Current Perspective on the Role of the Circadian Clock and Extracellular Matrix in Chronic Lung Diseases
Abstract
1. Introduction
2. The Circadian Clock Role in Chronic Lung Disease
3. The Multifactorial Role of Extracellular Matrix in Chronic Lung Disease
4. Transforming Growth Factor-β Signaling in ECM Remodeling and Chronic Lung Disease
5. Circadian Clock Modulation of the ECM
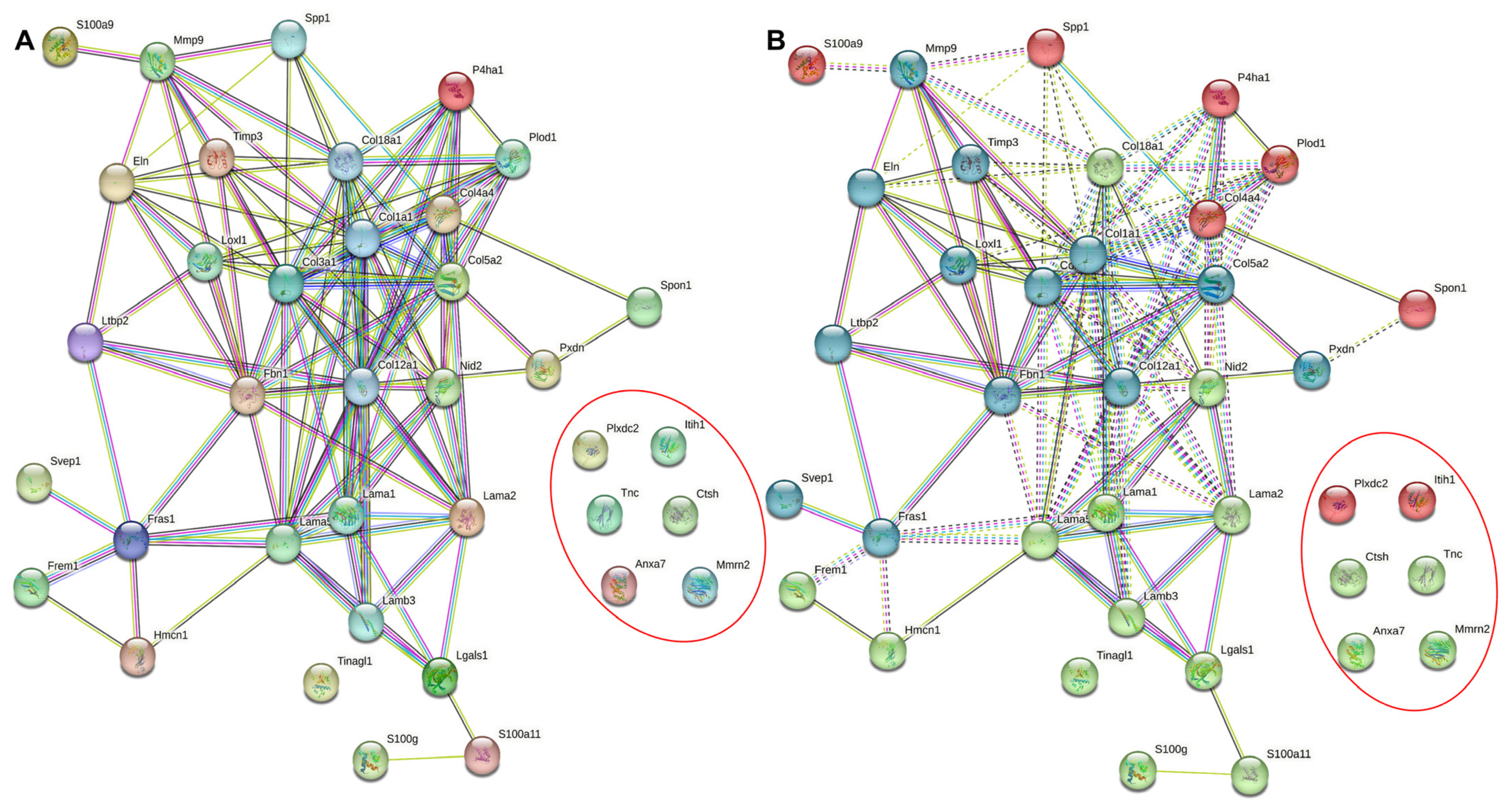
5.1. Circadian Modulation of the ECM throughout the Body
5.2. Circadian Modulation of ECM in Chronic Lung Disease
6. The Circadian Clock Influence on TGFβ Signaling in Chronic Lung Disease
7. Targeting the ECM with Circadian Clock-Based Therapeutics for the Treatment of Chronic Lung Disease
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Panda, S. Circadian physiology of metabolism. Science 2016, 354, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Saper, C.B.; Scammell, T.E.; Lu, J. Hypothalamic regulation of sleep and circadian rhythms. Nature 2005, 437, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Castanon-Cervantes, O.; Wu, M.; Ehlen, J.C.; Paul, K.; Gamble, K.L.; Johnson, R.L.; Besing, R.C.; Menaker, M.; Gewirtz, A.T.; Davidson, A.J. Dysregulation of Inflammatory Responses by Chronic Circadian Disruption. J. Immunol. 2010, 185, 5796–5805. [Google Scholar] [CrossRef] [PubMed]
- Froy, O. Circadian Rhythms, Aging, and Life Span in Mammals. Physiology 2011, 26, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Shetty, A.; Hsu, J.W.; Manka, P.P.; Syn, W.-K. Role of the Circadian Clock in the Metabolic Syndrome and Nonalcoholic Fatty Liver Disease. Dig. Dis. Sci. 2018, 63, 3187–3206. [Google Scholar] [CrossRef]
- Rabinovich-Nikitin, I.; Lieberman, B.; Martino, T.A.; Kirshenbaum, L.A. Circadian-Regulated Cell Death in Cardiovascular Diseases. Circulation 2019, 139, 965–980. [Google Scholar] [CrossRef]
- Li, H.; Song, S.; Wang, Y.; Huang, C.; Zhang, F.; Liu, J.; Hong, J.-S. Low-Grade Inflammation Aggravates Rotenone Neurotoxicity and Disrupts Circadian Clock Gene Expression in Rats. Neurotox. Res. 2019, 35, 421–431. [Google Scholar] [CrossRef]
- Sundar, I.K.; Ahmad, T.; Yao, H.; Hwang, J.-W.; Gerloff, J.; Lawrence, B.P.; Sellix, M.T.; Rahman, I. Influenza A virus-dependent remodeling of pulmonary clock function in a mouse model of COPD. Sci. Rep. 2015, 5, 9927. [Google Scholar] [CrossRef]
- Giri, A.; Wang, Q.; Rahman, I.; Sundar, I.K. Circadian molecular clock disruption in chronic pulmonary diseases. Trends Mol. Med. 2022, 28, 513–527. [Google Scholar] [CrossRef]
- Burgess, J.K.; Harmsen, M.C. Chronic lung diseases: Entangled in extracellular matrix. Eur. Respir. Rev. 2022, 31, 210202. [Google Scholar] [CrossRef]
