Evaluation of Chemical Elements, Lipid Profiles, Nutritional Indices and Health Risk Assessment of European Eel (Anguilla anguilla L.)
Abstract
1. Introduction
- To determine the chemical elements, including heavy metals, and the effect of biometric parameters (body weight and total length) on the content of the chemical elements in muscles of the European eel (Anguilla anguilla Linnaeus, 1758);
- To determine, based on quality indicators (THQ, HI, EDI, EWI, HQEFA, and BRQ), whether the level of heavy metal pollution is a concern for the health of the consumer after consumption of the studied fish species;
- To determine the load of all heavy metals (MPI) in the muscle tissue;
- To determine, based on the profile of fatty acids and lipid quality indices (PUFA/SFA, OFA, DFA, AI, TI, FLQ, HH, NVI, HPI, OFA, and de minimis EPA + DHA:Hg), whether the consumption of the examined fish is beneficial for human health.
2. Materials and Methods
2.1. Sampling and Sample Preparation
2.2. Element Analysis
2.2.1. Mercury
2.2.2. Copper, Zinc, Manganese, Iron, Cadmium, Magnesium, Calcium, Sodium, and Potassium
2.3. Fat and Fatty Acids Analysis
- Detector: flame ionization (FID);
- Capillary column (dimension 30 m × 0.25 μm with a 0.32 mm internal diameter, liquid phase StabilwaxR);
- Temperature:
2.4. Estimated Daily and Weekly Intake (EDI and EWI)
2.5. Target Hazard Quotient (THQ)
2.6. Hazardous Index (HI)
2.7. Metal Pollution Index (MPI)
2.8. The Lipid Quality Indices
2.8.1. Index of Atherogenicity (AI)
2.8.2. Index of Thrombogenicity (TI)
2.8.3. Flesh-Lipid Quality (FLQ)
2.8.4. Hypercholesterolaemic Fatty Acids (OFA)
2.8.5. Hypocholesterolaemic Fatty Acids (DFA)
2.8.6. The Hazard Quotient for the Benefit–Risk Ratio (HQEFA and BRQ)
DM = FP × c
HQ = DM/RfD × AW
HQEFA = (REFA/C) × c × (1/(RfD × AW)) = (REFA × c)/(C × RfD × AW)
QFA = RFA/CFA
QT = (RfD × bw)/c
2.8.7. Hypocholesterolemic/Hypercholesterolemic Ratio (HH)
2.8.8. Nutritive Value Index (NVI)
2.8.9. Health-Promoting Index (HPI)
2.8.10. The de Minimis EPA+DHA:Hg
2.9. Statistical Analysis
3. Results
3.1. Metal Bioaccumulation
3.2. Human Health Risk Assessment
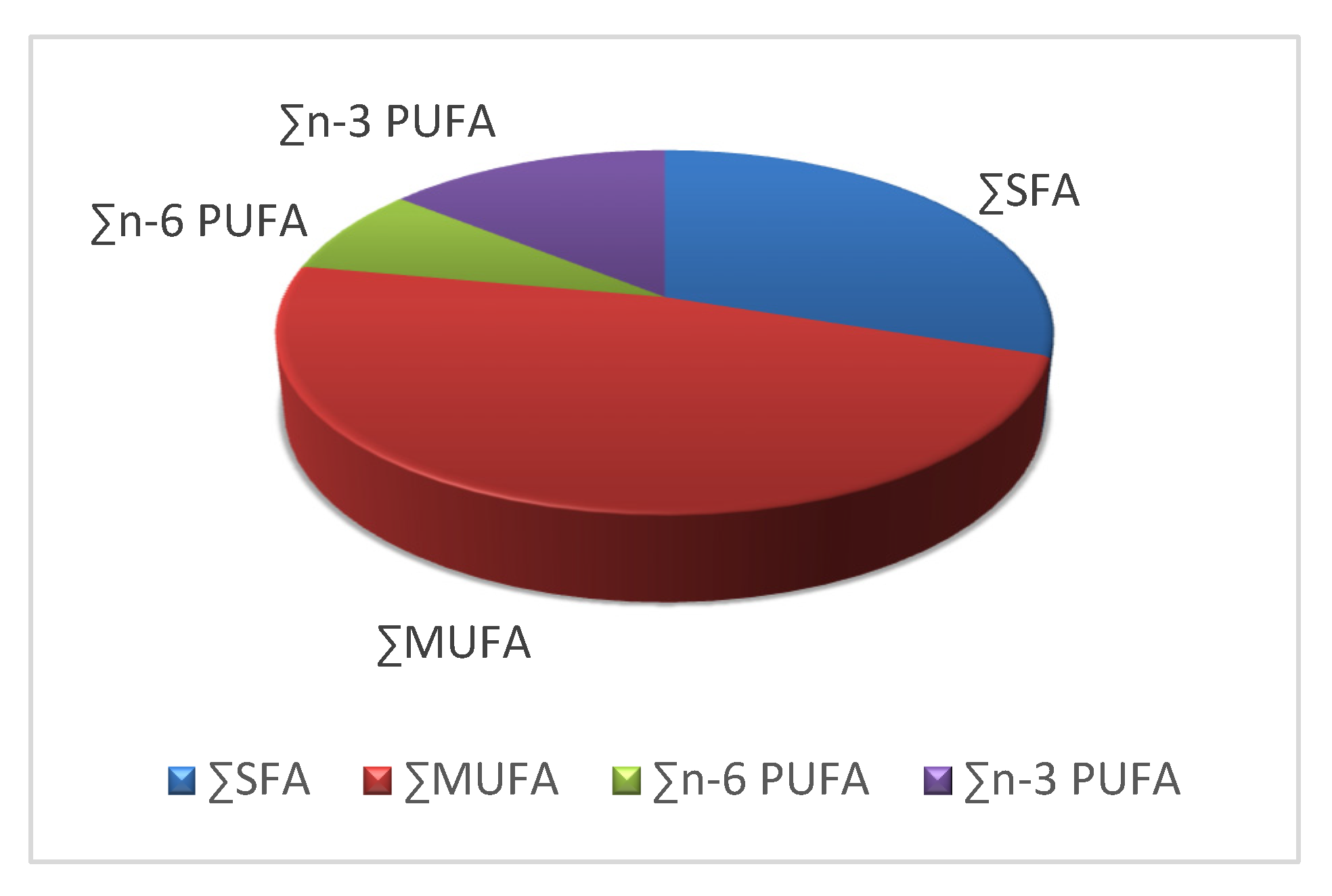
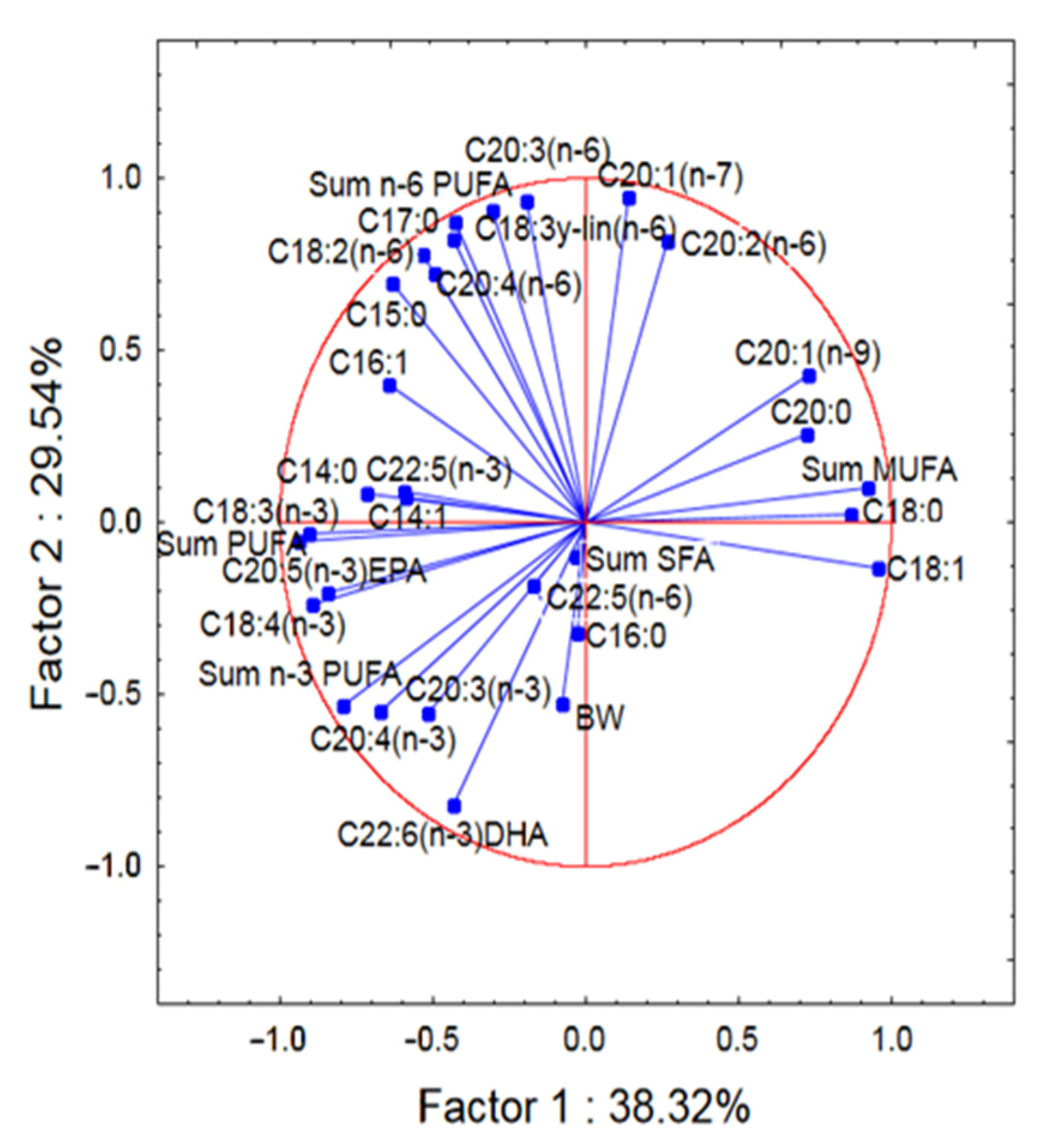
3.3. Fat and Fatty Acids Composition
4. Discussion
Health Risk
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van Ginneken, V.J.T.; Maes, G.E. The European eel (Anguilla anguilla, Linnaeus), its Lifecycle, Evolution and Reproduction: A Literature Review. Rev. Fish Biol. Fish. 2005, 15, 367–398. [Google Scholar] [CrossRef]
- Polak-Juszczak, L.; Robak, S. Macro- and microelements in eel (Anguilla anguilla) from the northern regions of Poland. J. Elem. 2015, 20, 385–394. [Google Scholar] [CrossRef]
- Lennox, R.J.; Økland, F.; Mitamura, H.; Cooke, S.; Thorstad, E.B.; Secor, D. European eel Anguilla anguilla compromise speed for safety in the early marine spawning migration. ICES J. Mar. Sci. 2018, 75, 1984–1991. [Google Scholar] [CrossRef]
- Parisi, G.; Terova, G.; Gasco, L.; Piccolo, G.; Roncarati, A.; Moretti, V.; Centoducati, G.; Gatta, P.P.; Pais, A. Current status and future perspectives of Italian finfish aquaculture. Rev. Fish Biol. Fish. 2013, 24, 15–73. [Google Scholar] [CrossRef]
