Compd. Drug Discov. Second. Ed. 2011, 525–550. [Google Scholar] [CrossRef]
- Picavet, E.; Dooms, M.; Cassiman, D.; Simoens, S. Orphan Drugs for Rare Diseases: Grounds for Special Status. Drug Dev. Res. 2012, 73, 115–119. [Google Scholar] [CrossRef]
- Pinxten, W.; Denier, Y.; Dooms, M.; Cassiman, J.J.; Dierickx, K. A Fair Share for the Orphans: Ethical Guidelines for a Fair Distribution of Resources within the Bounds of the 10-Year-Old European Orphan Drug Regulation. J. Med. Ethics 2012, 38, 148–153. [Google Scholar] [CrossRef]
- Webb, S.M.; Santos, A.; Valassi, E. The Value of a European Registry for Pituitary Adenomas: The Example of Cushing’s Syndrome Registry. Ann. Endocrinol. 2012, 73, 83–89. [Google Scholar] [CrossRef]
- Van Herle, K.; Behne, J.M.; Van Herle, A.; Blaschke, T.F.; Smith, T.J.; Yeaman, M.R. Integrative Continuum: Accelerating Therapeutic Advances in Rare Autoimmune Diseases. Annu. Rev. Pharmacol. Toxicol. 2012, 52, 523–547. [Google Scholar] [CrossRef]
- Maiella, S.; Rath, A.; Angin, C.; Mousson, F.; Kremp, O. Orphanet et Son Réseau: Où Trouver Une Information Validée Sur Les Maladies Rares. Rev. Neurol. 2013, 169, S3–S8. [Google Scholar] [CrossRef]
- Storf, H.; Hartz, T.; Tegtbauer, N.; Pfeiffer, W.; Schmidtke, J.; Graessner, H.; Wagner, T.; Ückert, F. Vision and Challenges of a Cartographic Representation of Expert Medical Centres for Rare Diseases. Stud. Health Technol. Inform. 2014, 205, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Bowlus, C.L.; Kenney, J.T.; Rice, G.; Navarro, R. Primary Biliary Cholangitis: Medical and Specialty Pharmacy Management Update. J. Manag. Care Spec. Pharm. 2016, 22, S3–S15. [Google Scholar] [CrossRef] [PubMed]
- Girard, N. Rare Thoracic Tumors. Rev. Malad Respir. Actual. 2014, 6, 540–551. [Google Scholar] [CrossRef]
- Shameer, K.; Readhead, B.T.; Dudley, J. Computational and Experimental Advances in Drug Repositioning for Accelerated Therapeutic Stratification. Curr. Top. Med. Chem. 2015, 15, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Roberts, E.A.; Herder, M.; Hollis, A. Fair Pricing of Old Orphan Drugs: Considerations for Canada’s Orphan Drug Policy. CMAJ 2015, 187, 422–425. [Google Scholar] [CrossRef] [PubMed]
- Hyry, H.I.; Roos, J.C.P.; Cox, T.M. Orphan Drugs: Expensive yet Necessary. QJM 2015, 108, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.R.; Xiang, W.; Zhang, F. International Reimbursement Policies on Drugs for Pulmonary Arterial Hypertension and Enlightenment to China. Chin. Pharm. J. 2015, 50, 1639–1642. [Google Scholar] [CrossRef]
- Bradford, D.; Reilly, K.M.; Widemann, B.C.; Sandler, A.; Kummar, S. Developing Therapies for Rare Tumors: Opportunities, Challenges and Progress. Expert Opin. Orphan Drugs 2016, 4, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Speid, L. Don’t Do Different Things—Do Things Differently! Drug Development in Rare Diseases: The Patient’s Perspective. Clin. Pharmacol. Ther. 2016, 100, 336–338. [Google Scholar] [CrossRef]
- Rhizobium, G.E. Complete Genome Sequence of the Sesbania Symbiont and Rice. Nucleic Acids Res. 2013, 1, 13–14. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, J.; Wang, J. PHP3 rare diseases, orphan drugs, and the legislation in China. Value Health 2010, 13, A533. [Google Scholar] [CrossRef]