- Burgstaller, G.; Oehrle, B.; Gerckens, M.; White, E.S.; Schiller, H.B.; Eickelberg, O. The instructive extracellular matrix of the lung: Basic composition and alterations in chronic lung disease. Eur. Respir. J. 2017, 50, 1601805. [Google Scholar] [CrossRef]
- Hung, C.F. Origin of Myofibroblasts in Lung Fibrosis. Curr. Tissue Microenviron. Rep. 2020, 1, 155–162. [Google Scholar] [CrossRef]
- Yue, X.; Shan, B.; Lasky, J.A. TGF-β: Titan of Lung Fibrogenesis. Curr. Enzym. Inhib. 2010, 6, 67–77. [Google Scholar] [CrossRef]
- Dong, C.; Gongora, R.; Sosulski, M.L.; Luo, F.; Sanchez, C.G. Regulation of transforming growth factor-beta1 (TGF-β1)-induced pro-fibrotic activities by circadian clock gene BMAL1. Respir. Res. 2016, 17, 4. [Google Scholar] [CrossRef]
- Cunningham Peter, S.; Meijer, P.; Nazgiewicz, A.; Anderson Simon, G.; Borthwick Lee, A.; Bagnall, J.; Kitchen Gareth, B.; Lodyga, M.; Begley, N.; Venkateswaran Rajamiyer, V.; et al. The circadian clock protein REVERBα inhibits pulmonary fibrosis development. Proc. Natl. Acad. Sci. USA 2020, 117, 1139–1147. [Google Scholar] [CrossRef]
- Mohawk, J.A.; Green, C.B.; Takahashi, J.S. Central and peripheral circadian clocks in mammals. Annu. Rev. Neurosci. 2012, 35, 445–462. [Google Scholar] [CrossRef]
- Preitner, N.; Damiola, F.; Luis Lopez, M.; Zakany, J.; Duboule, D.; Albrecht, U.; Schibler, U. The Orphan Nuclear Receptor REV-ERBα Controls Circadian Transcription within the Positive Limb of the Mammalian Circadian Oscillator. Cell 2002, 110, 251–260. [Google Scholar] [CrossRef]
- Sato, T.K.; Panda, S.; Miraglia, L.J.; Reyes, T.M.; Rudic, R.D.; McNamara, P.; Naik, K.A.; FitzGerald, G.A.; Kay, S.A.; Hogenesch, J.B. A Functional Genomics Strategy Reveals Rora as a Component of the Mammalian Circadian Clock. Neuron 2004, 43, 527–537. [Google Scholar] [CrossRef]
- Agusti, A.; Hedner, J.; Marin, J.M.; Barbé, F.; Cazzola, M.; Rennard, S. Night-time symptoms: A forgotten dimension of COPD. Eur. Respir. Rev. 2011, 20, 183. [Google Scholar] [CrossRef]
- Scheer Frank, A.J.L.; Hilton Michael, F.; Evoniuk Heather, L.; Shiels Sally, A.; Malhotra, A.; Sugarbaker, R.; Ayers, R.T.; Israel, E.; Massaro Anthony, F.; Shea Steven, A. The endogenous circadian system worsens asthma at night independent of sleep and other daily behavioral or environmental cycles. Proc. Natl. Acad. Sci. USA 2021, 118, e2018486118. [Google Scholar] [CrossRef]
- Durrington, H.J.; Krakowiak, K.; Meijer, P.; Begley, N.; Maidstone, R.; Goosey, L.; Gibbs, J.E.; Blaikley, J.F.; Gregory, L.G.; Lloyd, C.M.; et al. Circadian asthma airway responses are gated by REV-ERBα. Eur. Respir. J. 2020, 56, 1902407. [Google Scholar] [CrossRef] [PubMed]
- Lelièvre, S.A.; Weaver, V.M.; Nickerson, J.A.; Larabell, C.A.; Bhaumik, A.; Petersen, O.W.; Bissell, M.J. Tissue phenotype depends on reciprocal interactions between the extracellular matrix and the structural organization of the nucleus. Proc. Natl. Acad. Sci. USA 1998, 95, 14711–14716. [Google Scholar] [CrossRef] [PubMed]