- Podda, C.; Palmas, F.; Pusceddu, A.; Sabatini, A. Hard times for catadromous fish: The case of the European eel Anguilla anguilla (L. 1758). Adv. Oceanogr. Limnol. 2021, 12, 9997. [Google Scholar] [CrossRef]
- Chanda, S.; Paul, B.N.; Ghosh, K.; Giri, S.S. Dietary essentiality of trace minerals in aquaculture—A Review. Agric. Rev. 2015, 36, 100–112. [Google Scholar] [CrossRef]
- Lovell, T. Nutrition and Feeding of Fish; Van Nostrand Reinhold: New York, NY, USA, 1989; p. 262. [Google Scholar] [CrossRef]
- Belitz, H.-D.; Grosch, W.; Schieberle, P. Food Chemistry, 3rd ed.; Springer-Verlag: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Solgi, E.; Beigzadeh-Shahraki, F. Accumulation and human health risk of heavy metals in cultured Rainbow Trout (Oncorhynchus mykiss) from different fish farms of eight cities of Chaharmahal and Bakhtiari Province, Iran. Thalass. Int. J. Mar. Sci. 2019, 35, 305–317. [Google Scholar] [CrossRef]
- Cieślik, I.; Migdał, W.; Topolska, K.; Gambuś, F.; Szczurowska, K.; Cieślik, E. Changes in the content of heavy metals (Pb, Cd, Hg, As, Ni, Cr) in freshwater fish after processing—The consumer’s exposure. J. Elem. 2017, 23, 247–259. [Google Scholar] [CrossRef]
- García-Gallego, M.; Akharbach, H. Evolution of body composition of European eels during their growth phase in a fish farm, with special emphasis on the lipid component. Aquac. Int. 1998, 6, 345–356. [Google Scholar] [CrossRef]
- Howell, N.K. The Chemistry of Quality Enhancement in Low-Value Fish. In Quality of Fresh and Processed Foods; Springer: Boston, MA, USA, 2004; pp. 135–145. [Google Scholar] [CrossRef]
- Mouritsen, O.G. SUSHI Food for the Eye, the Body & the Soul, 1st ed.; Springer Science & Business Media: New York, NY, USA, 2009; p. 330. [Google Scholar] [CrossRef]
- Quintaes, K.D.; Diez-Garcia, R.W. The importance of minerals in the human diet. In Handbook of Mineral Elements in Food, 1st ed.; de la Guardia, M., Garrigues, S., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 1–21. [Google Scholar] [CrossRef]
- Lieu, P.T.; Heiskala, M.; Peterson, P.A.; Yang, Y. The roles of iron in health and disease. Mol. Asp. Med. 2001, 22, 1–87. [Google Scholar] [CrossRef]
- Azaman, F.; Juahir, H.; Yunus, K.; Azid, A.; Dasuki, A.; Amran, M.; Kamarudin, M.; Toriman, M.; Hasnam, C.; Mohd Saudi, A.S. Heavy metal in fish: Analysis and human health—A review. J. Pengur. 2015, 77, 61–69. [Google Scholar] [CrossRef]
- Agbugui, M.O.; Abe, G.O. Heavy Metals in Fish: Bioaccumulation and Health. BJESR 2022, 10, 47–66. [Google Scholar] [CrossRef]
- Boran, M.; Altinok, I. A Review of Heavy Metals in Water, Sediment and Living Organisms in the Black Sea. Turk. J. Fish. Aquat. Sci. 2010, 10, 565–572. [Google Scholar] [CrossRef]
- Tahity, T.; Islam, M.R.U.; Bhuiyan, N.Z.; Choudhury, T.R.; Yu, J.; Noman, M.A.; Hosen, M.M.; Quraishi, S.B.; Paray, B.A.; Arai, T.; et al. Heavy Metals Accumulation in Tissues of Wild and Farmed Barramundi from the Northern Bay of Bengal Coast, and Its Estimated Human Health Risks. Toxics 2022, 10, 410. [Google Scholar] [CrossRef]
- Yousif, R.; Choudhary, M.I.; Ahmed, S.; Ahmed, Q. Review: Bioaccumulation of heavy metals in fish and other aquatic organisms from Karachi Coast, Pakistan. Nus. Biosci. 2021, 13, 73–84. [Google Scholar] [CrossRef]