- Postma, M.J.; Noone, D.; Rozenbaum, M.H.; Carter, J.A.; Botteman, M.F.; Fenwick, E.; Garrison, L.P. Assessing the value of orphan drugs using conventional cost-effectiveness analysis: Is it fit for purpose? Orphanet J. Rare Dis. 2022, 17, 157. [Google Scholar] [CrossRef]
- Rollet, P.; Lemoine, A.; Dunoyer, M. Sustainable Rare Diseases Business and Drug Access: No Time for Misconceptions. Orphanet J. Rare Dis. 2013, 8, 109. [Google Scholar] [CrossRef]
- Stolk, P.; Willemen, M.J.; Leufkens, H.G.M. “Rare Essentials”: Drugs for Rare Diseases as Essential Medicines. Bull. World Health Organ 2006, 84, 745–751. [Google Scholar] [CrossRef]
- Stolk, P.; Heemstra, H.E.; Leufkens, H.G.; Bloechl-Daum, B.; Heerdink, E.R. No Difference in Between-Country Variability in Use of Newly Approved Orphan and Non-Orphan Medicinal Products-a Pilot Study. Orphanet J. Rare Dis. 2009, 4, 27. [Google Scholar] [CrossRef]
- Verma, I.C.; El-Beshlawy, A.; Tylki-Szymańska, A.; Martins, A.; Duan, Y.-L.; Collin-Histed, T.; Schoneveld Van Der Linde, M.; Mansour, R.; Dũng, V.C.; Mistry, P.K. Transformative effect of a Humanitarian Program for individuals affected by rare diseases: Building support systems and creating local expertise. Orphanet J. Rare Dis. 2022, 17, 87. [Google Scholar] [CrossRef] [PubMed]
- Schlander, M.; Dintsios, C.M.; Gandjour, A. Budgetary Impact and Cost Drivers of Drugs for Rare and Ultrarare Diseases. Value Health 2018, 21, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Kessel, M.; Hannemann-Weber, H.; Kratzer, J. Innovative Work Behavior in Healthcare: The Benefit of Operational Guidelines in the Treatment of Rare Diseases. Health Policy 2012, 105, 146–153. [Google Scholar] [CrossRef]
- Winquist, E.; Bell, C.M.; Clarke, J.T.R.; Evans, G.; Martin, J.; Sabharwal, M.; Gadhok, A.; Stevenson, H.; Coyle, D. An Evaluation Framework for Funding Drugs for Rare Diseases. Value Health 2012, 15, 982–986. [Google Scholar] [CrossRef]
- Faulkner, E.; Spinner, D.S.; Ringo, M.; Carroll, M. Are Global Health Systems Ready for Transformative Therapies? Value Health 2019, 22, 627–641. [Google Scholar] [CrossRef] [PubMed]
- Clarke, J.T.R.; Coyle, D.; Evans, G.; Martin, J.; Winquist, E. Toward a Functional Definition of a “Rare Disease” for Regulatory Authorities and Funding Agencies. Value Health 2014, 17, 757–761. [Google Scholar] [CrossRef]
- Ollendorf, D.A.; Chapman, R.H.; Pearson, S.D. Evaluating and Valuing Drugs for Rare Conditions: No Easy Answers. Value Health 2018, 21, 547–552. [Google Scholar] [CrossRef]
- Rodwell, C.; Aymé, S. Rare Disease Policies to Improve Care for Patients in Europe. Biochim. Et Biophys. Acta (BBA)—Mol. Basis Dis. 2015, 1852, 2329–2335. [Google Scholar] [CrossRef] [PubMed]
- Richter, T.; Nestler-Parr, S.; Babela, R.; Khan, Z.M.; Tesoro, T.; Molsen, E.; Hughes, D.A. Rare Disease Terminology and Definitions—A Systematic Global Review: Report of the ISPOR Rare Disease Special Interest Group. Value Health 2015, 18, 906–914. [Google Scholar] [CrossRef]