- Mauad, T.; Silva, L.F.F.; Santos, M.A.; Grinberg, L.; Bernardi, F.D.C.; Martins, M.A.; Saldiva, P.H.N.; Dolhnikoff, M. Abnormal Alveolar Attachments with Decreased Elastic Fiber Content in Distal Lung in Fatal Asthma. Am. J. Respir. Crit. Care Med. 2004, 170, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Grainge, C.L.; Lau, L.C.K.; Ward, J.A.; Dulay, V.; Lahiff, G.; Wilson, S.; Holgate, S.; Davies, D.E.; Howarth, P.H. Effect of Bronchoconstriction on Airway Remodeling in Asthma. N. Engl. J. Med. 2011, 364, 2006–2015. [Google Scholar] [CrossRef] [PubMed]
- Ramis, J.; Middlewick, R.; Pappalardo, F.; Cairns, J.T.; Stewart, I.D.; John, A.E.; Naveed, S.-u.N.; Krishnan, R.; Miller, S.; Shaw, D.E.; et al. LOXL2 Mediates Airway Smooth Muscle Cell Matrix Stiffness and Drives Asthmatic Airway Remodelling. bioRxiv 2020. [Google Scholar] [CrossRef]
- Joseph, C.; Tatler, A. Pathobiology of Airway Remodeling in Asthma: The Emerging Role of Integrins. J. Asthma Allergy 2022, 15, 595–610. [Google Scholar] [CrossRef]
- Hinz, B. Mechanical Aspects of Lung Fibrosis. Proc. Am. Thorac. Soc. 2012, 9, 137–147. [Google Scholar] [CrossRef]
- Kong, J.; Tian, H.; Zhang, F.; Zhang, Z.; Li, J.; Liu, X.; Li, X.; Liu, J.; Li, X.; Jin, D.; et al. Extracellular vesicles of carcinoma-associated fibroblasts creates a pre-metastatic niche in the lung through activating fibroblasts. Mol. Cancer 2019, 18, 175. [Google Scholar] [CrossRef]
- Genschmer, K.R.; Russell, D.W.; Lal, C.; Szul, T.; Bratcher, P.E.; Noerager, B.D.; Abdul Roda, M.; Xu, X.; Rezonzew, G.; Viera, L.; et al. Activated PMN Exosomes: Pathogenic Entities Causing Matrix Destruction and Disease in the Lung. Cell 2019, 176, 113–126.e115. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.; Wang, S.; Bi, J.; Huo, R. Exosomes from human adipose-derived mesenchymal stem cells inhibit production of extracellular matrix in keloid fibroblasts via downregulating transforming growth factor-β2 and Notch-1 expression. Bioengineered 2022, 13, 8515–8525. [Google Scholar] [CrossRef]
- Batlle, E.; Massagué, J. Transforming Growth Factor-β Signaling in Immunity and Cancer. Immunity 2019, 50, 924–940. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X.; Zhao, D.; Liu, B.; Wang, B.; Yu, W.; Li, J.; Yu, X.; Cao, F.; Zheng, G.; et al. TGF-β1 promotes the osteoinduction of human osteoblasts via the PI3K/AKT/mTOR/S6K1 signalling pathway. Mol. Med. Rep. 2019, 19, 3505–3518. [Google Scholar] [CrossRef]
- Li, M.O.; Flavell, R.A. TGF-β: A Master of All T Cell Trades. Cell 2008, 134, 392–404. [Google Scholar] [CrossRef]