- Afzaal, M.; Hameed, S.; Liaqat, I.; Ali Khan, A.A.; abdul Manan, H.; Shahid, R.; Altaf, M. Heavy metals contamination in water, sediments and fish of freshwater ecosystems in Pakistan. Water Pract. Technol. 2022, 17, 1253–1272. [Google Scholar] [CrossRef]
- Garai, P.; Banerjee, P.; Mondal, P. Effect of Heavy Metals on Fishes: Toxicity and Bioaccumulation. J. Clin. Toxicol. 2021, 11, 001. [Google Scholar]
- Amilhat, E.; Fazio, G.; Simon, G.; Manetti, M.; Paris, S.; Delahaut, L.; Farrugio, H.; Lecomte-Finiger, R.; Sasal, P.; Faliex, E. Silver European eels health in Mediterranean habitats. Ecol. Freshw. Fish 2014, 23, 49–64. [Google Scholar] [CrossRef]
- Ribeiro, C.A.O.; Vollaire, Y.; Sanchez-Chardi, A.; Roche, H. Bioaccumulation and the effects of organochlorine pesticides, PAH and heavy metals in the Eel (Anguilla anguilla) at the Camargue Nature Reserve, France. Aquat. Toxicol. 2005, 74, 53–69. [Google Scholar] [CrossRef]
- Elbeshti, R.; Elderwish, N.; Abdelali, K.; Taştan, Y. Effects of Heavy Metals on Fish. Menba J. Fish. Fac. 2018, 4, 36–47. [Google Scholar]
- Chung, S.W.C.; Tong, S.K.; Xiao, Y.; Ho, Y.Y. Methylmercury and long-chain n-3 fatty acids of 88 fish species commonly consumed in Hong Kong. J. Anal. Sci. Technol. 2015, 6, 5. [Google Scholar] [CrossRef]
- Valfre, F.; Caprino, F.; Turchini, G.M. The health benefit of seafood. Vet. Res. Commun. 2003, 27, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, H. Nutritional Indices for Assessing Fatty Acids: A Mini-Review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef] [PubMed]
- Senso, L.; Suárez, M.D.; Ruiz-Cara, T.; García-Gallego, M. On the possible effects of harvesting season and chilled storage on the fatty acid profile of the fillet of farmed gilthead sea bream (Sparus aurata). Food Chem. 2007, 101, 298–307. [Google Scholar] [CrossRef]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Bobe, G.; Zimmerman, S.; Hammond, E.G.; Luhman, C.M.; Boylston, T.D.; Freeman, A.E.; Beitz, D.C. Physical and Sensory Properties of Dairy Products from Cows with Various Milk Fatty Acid Compositions. J. Agric. Food Chem. 2004, 52, 3422–3428. [Google Scholar] [CrossRef] [PubMed]
- Łuczyńska, J.; Łuczyński, M.J.; Paszczyk, B.; Tońska, E. Concentration of mercury in muscles of predatory and non-predatory fish from lake Pluszne (Poland). J. Vet. Res. 2016, 60, 43–47. [Google Scholar] [CrossRef]
- Christie, W.W. The isolation of lipids from tissues. Recommended Procedures. Chloroform-methanol (2:1, v/v) extraction and “Folch” wash. In Lipid Analysis. Isolation, Separation, Identification and Structural Analysis of Lipids; Christie, W.W., Ed.; Pergamon Press Oxford: New York, NY, USA; Toronto, ON, Canada; Sydney, Australia; Braunschweig, Germany, 1973; pp. 39–40. [Google Scholar]
- Zegarska, Z.; Jaworski, J.; Borejszo, Z. Evaluation of the Peisker modified method for extracting methyl esters from fatty acids. Acta Acad. Agri. Techn. Olst. 1991, 24, 25–33. (In Polish) [Google Scholar]
- Ahmed, M.K.; Baki, M.A.; Kundu, G.K.; Saiful Islam, M.; Monirul Islam, M.; Muzammel Hossain, M. Human health risks from heavy metals in fish of Buriganga river, Bangladesh. SpringerPlus 2016, 5, 1697. [Google Scholar] [CrossRef]
- US EPA. Regional Screening Levels (RSLs)—Generic Tables. Tables as of: May 2022. 2022. Available online: https://www.epa.gov/risk/regional-screening-levels-rsls-generic-tables (accessed on 22 January 2023).