- Aranda-Reneo, I.; Rodríguez-Sánchez, B.; Peña-Longobardo, L.M.; Oliva-Moreno, J.; López-Bastida, J. Can the Consideration of Societal Costs Change the Recommendation of Economic Evaluations in the Field of Rare Diseases? An Empirical Analysis. Value Health 2021, 24, 431–442. [Google Scholar] [CrossRef]
- Belousova, O.A.; Groen, A.J.; Ouendag, A.M. Opportunities and Barriers for Innovation and Entrepreneurship in Orphan Drug Development. Technol. Forecast. Soc. Change 2020, 161, 120333. [Google Scholar] [CrossRef]
- Bai, J.P.F.; Barrett, J.S.; Burckart, G.J.; Meibohm, B.; Sachs, H.C.; Yao, L. Strategic Biomarkers for Drug Development in Treating Rare Diseases and Diseases in Neonates and Infants. AAPS J. 2013, 15, 447–454. [Google Scholar] [CrossRef]
- Putzeist, M.; Mantel-Teeuwisse, A.K.; Gispen-De Wied, C.C.; Hoes, A.W.; Leufkens, H.G.; La De Vrueh, R. Drug Development for Exceptionally Rare Metabolic Diseases: Challenging but Not Impossible. Orphanet J. Rare Dis. 2013, 8, 179. [Google Scholar] [CrossRef] [PubMed]
- Harari, S. Why We Should Care about Ultra-Rare Disease. Eur. Respir. Rev. 2016, 25, 101–103. [Google Scholar] [CrossRef]
- Janoudi, G.; Amegatse, W.; Mcintosh, B.; Sehgal, C.; Richter, T. Health Technology Assessment of Drugs for Rare Diseases: Insights, Trends, and Reasons for Negative Recommendations from the CADTH Common Drug Review. Orphanet J. Rare Dis. 2016, 11, 164. [Google Scholar] [CrossRef]
- Annemans, L.; Aymé, S.; Le Cam, Y.; Facey, K.; Gunther, P.; Nicod, E.; Reni, M.; Roux, J.-L.; Schlander, M.; Taylor, D.; et al. Recommendations from the European Working Group for Value Assessment and Funding Processes in Rare Diseases (ORPH-VAL). Orphanet J. Rare Dis. 2017, 12, 50. [Google Scholar] [CrossRef] [PubMed]
- Volmar, C.-H.; Brothers, S. Orphan Diseases: State of the Drug Discovery Art. Wien. Med. Wochenschr. 2017, 167, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Southall, N.T.; Natarajan, M.; Pek, L.; Lau, L.; Jonker, A.H.; Deprez, B.; Guilliams, T.; Hunter, L.; Rademaker, C.M.; Hivert, V.; et al. The Use or Generation of Biomedical Data and Existing Medicines to Discover and Establish New Treatments for Patients with Rare Diseases-Recommendations of the IRDiRC Data Mining and Repurposing Task Force on Behalf of the IRDiRC Data Mining and Repurposing Task Force. Orphanet J. Rare Dis. 2019, 14, 1–8. [Google Scholar] [CrossRef]
- Pires De Mello, C.P.; Rumsey, J.; Slaughter, V.; Hickman, J.J. A Human-on-a-Chip Approach to Tackling Rare Diseases. Drug Discov. Today 2019, 24, 2139–2151. [Google Scholar] [CrossRef] [PubMed]
- Lanar, S.; Acquadro, C.; Seaton, J.; Savre, I.; Arnould, B. To What Degree Are Orphan Drugs Patient-Centered? A Review of the Current State of Clinical Research in Rare Diseases. Lanaret Al. Orphanet J. Rare Dis. 2020. [Google Scholar] [CrossRef]
- Armeni, P.; Cavazza, M.; Xoxi, E.; Taruscio, D.; Kodra, Y. Reflections on the Importance of Cost of Illness Analysis in Rare Diseases: A Proposal. J. Environ. Res. Public Health 2021, 18, 1101. [Google Scholar] [CrossRef] [PubMed]