- Ojiaku, C.A.; Yoo, E.J.; Panettieri, R.A. Transforming Growth Factor β1 Function in Airway Remodeling and Hyperresponsiveness. The Missing Link? Am. J. Respir. Cell Mol. Biol. 2017, 56, 432–442. [Google Scholar] [CrossRef]
- Gu, L.; Zhu, Y.-J.; Yang, X.; Guo, Z.-J.; Xu, W.-B.; Tian, X.-L. Effect of TGF-?/Smad signaling pathway on lung myofibroblast differentiation. Acta Pharmacol. Sin. 2007, 28, 382–391. [Google Scholar] [CrossRef]
- Guo, Y.; Xiao, L.; Sun, L.; Liu, F. Wnt/beta-catenin signaling: A promising new target for fibrosis diseases. Physiol. Res. 2012, 61, 337–346. [Google Scholar] [CrossRef]
- Wan, X.; Chen, S.; Li, P.; Zhao, T.; Xie, S.; Fang, Y. Sinensetin protects against pulmonary fibrosis via inhibiting Wnt/β-Catenin signaling pathway. Tissue Cell 2022, 78, 101866. [Google Scholar] [CrossRef]
- Piersma, B.; Bank, R.A.; Boersema, M. Signaling in Fibrosis: TGF-β, WNT, and YAP/TAZ Converge. Front. Med. 2015, 2, 59. [Google Scholar] [CrossRef]
- Streuli, C.H.; Schmidhauser, C.; Kobrin, M.; Bissell, M.J.; Derynck, R. Extracellular matrix regulates expression of the TGF-beta 1 gene. J. Cell Biol. 1993, 120, 253–260. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, J.; Hu, Y.; Pan, T.; Xu, Y.; Yu, J.; Xiong, W.; Zhou, Q.; Wang, Y. Local administration of liposomal-based Srpx2 gene therapy reverses pulmonary fibrosis by blockading fibroblast-to-myofibroblast transition. Theranostics 2021, 11, 7110–7125. [Google Scholar] [CrossRef]
- Pantazopoulos, H.; Gisabella, B.; Rexrode, L.; Benefield, D.; Yildiz, E.; Seltzer, P.; Valeri, J.; Chelini, G.; Reich, A.; Ardelt, M.; et al. Circadian Rhythms of Perineuronal Net Composition. Eneuro 2020, 7, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Takayama, F.; Zhang, X.; Hayashi, Y.; Wu, Z.; Nakanishi, H. Dysfunction in diurnal synaptic responses and social behavior abnormalities in cathepsin S-deficient mice. Biochem. Biophys. Res. Commun. 2017, 490, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, S.; Al Kassas, R.; Dykes, I.M.; Hughes, A.T.; Ghali, F.; Ross, K. A time to heal: microRNA and circadian dynamics in cutaneous wound repair. Clin. Sci. 2022, 136, 579–597. [Google Scholar] [CrossRef] [PubMed]
- Bekki, H.; Duffy, T.; Okubo, N.; Olmer, M.; Alvarez-Garcia, O.; Lamia, K.; Kay, S.; Lotz, M. Suppression of circadian clock protein cryptochrome 2 promotes osteoarthritis. Osteoarthr. Cartil. 2020, 28, 966–976. [Google Scholar] [CrossRef] [PubMed]
- Duffy, T.; Bekki, H.; Lotz, M.K. Genome-Wide Occupancy Profiling Reveals Critical Roles of FoxO1 in Regulating Extracellular Matrix and Circadian Rhythm Genes in Human Chondrocytes. Arthritis Rheumatol. 2020, 72, 1514–1523. [Google Scholar] [CrossRef]