- Usero, J.; González-Regalado, E.; Gracia, I. Trace metals in the bivalve mollusc Chamelea gallina from the Atlantic coast of southern Spain. Mar. Pollut. Bull. 1996, 32, 305–310. [Google Scholar] [CrossRef]
- Usero, J.; González-Regalado, E.; Gracia, I. Trace metals in the bivalve molluscs Ruditapes decussatus and Ruditapes philippinarum from the atlantic coast of Southern Spain. Environ. Int. 1997, 23, 291–298. [Google Scholar] [CrossRef]
- Abdel-Khalek, A.A.; Elhaddad, E.; Mamdouh, S.; Saed Marie, M.-A. Assessment of metal pollution around Sabal Drainage in River Nile and its impacts on bioaccumulation level, metals correlation and human risk hazard using Oreochromis niloticus as a bioindicator. Turk. J. Fish. Aquat. Sci. 2016, 16, 227–239. [Google Scholar] [CrossRef]
- Garaffo, M.A.; Vassallo-Agius, R.; Nengas, Y.; Lembo, E.; Rando, R.; Maisano, R.; Dugo, G.; Giuffrida, D. Fatty Acids Profile, Atherogenic (IA) and Thrombogenic (IT) Health Lipid Indices, of Raw Roe of Blue Fin Tuna (Thunnus thynnus L.) and Their Salted Product “Bottarga”. Food Nutr. Sci. 2011, 02, 736–743. [Google Scholar] [CrossRef]
- Telahigue, K.; Hajji, T.; Rabeh, I.; El Cafsi, M. The changes of fatty acid composition in sun dried, oven dried and frozen hake (Merluccius merluccius) and sardinella (Sardinella aurita). Afr. J. Biochem. Res. 2013, 7, 158–164. [Google Scholar] [CrossRef]
- Abrami, G.; Natiello, F.; Bronzi, P.; McKenzie, D.; Bolis, L.; Agradi, E. A comparison of highly unsaturated fatty acid levels in wild and farmed eels (Anguilla anguilla). Comp. Biochem. Physiol. 1992, 101, 79–81. [Google Scholar] [CrossRef]
- Gladyshev, M.I.; Sushchik, N.N.; Anishchenko, O.V.; Makhutova, O.N.; Kalachova, G.S.; Gribovskaya, I.V. Benefit-risk ratio of food fish intake as the source of essential fatty acids vs. heavy metals: A case study of Siberian grayling from the Yenisei River. Food Chem. 2009, 115, 545–550. [Google Scholar] [CrossRef]
- Branciari, R.; Franceschini, R.; Roila, R.; Valiani, A.; Pecorelli, I.; Piersanti, A.; Haouet, N.; Framboas, M.; Ranucci, D. Nutritional Value and Contaminant Risk Assessment of Some Commercially Important Fishes and Crawfish of Lake Trasimeno, Italy. Int. J. Environ. Res. Public Health 2020, 17, 2545. [Google Scholar] [CrossRef]
- Özden, Ö.; Erkan, N.; Kaplan, M.; Karakulak, F.S. Toxic Metals and Omega-3 Fatty Acids of Bluefin Tuna from Aquaculture: Health Risk and Benefits. Expo. Health 2020, 12, 9–18. [Google Scholar] [CrossRef]
- Greenfield, H.; Southgate, D.A.T. Food Composition Data. Production, Management and Use, 2nd ed.; Burlingname, B.A., Charrondiere, U.R., Eds.; Food and Agriculture Organization of the United Nations: Rome, Italy, 2003; 288p. [Google Scholar] [CrossRef]
- Chen, Y.; Qiao, Y.; Xiao, Y.; Chen, H.; Zhao, L.; Huang, M.; Zhou, G. Differences in Physicochemical and Nutritional Properties of Breast and Thigh Meat from Crossbred Chickens, Commercial Broilers, and Spent Hens. Asian Australas. J. Anim. Sci. 2015, 29, 855–864. [Google Scholar] [CrossRef]
- Zula, A.T.; Desta, D.T.; Apetrei, C. Fatty Acid-Related Health Lipid Index of Raw and Fried Nile Tilapia (Oreochromis niloticus) Fish Muscle. J. Food Qual. 2021, 2021, 6676528. [Google Scholar] [CrossRef]
- Santos-Silva, J.; Bessa, R.J.B.; Santos-Silva, F. Effect of genotype, feeding system and slaughter weight on the quality of light lambs. Livest. Prod. Sci. 2002, 77, 187–194. [Google Scholar] [CrossRef]