- Aartsma-Rus, A.; Dooms, M.; Le Cam, Y.; Economics, C. Orphan Medicine Incentives: How to Address the Unmet Needs of Rare Disease Patients by Optimizing the European Orphan Medicinal Product Landscape Guiding Principles and Policy Proposals by the European Expert Group for Orphan Drug Incentives (OD Expert Gr. Front. Pharmacol. 2021, 12, 3666. [Google Scholar] [CrossRef]
- Gorini, F.; Santoro, M.; Pierini, A.; Mezzasalma, L.; Baldacci, S.; Bargagli, E.; Boncristiano, A.; Rossana Brunetto, M.; Cameli, P.; Cappelli, F.; et al. Orphan Drug Use in Patients With Rare Diseases: A Population-Based Cohort Study. Front. Pharmacol. 2022, 13, 1. [Google Scholar] [CrossRef]
- Directive 2011/24/EU of the European Parliament and of the Council of 9 March 2011 on the Application of Patients’ Rights in Cross-Border Healthcare. 2011, Vol. 088. L 88/45. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32011L0024 (accessed on 17 September 2022).
- Władysiuk, M.; Skweres-Kuchta, M. Rodziny Dzieci Dotkniętych Chorobą Rzadką Muszą Wyjeżdżać z Polski o. Available online: https://politykazdrowotna.com/artykul/rodziny-dzieci-dotknietych-choroba-rzadka-musza-wyjezdzac-z-polski/831472 (accessed on 10 November 2022).
- Hivert, V.; Jonker, A.H.; O’Connor, D.; Ardigo, D. IRDiRC: 1000 New Rare Diseases Treatments by 2027, Identifying and Bringing Forward Strategic Actions. Rare Dis. Orphan Drugs J. 2021, 1, 3. [Google Scholar] [CrossRef]
- Rare 2030. Panel of Experts. Everything you would like to know about foresight but were afraid to ask. Available online: https://www.rare2030.eu/wp-content/uploads/2019/09/Explaining-Foresight-resource-for-the-Rare2030-project-panel-of-experts.pdf (accessed on 27 January 2023).
- United Nations. Transforming Our World: The 2030 Agenda for Sustainable Development; United Nations: New York, NY, USA, 2015. [Google Scholar]
- United Nations. Addressing the Challenges Of Persons Living With A Rare Disease And Their Families: Resolution / adopted by the General Assembly; United Nations: New York, NY, USA, 2021. [Google Scholar]
- Władysiuk, M.; Plisko, R.; Kostrzewska, K.; Tóth, A.; Molnar, M.G.A.P. Gearing up. Improving Time to Patient Access to Innovative Therapies in V4. Executive Summary; G.A.P.: Kraków, Poland; Warsaw, Poland; Budapest, Hungary, 2022. [Google Scholar]
- Senior, M.; Hadjivasiliou, A. Orphan Drug. Report 2022; Evaluate Pharma: London, UK, 2022. [Google Scholar]
- European Commission. Pharmaceutical Strategy for Europe; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- European Commission. European Medicines Agency Pilot Project “Market Launch of Centrally Authorised Products”; European Commission: Brussels, Belgium, 2021. [Google Scholar]
- Minister Zdrowia. Komunikat Ministra Zdrowia w Sprawie Produktów Leczniczych Niepodlegających Finansowaniu w Ramach Procedury Ratunkowego Dostępu Do Technologii Lekowych; Ministerstwo Zdrowia: Warsaw, Poland, 2022.
- EURORDIS-Rare Diseases Europe. Breaking the Access Deadlock to Leave No One Behind. A contribution by EURORDIS and its Members on possibilities for patients’ full and equitable access to rare disease therapies in Europe; EURORDIS-Rare Diseases Europe: Paris, France, 2018. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).