- Mengatto, C.M.; Mussano, F.; Honda, Y.; Colwell, C.S.; Nishimura, I. Circadian Rhythm and Cartilage Extracellular Matrix Genes in Osseointegration: A Genome-Wide Screening of Implant Failure by Vitamin D Deficiency. PLoS ONE 2011, 6, e15848. [Google Scholar] [CrossRef]
- Chen, P.; Kakan, X.; Wang, S.; Dong, W.; Jia, A.; Cai, C.; Zhang, J. Deletion of clock gene Per2 exacerbates cholestatic liver injury and fibrosis in mice. Exp. Toxicol. Pathol. 2013, 65, 427–432. [Google Scholar] [CrossRef]
- Kwon, E.-Y.; Shin, S.-K.; Choi, M.-S. Ursolic Acid Attenuates Hepatic Steatosis, Fibrosis, and Insulin Resistance by Modulating the Circadian Rhythm Pathway in Diet-Induced Obese Mice. Nutrients 2018, 10, 1719. [Google Scholar] [CrossRef]
- Hu, C.; Beebe, K.; Hernandez, E.J.; Lazaro-Guevara, J.M.; Revelo, M.P.; Huang, Y.; Maschek, J.A.; Cox, J.E.; Kohan, D.E. Multiomic identification of factors associated with progression to cystic kidney disease in mice with nephron Ift88 disruption. Am. J. Physiol.-Ren. Physiol. 2022, 322, F175–F192. [Google Scholar] [CrossRef]
- Ingle, K.A.; Kain, V.; Goel, M.; Prabhu, S.D.; Young, M.E.; Halade, G.V. Cardiomyocyte-specific Bmal1 deletion in mice triggers diastolic dysfunction, extracellular matrix response, and impaired resolution of inflammation. Am. J. Physiol.-Heart Circ. Physiol. 2015, 309, H1827–H1836. [Google Scholar] [CrossRef]
- Yeung, C.-Y.C.; Dondelinger, F.; Schoof, E.M.; Georg, B.; Lu, Y.; Zheng, Z.; Zhang, J.; Hannibal, J.; Fahrenkrug, J.; Kjaer, M. Circadian regulation of protein cargo in extracellular vesicles. Sci. Adv. 2022, 8, eabc9061. [Google Scholar] [CrossRef]
- Tao, S.-C.; Guo, S.-C. Extracellular Vesicles: Potential Participants in Circadian Rhythm Synchronization. Int. J. Biol. Sci. 2018, 14, 1610–1620. [Google Scholar] [CrossRef]
- Sundar, I.K.; Rashid, K.; Sellix, M.T.; Rahman, I. The nuclear receptor and clock gene REV-ERBα regulates cigarette smoke-induced lung inflammation. Biochem. Biophys. Res. Commun. 2017, 493, 1390–1395. [Google Scholar] [CrossRef]
- Yao, H.; Sundar, I.K.; Huang, Y.; Gerloff, J.; Sellix, M.T.; Sime, P.J.; Rahman, I. Disruption of Sirtuin 1–Mediated Control of Circadian Molecular Clock and Inflammation in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Cell Mol. Biol. 2015, 53, 782–792. [Google Scholar] [CrossRef]
- Wang, Q.; Sundar, I.K.; Lucas, J.H.; Muthumalage, T.; Rahman, I. Molecular clock REV-ERBα regulates cigarette smoke–induced pulmonary inflammation and epithelial-mesenchymal transition. JCI Insight 2021, 6, e145200. [Google Scholar] [CrossRef]
- Yeung, L.; Hickey, M.; Wright, M. The Many and Varied Roles of Tetraspanins in Immune Cell Recruitment and Migration. Front. Immunol. 2018, 9, 1644. [Google Scholar] [CrossRef]