- Zhang, X.; Ning, X.; He, X.; Sun, X.; Yu, X.; Cheng, Y.; Yu, R.-Q.; Wu, Y. Fatty acid composition analyses of commercially important fish species from the Pearl River Estuary, China. PLoS ONE 2020, 15, e0228276. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Manzoor, M.F.; Shabbir, U.; Ahmed, S.; Ismail, T.; Saeed, F.; Nisa, M.; Anjum, F.M.; Hussain, S. Health lipid indices and physicochemical properties of dual fortified yogurt with extruded flaxseed omega fatty acids and fibers for hypercholesterolemic subjects. Food Sci. Nutr. 2019, 8, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Sulimanec Grgec, A.; Jurasović, J.; Kljaković-Gašpić, Z.; Orct, T.; Rumora Samarin, I.; Janči, T.; Sekovanić, A.; Grzunov Letinić, J.; Matek Sarić, M.; Benutić, A.; et al. Potential risks and health benefits of fish in the diet during the childbearing period: Focus on trace elements and n-3 fatty acid content in commonly consumed fish species from the Adriatic Sea. Environ. Adv. 2022, 8, 100226. [Google Scholar] [CrossRef]
- Statistics Poland. Statistical Yearbook of Agriculture; Editorial Board of Statistics, Ed.; Statistics Poland: Warsaw, Poland, 2021; 449p. [Google Scholar]
- Geeraerts, C.; Belpaire, C. The effects of contaminants in European eel: A review. Ecotoxicology 2009, 19, 239–266. [Google Scholar] [CrossRef]
- Rakocevic, J.; Sukovic, D.; Maric, D. Distribution and relationships of eleven trace elements in muscle of six fish species from Skadar Lake (Montenegro). Turk. J. Fish. Aquat. Sci. 2018, 18, 647–657. [Google Scholar] [CrossRef]
- Noël, L.; Chekri, R.; Millour, S.; Merlo, M.; Leblanc, J.-C.; Guérin, T. Distribution and relationships of As, Cd, Pb and Hg in freshwater fish from five French fishing areas. Chemosphere 2013, 90, 1900–1910. [Google Scholar] [CrossRef]
- Linde, A.R.; Sanchez-Galan, S.; Garcia-Vazquez, E. Heavy Metal Contamination of European Eel (Anguilla anguilla) and Brown Trout (Salmo trutta) Caught in Wild Ecosystems in Spain. J. Food Prot. 2004, 67, 2332–2336. [Google Scholar] [CrossRef]
- Polak-Juszczak, L. Distribution of organic and inorganic mercury in the tissues and organs of fish from the southern Baltic Sea. Environ. Sci. Pollut. Res. 2018, 25, 34181–34189. [Google Scholar] [CrossRef]
- Achouri, N.; Kharrat, N.; Smichi, N.; Miled, N.; Gargouri, Y.; Fendri, A. Nutritional properties, oxidative stability, and in vitro digestibility of oils extracted from muscles of wild and breeding eels (Anguilla anguilla). J. Food Process. Preserv. 2017, 42, e13519. [Google Scholar] [CrossRef]
- El Morhit, M.; Mohamed, F.; Elie, P.; Girard, P.; Yahyaoui, A.; El Abidi, M.; Jbilou, M. Heavy metals in sediment, water and the European glass eel, Anguilla anguilla (Osteichthyes: Anguillidae) from Loukkos River estuary (Morocco, Atlantic). Cybium 2009, 33, 219–228. [Google Scholar]
- Yorulmaz, B.; Yilmaz, F.; Okan Genz, T. Heavy metal concentrations in European eel (Anguilla anguilla L., 1758) from Kyocegiz-Dalyan lagoon system. Fresenius Environ. Bull. 2015, 24, 1607–1613. [Google Scholar]
- Rudovica, V.; Bartkevics, V. Chemical elements in the muscle tissues of European eel (Anguilla anguilla) from selected lakes in Latvia. Environ. Monit. Assess. 2015, 187, 608. [Google Scholar] [CrossRef] [PubMed]
- Genç, T.O.; Yilmaz, F. Metal Accumulations in Water, Sediment, Crab (Callinectes sapidus) and Two Fish Species (Mugil cephalus and Anguilla anguilla) from the Köyceğiz Lagoon System–Turkey: An Index Analysis Approach. Bull. Environ. Contam. Toxicol. 2017, 99, 173–181. [Google Scholar] [CrossRef]
- Wariaghli, F.; Tilghman-Sibille, A.; El Abidi, A.; El Hamri, H.; Fekhaoui, M.; Yahyaoui, A. Anguilla anguilla L.: Evaluation of the degree of heavy metal contamination in the Sebou estuary and in Moulay Bousselham lagoon reserve (Morocco). Int. J. Aqu. Sci. 2013, 4, 69–82. [Google Scholar]
- Maes, G.E.; Raeymaekers, J.A.M.; Pampoulie, C.; Seynaeve, A.; Goemans, G.; Belpaire, C.; Volckaert, F.A.M. The catadromous European eel Anguilla anguilla (L.) as a model for freshwater evolutionary ecotoxicology: Relationship between heavy metal bioaccumulation, condition and genetic variability. Aquat. Toxicol. 2005, 73, 99–114. [Google Scholar] [CrossRef]
- Mazrouh, M.M. Effects of Some Heavy Metals in Different Organs and Some Hepatic Enzymes for European eel (Anguilla anguilla) at Lake Edku. IJSR 2016, 5, 1872–1876. [Google Scholar]
- Baillon, L.; Pierron, F.; Coudret, R.; Normendeau, E.; Caron, A.; Peluhet, L.; Labadie, P.; Budzinski, H.; Durrieu, G.; Sarraco, J.; et al. Transcriptome profile analysis reveals specific signatures of pollutants in Atlantic eels. Ecotoxicology 2014, 24, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Has-Schon, E.; Bogut, I.; Rajkovic, V.; Bogut, S.; Cacic, M.; Horvatic, J. Heavy metal distribution in tissues of six fish species included in human diet, inhabiting freshwaters of the Nature Park “Hutovo Blato” (Bosnia and Herzegovina). Arch. Environ. Contam. Toxicol. 2008, 54, 75–83. [Google Scholar] [CrossRef]
- Capoccioni, F.; Leone, C.; Belpaire, C.; Malarvannan, G.; Poma, G.; De Matteis, G.; Tancioni, L.; Contò, M.; Failla, S.; Covaci, A.; et al. Quality assessment of escaping silver eel (Anguilla anguilla L.) to support management and conservation strategies in Mediterranean coastal lagoons. Environ. Monit. Assess. 2020, 192, 570. [Google Scholar] [CrossRef]
- Kasimoglu, C. The Effect of Fish Size, Age and Condition Factor on the Contents of Seven Essential Elements in Anguilla anguilla from Tersakan Stream Mugla (Turkey). J. Pollut. Eff. Cont. 2014, 2, 123. [Google Scholar] [CrossRef]
- Kasimoglu, C.; Yilmaz, F. Toxic metals (Cd and Pb) levels in the muscle of commercially important fish, Anguilla anguilla from Tersakan Stream Mugla (Turkey): Correlations between Cd and Pb concentrations with biological features. DUFED 2016, 5, 26–33. [Google Scholar]
- Guhl, B. Contaminant levels in the European eel (Anguilla anguilla) in North Rhine-Westphalian rivers. Environ. Sci. Eur. 2014, 26, 26. [Google Scholar] [CrossRef]
- Teunen, L.; Belpaire, C.; De Boeck, G.; Blust, R.; Bervoets, L. Mercury accumulation in muscle and liver tissue and human health risk assessment of two resident freshwater fish species in Flanders (Belgium): A multilocation approach. Environ. Sci. Pollut. Res. 2021, 29, 7853–7865. [Google Scholar] [CrossRef]
- Commission Regulation (EC). No 629/2008 of 2 July 2008 Amending Regulation (EC) No 1881/2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs; European Commission: Brussels, Belgium, 2008. [Google Scholar]
- Nauen, C.E. Compilation of legal limits for hazardous substances in fish and fishery products. FAO Fish. Circ. 1983, 764, 102. [Google Scholar]
- Zencir, Ö. Determination of heavy metals in some tissues of four fish species from the Karasu River (Erzincan, Turkey) for public consumption. Oceanol. Hydrobiol. Stud. 2021, 50, 232–246. [Google Scholar] [CrossRef]
- Romero, D.; Barcala, E.; Maria-Dolores, E.; Muñoz, P. European eels and heavy metals from the Mar Menor lagoon (SE Spain). Mar. Pollut. Bull. 2020, 158, 111368. [Google Scholar] [CrossRef]
- Gómez-Limia, L.; Cobas, N.; Martínez, S. Proximate composition, fatty acid profile and total amino acid contents in samples of the European eel (Anguilla anguilla) of different weights. Int. J. Gastron. Food Sci. 2021, 25, 100364. [Google Scholar] [CrossRef]
- Đikić, D.; Landeka, I.; Fuchs, R.; Skaramuca, D.; Matić-Skoko, S.; Tutman, P.; Franić, Z.; Cvetković, I.; Skaramuca, B. Lipid profiles of Mediterranean moray, Muraena helena, European conger, Conger conger, and European eel, Anguilla anguilla (Actinopterygii: Anguilliformes). Acta Ichthyol. Piscat. 2017, 47, 1–11. [Google Scholar] [CrossRef]