- Tripathi, L.P.; Itoh, M.N.; Takeda, Y.; Tsujino, K.; Kondo, Y.; Kumanogoh, A.; Mizuguchi, K. Integrative Analysis Reveals Common and Unique Roles of Tetraspanins in Fibrosis and Emphysema. Front. Genet. 2020, 11, 585998. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, S.; Yu, L.; Deng, Y.; Li, D.; Yu, X.; Chen, D.; Lu, Y.; Liu, S.; Chen, R. Pemafibrate attenuates pulmonary fibrosis by inhibiting myofibroblast differentiation. Int. Immunopharmacol. 2022, 108, 108728. [Google Scholar] [CrossRef]
- Van Raalte, D.H.; Li, M.; Pritchard, P.H.; Wasan, K.M. Peroxisome Proliferator-Activated Receptor (PPAR)-: A Pharmacological Target with a Promising Future. Pharm. Res. 2004, 21, 1531–1538. [Google Scholar] [CrossRef]
- Oishi, K.; Shirai, H.; Ishida, N. CLOCK is involved in the circadian transactivation of peroxisome-proliferator-activated receptor α (PPARα) in mice. Biochem. J. 2005, 386, 575–581. [Google Scholar] [CrossRef]
- Shao, X.; Taha, I.N.; Clauser, K.R.; Gao, Y.T.; Naba, A. MatrisomeDB: The ECM-protein knowledge database. Nucleic Acids Res. 2020, 48, D1136–D1144. [Google Scholar] [CrossRef] [PubMed]
- Pizarro, A.; Hayer, K.; Lahens, N.F.; Hogenesch, J.B. CircaDB: A database of mammalian circadian gene expression profiles. Nucleic Acids Res. 2013, 41, D1009–D1013. [Google Scholar] [CrossRef] [PubMed]
- Nainwal, N. Chronotherapeutics—A chronopharmaceutical approach to drug delivery in the treatment of asthma. J. Control. Release 2012, 163, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Jacob, H.; Curtis, A.M.; Kearney, C.J. Therapeutics on the clock: Circadian medicine in the treatment of chronic inflammatory diseases. Biochem. Pharmacol. 2020, 182, 114254. [Google Scholar] [CrossRef]
- Giri, A.; Rahman, I.; Sundar, I.K. Circadian clock-based therapeutics in chronic pulmonary diseases. Trends Pharm. Sci. 2022, 43, 1014–1029. [Google Scholar] [CrossRef]
- Nicolaides, N.C.; Charmandari, E.; Chrousos, G.P.; Kino, T. Circadian endocrine rhythms: The hypothalamic-pituitary-adrenal axis and its actions. Ann. N.Y. Acad. Sci. 2014, 1318, 71–80. [Google Scholar] [CrossRef]
- Solt, L.A.; Wang, Y.; Banerjee, S.; Hughes, T.; Kojetin, D.J.; Lundasen, T.; Shin, Y.; Liu, J.; Cameron, M.D.; Noel, R.; et al. Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature 2012, 485, 62–68. [Google Scholar] [CrossRef]
- Amador, A.; Huitron-Resendiz, S.; Roberts, A.J.; Kamenecka, T.M.; Solt, L.A.; Burris, T.P. Pharmacological Targeting the REV-ERBs in Sleep/Wake Regulation. PLoS ONE 2016, 11, e0162452. [Google Scholar] [CrossRef]
- He, B.; Chen, Z. Molecular Targets for Small-Molecule Modulators of Circadian Clocks. Curr. Drug Metab. 2016, 17, 503–512. [Google Scholar] [CrossRef]

| Gene Symbols | Gene Names | JTK Period | JTK Phase | JTK p-Value | JTK q-Value | Confidence Score |
|---|---|---|---|---|---|---|
| Lama5 | Laminin, alpha 5 | 26.0 | 7.0 | 7.35e-06 | 0.0006 | 100,033 |