- Can Tunçelli, İ.; Özden, Ö.; Erkan Özden, N. Seasonal Differences in Lipid and Fatty Acid Composition of European Eels (Anguilla anguilla, Linnaeus 1758) from Orontes River, Turkiye. Aquat. Sci. Eng. 2022, 37, 169–174. [Google Scholar] [CrossRef]
- Hameed, A.; Hussain, S.A.; Shabbir, M.A.; Pasha, I.; Song, Y. Nutritional and fatty acids profile analyses of commonly consumed fresh water fish species in Pakistan. Am. J. Biochem. Biotechnol. 2017, 13, 15–26. [Google Scholar] [CrossRef]
- Prigge, E.; Malzahn, A.M.; Zumholz, K.; Hanel, R. Dietary effects on fatty acid composition in muscle tissue of juvenile European eel, Anguilla anguilla (L.). Helgol. Mar. Res. 2011, 66, 51–61. [Google Scholar] [CrossRef]
- Parzanini, C.; Colombo, S.; Kainz, M.; Wacker, A.; Parrish, C.; Arts, M. Discrimination between Freshwater and Marine Fish using Fatty Acids: Ecological Implications and Future Perspectives. Environ. Rev. 2020, 28, 546–559. [Google Scholar] [CrossRef]
- Parzanini, C.; Arts, M.; Power, M.; Rohtla, M.; Skiftesvik, A.B.; Koprivnikar, J.; Browman, H.I.; Milotic, D.; Durif, C.M.F. Trophic ecology of the European eel (Anguilla anguilla) across different salinity habitats inferred from fatty acid and stable isotope analysis. Can. J. Fish. Aquat. Sci. 2021, 78, 1721–1731. [Google Scholar] [CrossRef]
- Parzanini, C.; Arts, M.; Rohtla, M.; Koprivnikar, J.; Power, M.; Skiftesvik, A.; Browman, H.; Milotic, D.; Durif, C. Feeding Habitat and Silvering Stage Affect Lipid Content and Fatty Acid Composition of European Eel Anguilla anguilla Tissues. J. Fish Biol. 2021, 99, 1110–1124. [Google Scholar] [CrossRef] [PubMed]
- Pleadin, J.; Lesic, T.; Kresic, G.; Baric, R.; Bogdanovic, T.; Oraic, D.; Vulic, A.; Legac, A.; Zrncic, S. Nutritional quality of different fish species farmed in the Adriatic Sea. Ital. J. Food Sci. 2017, 29, 537–549. [Google Scholar] [CrossRef]
- Polak-Juszczak, L.; Komar-Szymczak, K. Fatty acid profiles and fat contents of commercially important fish from Vistula Lagoon. Pol. J. Food Nutr. Sci. 2009, 59, 225–229. [Google Scholar]
- Karsli, B. Comparative analysis of the fatty acid composition of commercially available fish oil supplements in Turkey: Public health risks and benefits. J. Food Compost. Anal. 2021, 103, 104105. [Google Scholar] [CrossRef]
- Bazarsadueva, S.V.; Radnaeva, L.D.; Nikitina, E.P.; Popov, P.A. Fatty acid composition and lipid quality indices of bream Abramis brama (Linnaeus, 1758) of Lake Kotokel (Western Transbaikalia). IOP Conf. Ser. Earth Environ. Sci. 2021, 885, 012062. [Google Scholar] [CrossRef]
- Perneel, M. The Influence of Artificial Canal Dynamics on European Eel Recruits. Master’s Thesis, Ghent Univerity, Ghent, Belgium, 2019; p. 71. [Google Scholar]
- Strandberg, U.; Palviainen, M.; Eronen, A.; Piirainen, S.; Laurén, A.; Akkanen, J.; Kankaala, P. Spatial variability of mercury and polyunsaturated fatty acids in the European perch (Perca fluviatilis)—Implications for risk-benefit analyses of fish consumption. Environ. Pollut. 2016, 219, 305–314. [Google Scholar] [CrossRef]
- Laird, M.J.; Henao, J.J.A.; Reyes, E.S.; Stark, K.D.; Low, G.; Swanson, H.K.; Laird, B.D. Mercury and omega-3 fatty acid profiles in freshwater fish of the Dehcho Region, Northwest Territories: Informing risk benefit assessments. Sci. Total Environ. 2018, 637–638, 1508–1517. [Google Scholar] [CrossRef] [PubMed]
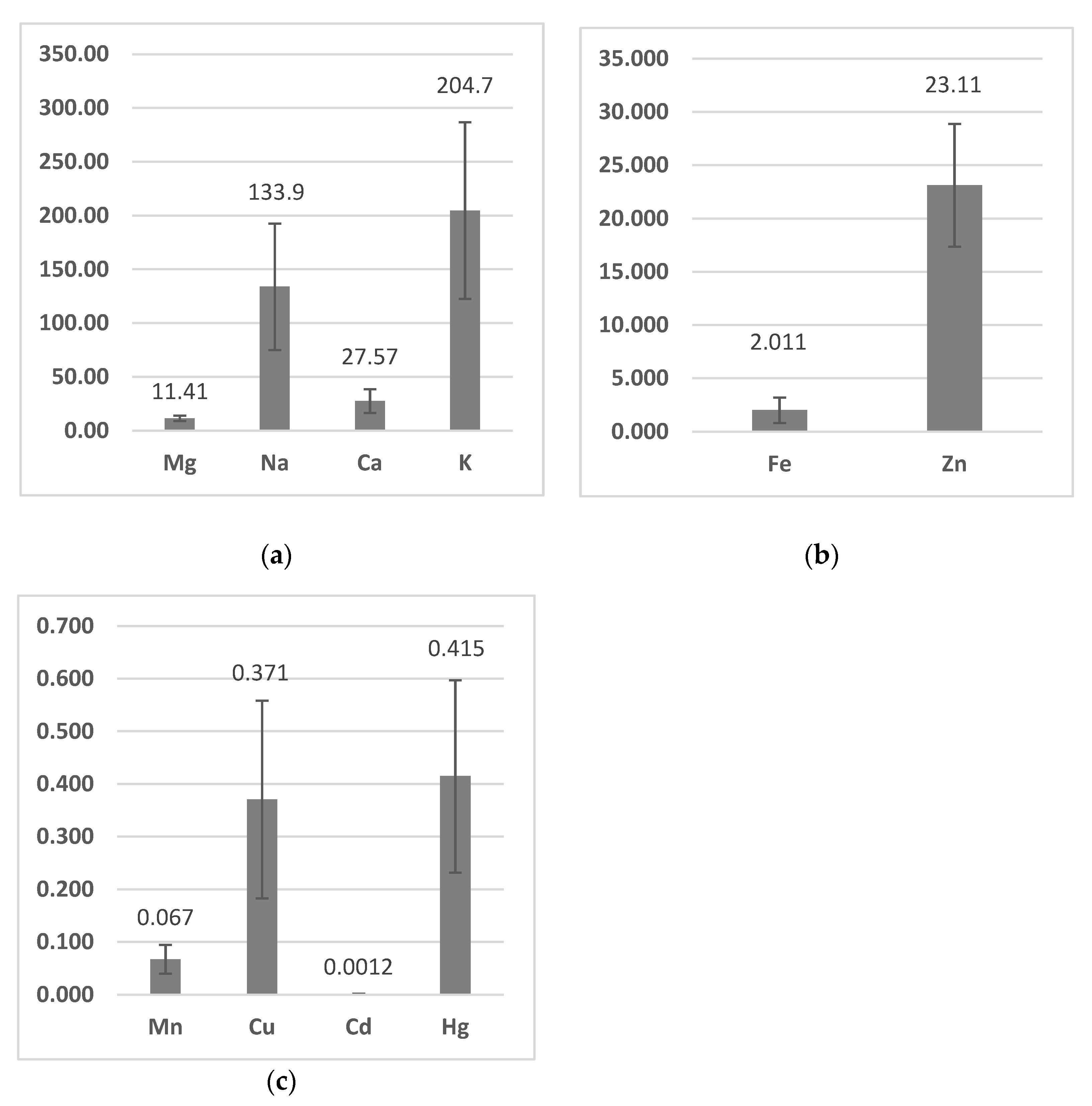
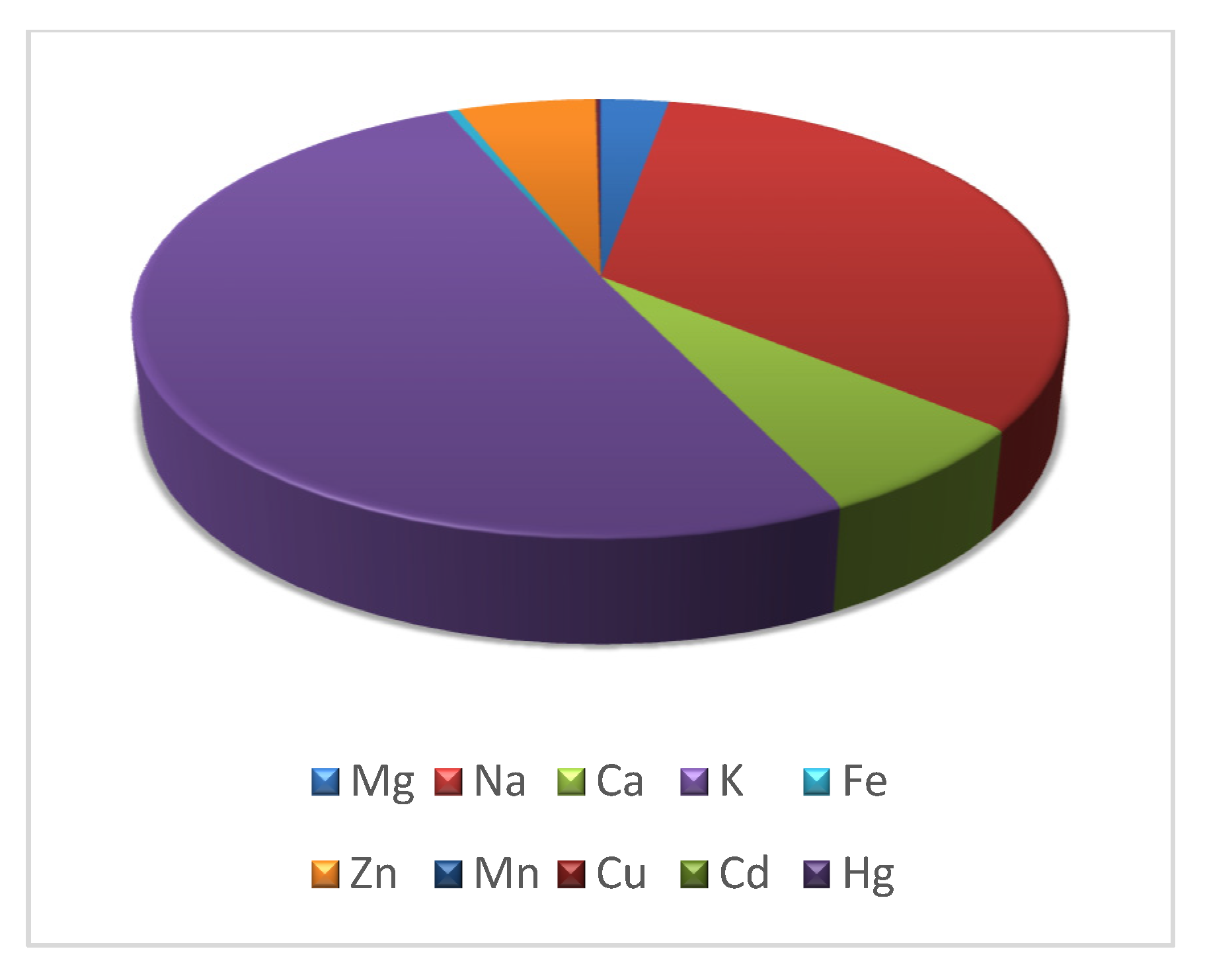
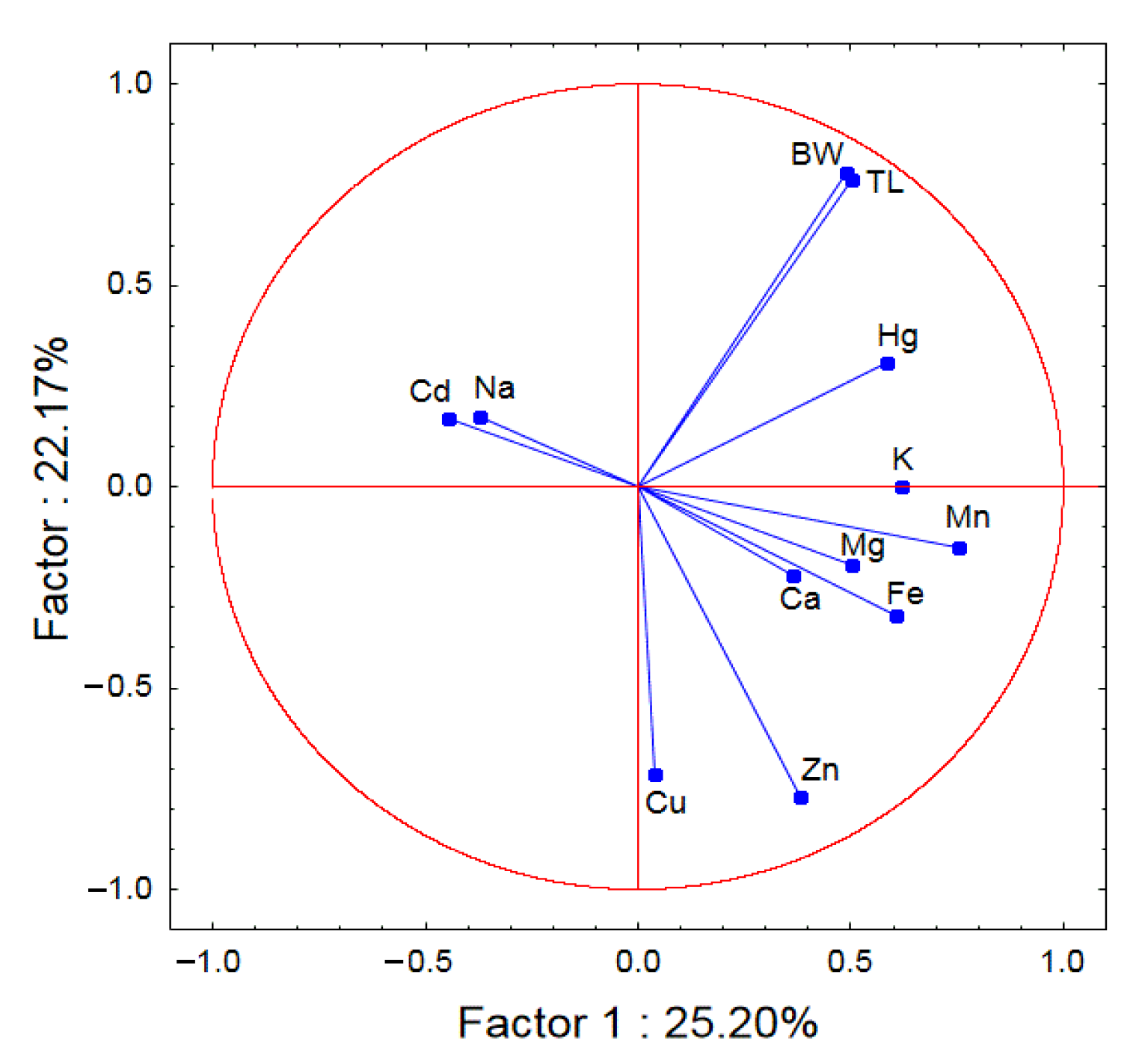
| Weight | Length | Mg | Na | Ca | K | Fe | Zn | Mn | Cu | Cd | Hg | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| weight | - | 0.9130 | 0.1847 | 0.0893 | −0.0221 | 0.1875 | 0.0380 | −0.2892 | 0.2555 | −0.4030 | −0.3609 | 0.4884 |