| Col1a1 | Collagen, type I, alpha 1 | 24.0 | 4.0 | 2.48e-10 | 3.53e-07 | 43094 |
| Lamb3 | Laminin, beta 3 | 22.0 | 12.0 | 0.001 | 0.020 | 35,555.7 |
| Col12a1 | Collagen, type XII, alpha 1 | 24.0 | 15.0 | 0.0001 | 0.006 | 25,065.2 |
| S100a11 | S100 calcium binding protein A11 | 22.0 | 9.0 | 0.001 | 0.024 | 24,752.1 |
| Eln | Elastin | 24.0 | 23.0 | 2.36e-07 | 5.35e-05 | 23,947.5 |
| Col3a1 | Collagen type III, alpha 1 | 24.0 | 2.0 | 3.78e-07 | 7.30e-05 | 20,549.2 |
| Lama2 | Laminin, alpha 2 | 24.0 | 7.0 | 3.24e-05 | 0.001 | 17,516.3 |
| Nid2 | Nidogen 2 | 24.0 | 16.0 | 2.36e-07 | 5.35e-05 | 13,971.3 |
| S100a9 | S100 calcium binding protein A9 | 25.0 | 2.5 | 0.0004 | 0.011 | 10,646.8 |
| Tnc | Tenascin C | 24.0 | 5.5 | 0.0002 | 0.007 | 7974.4 |
| Tinagl1 | Tubulointerstitial nephritis antigen-like 1 | 24.0 | 14.5 | 0.001 | 0.032 | 7707.24 |
| Hmcn1 | Hemicentin 1 | 26.0 | 6.0 | 0.0002 | 0.007 | 7150.28 |
| Itih1 | Inter-alpha trypsin inhibitor, heavy chain 1 | 24.0 | 4.0 | 0.002 | 0.040 | 5836.89 |
| Fbn1 | Fibrillin 1 | 24.0 | 2.0 | 8.88e-08 | 2.74e-05 | 5221.73 |
| Anxa7 | Annexin A7 | 24.0 | 9.0 | 0.0001 | 0.004 | 4549.21 |
| Pxdn | Peroxidasin homolog | 22.0 | 8.0 | 9.32e-07 | 0.0001 | 4398.3 |
| Col5a2 | Collagen, type V alpha 2 | 26.0 | 22.0 | 0.0003 | 0.009 | 3921.87 |
| Fras1 | Fraser syndrome 1 homolog | 26.0 | 6.0 | 0.002 | 0.036 | 3695.19 |
| Col18a1 | Collagen, type XVIII, alpha 1 | 24.0 | 4.0 | 0.002 | 0.040 | 3506.24 |
| Frem1 | FRAS1-related extracellular matrix protein 1 | 26.0 | 8.0 | 0.0001 | 0.004 | 3063.51 |
| Mmrn2 | Multimerin 2 | 22.0 | 9.0 | 6.48e-05 | 0.003 | 2930.29 |
| Spon1 | Spondin 1 | 220. | 19.5 | 0.002 | 0.036 | 2808.89 |
| Spp1 | Osteopontin | 24.0 | 19.0 | 7.64e-07 | 0.0001 | 2684.34 |
| Ctsh | Cathepsin H | 24.0 | 17.0 | 0.0002 | 0.007 | 2621.88 |
| Lgals1 | Lectin galactose binding, soluble 1 | 22.0 | 9.0 | 0.003 | 0.043 | 2586.67 |
| Loxl1 | Lysyl oxidase-like 1 | 24.0 | 3.0 | 5.92e-09 | 3.57e-06 | 2012 |
| Col4a4 | Collagen, type IV alpha 4 | 24.0 | 3.0 | 0.0005 | 0.014 | 1991.64 |
| Svep1 | Sushi, von Willebrand factor type A | 22.0 | 15.0 | 0.001 | 0.024 | 1968.1 |
| Mmp9 | Matrix metalloproteinase 9 | 28.0 | 1.0 | 0.0005 | 0.014 | 1904.63 |
| Plxdc2 | Plexin domain containing protein 2 | 26.0 | 9.0 | 6.48e-05 | 0.003 | 1853.26 |
| Col12a1 | Collagen, type XII alpha 1 | 24.0 | 15.0 | 0.0001 | 0.006 | 1834.77 |
| P4ha1 | Prolyl 4-hydroxylase alpha 1 | 24.0 | 5.5 | 0.0003 | 0.009 | 1823.97 |
| S100g | S100 calcium binding protein G | 26.0 | 4.0 | 2.19e-06 | 0.0002 | 1797.8 |
| Lama1 | Laminin, alpha 1 | 28.0 | 7.5 | 0.001 | 0.028 | 1772.29 |
| Plod1 | Procollagen-lysine | 24.0 | 17.0 | 1.57e-05 | 0.001 | 1676.2 |
| Ltbp2 | Latent transforming growth factor beta binding protein 2 | 22.0 | 19.0 | 1.08e-05 | 0.0008 | 1423.32 |
| Timp3 | Tissue inhibitor of metalloproteinase 3 | 24.0 | 15.0 | 1.77e-09 | 1.46e-06 | 1138.08 |
| GO-Term/Pathway | Description | Count in Network | Strength | False Discovery Rate |
|---|---|---|---|---|
| Biological Process (GO) | ||||
| GO:0007155 | Cell adhesion | 17 | 1.13 | 1.23E-11 |
| GO:0030198 | Extracellular matrix organization | 11 | 1.45 | 6.23E-10 |
| GO:0009887 | Animal organ morphogenesis | 14 | 0.91 | 1.40E-06 |
| GO:0009888 | Tissue development | 17 | 0.77 | 1.40E-06 |
| GO:0009653 | Anatomical structure morphogenesis | 18 | 0.68 | 6.91E-06 |
| Molecular Function (GO) | ||||
| GO:0005201 | Extracellular matrix structural constituent | 10 | 2.02 | 3.45E-14 |
| GO:0046872 | Metal ion binding | 22 | 0.57 | 3.77E-06 |
| GO:0005178 | Integrin binding | 6 | 1.42 | 8.10E-05 |
| GO:0005509 | Calcium ion binding | 9 | 0.95 | 0.00029 |
| GO:0050840 | Extracellular matrix binding | 4 | 1.61 | 0.0017 |
| Cellular Component (GO) | ||||
| GO:0062023 | Collagen-containing extracellular matrix | 31 | 1.71 | 4.87E-46 |
| GO:0031012 | Extracellular matrix | 32 | 1.61 | 2.80E-45 |
| GO:0005576 | Extracellular region | 35 | 0.97 | 5.75E-30 |
| GO:0005604 | Basement membrane | 15 | 1.93 | 1.54E-22 |
| GO:0005615 | Extracellular space | 20 | 0.92 | 3.31E-12 |
| KEGG Pathways | ||||
| mmu04512 | ECM-receptor interaction | 10 | 1.84 | 1.74E-13 |
| mmu04974 | Protein digestion and absorption | 7 | 1.59 | 1.08E-07 |
| mmu05146 | Amoebiasis | 7 | 1.6 | 1.08E-07 |
| mmu04510 | Focal adhesion | 8 | 1.39 | 1.18E-07 |
| mmu05165 | Human papillomavirus infection | 8 | 1.14 | 6.31E-06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hahn, K.; Sundar, I.K. Current Perspective on the Role of the Circadian Clock and Extracellular Matrix in Chronic Lung Diseases. Int. J. Environ. Res. Public Health 2023, 20, 2455. https://doi.org/10.3390/ijerph20032455
Hahn K, Sundar IK. Current Perspective on the Role of the Circadian Clock and Extracellular Matrix in Chronic Lung Diseases. International Journal of Environmental Research and Public Health. 2023; 20(3):2455. https://doi.org/10.3390/ijerph20032455
Chicago/Turabian StyleHahn, Kameron, and Isaac Kirubakaran Sundar. 2023. "Current Perspective on the Role of the Circadian Clock and Extracellular Matrix in Chronic Lung Diseases" International Journal of Environmental Research and Public Health 20, no. 3: 2455. https://doi.org/10.3390/ijerph20032455
APA StyleHahn, K., & Sundar, I. K. (2023). Current Perspective on the Role of the Circadian Clock and Extracellular Matrix in Chronic Lung Diseases. International Journal of Environmental Research and Public Health, 20(3), 2455. https://doi.org/10.3390/ijerph20032455