| p = 0.000 | p = 0.839 | p = 0.716 | p = 0.928 | p = 0.442 | p = 0.877 | p = 0.230 | p = 0.291 | p = 0.087 | p = 0.141 | p = 0.034 | ||
| length | 0.9130 | - | 0.0049 | 0.0424 | −0.1348 | 0.1632 | 0.1408 | −0.3305 | 0.3699 | −0.3112 | −0.0542 | 0.4855 |
| p = 0.000 | p = 0.984 | p = 0.863 | p = 0.582 | p = 0.504 | p = 0.565 | p = 0.167 | p = 0.119 | p = 0.195 | p = 0.826 | p = 0.035 | ||
| Mg | 0.1847 | 0.0049 | - | −0.0554 | 0.4019 | 0.3912 | 0.2373 | 0.3480 | 0.2101 | −0.5638 | −0.0243 | 0.2426 |
| p = 0.839 | p = 0.984 | p = 0.822 | p = 0.088 | p = 0.098 | p = 0.328 | p = 0.144 | p = 0.388 | p = 819 | p = 0.921 | p = 0.317 | ||
| Na | 0.0893 | 0.0424 | −0.0554 | - | 0.1119 | −0.6420 | −0.1968 | −0.2994 | −0.0558 | 0.1184 | 0.1764 | −0.1456 |
| p = 0.716 | p = 0.863 | p = 0.822 | p = 0.648 | p = 0.003 | p = 0.419 | p = 0.213 | p = 0.821 | p = 0.629 | p = 0.470 | p = 0.552 | ||
| Ca | −0.0221 | −0.1348 | 0.4019 | 0.1119 | - | 0.1259 | 0.1766 | 0.1545 | 0.4425 | 0.0860 | 0.0860 | 0.0401 |
| p = 0.928 | p = 0.582 | p = 0.088 | p = 0.648 | p = 0.608 | p = 0.469 | p = 0.528 | p = 0.058 | p = 0.726 | p = 0.726 | p = 0.871 | ||
| K | 0.1875 | 0.1632 | 0.3912 | −0.6420 | 0.1259 | - | 0.1420 | 0.2418 | 0.2189 | −0.3365 | −0.4170 | 0.1402 |
| p = 0.442 | p = 0.504 | p = 0.098 | p = 0.003 | p = 0.608 | p = 0.562 | p = 0.319 | p = 0.368 | p = 0.159 | p = 0.076 | p = 0.567 | ||
| Fe | 0.0380 | 0.1408 | 0.2373 | −0.1968 | 0.1766 | 0.1420 | - | 0.3332 | 0.5611 | 0.4809 | 0.0502 | 0.4252 |
| p = 0.877 | p = 0.565 | p = 0.328 | p = 0.419 | p = 0.469 | p = 0.562 | p = 0.163 | p = 0.012 | p = 0.037 | p = 0.838 | p = 0.070 | ||
| Zn | −0.2892 | −0.3305 | 0.3480 | −0.2994 | 0.1545 | 0.2418 | 0.3332 | - | 0.2674 | 0.6232 | −0.3482 | −0.0004 |
| p = 0.2300 | p = 0.167 | p = 0.144 | p = 0.213 | p = 0.528 | p = 0.319 | p = 0.163 | p = 0.268 | p = 0.004 | p = 0.144 | p = 0.999 | ||
| Mn | 0.2555 | 0.3699 | 0.2101 | −0.0558 | 0.4425 | 0.2189 | 0.5611 | 0.2674 | - | 0.2730 | −0.4577 | 0.2304 |
| p = 0.291 | p = 0.119 | p = 0.388 | p = 0.821 | p = 0.058 | p = 0.368 | p = 0.012 | p = 0.268 | p = 0.258 | p = 0.049 | p = 0.343 | ||
| Cu | −0.4030 | −0.3112 | −0.5638 | 0.1184 | 0.0860 | −0.3365 | 0.4809 | 0.6232 | 0.2730 | - | −0.0348 | −0.0096 |
| p = 0.087 | p = 0.195 | p = 819 | p = 0.629 | p = 0.726 | p = 0.159 | p = 0.037 | p = 0.004 | p = 0.258 | p = 0.888 | p = 0.969 | ||
| Cd | −0.3609 | −0.0542 | −0.0243 | 0.1764 | 0.0860 | −0.4170 | 0.0502 | −0.3482 | −0.4577 | −0.0348 | - | 0.01392 |
| p = 0.141 | p = 0.826 | p = 0.921 | p = 0.470 | p = 0.726 | p = 0.076 | p = 0.838 | p = 0.144 | p = 0.049 | p = 0.888 | p = 0.955 | ||
| Hg | 0.4884 | 0.4855 | 0.2426 | −0.1456 | 0.0401 | 0.1402 | 0.4252 | −0.0004 | 0.2304 | −0.0096 | 0.01392 | - |
| p = 0.034 | p = 0.035 | p = 0.317 | p = 0.552 | p = 0.871 | p = 0.5671 | p = 0.070 | p = 0.999 | p = 0.343 | p = 0.969 | p = 0.955 |
| Cu | Zn | Mn | Fe | Hg | Cd | |
|---|---|---|---|---|---|---|
| RfD (mg/kg/day) | 4.00 × 10−2 | 3.00 × 10−1 | 1.4 × 10−1 | 7.00 × 10−1 | 3.00 × 10−4 | 1.00 × 10−4 |
| EDI | 0.1828 | 11.3989 | 0.0331 | 0.9918 | 0.2045 | 0.0006 |
| EWI | 1.2798 | 79.7923 | 0.2317 | 6.9428 | 1.4317 | 0.0041 |
| THQ | 0.0046 | 0.0380 | 0.0002 | 0.0014 | 0.6818 | 0.0059 |
| HI | 0.7319 | |||||
| MPI | 0.2903 | |||||
| HQEFA | 0.0026 | 0.0217 | 0.0001 | 0.0008 | 0.3897 | 0.0034 |
| BRQ | 0.0026 | 0.0217 | 0.0001 | 0.0008 | 0.3897 | 0.0034 |
| Mean | SD | Systematic Name | Trivial Name | Mean | SD | |
|---|---|---|---|---|---|---|
| fat | 21.26 | 5.10 | ||||
| expressed as % of the total fatty acids | expressed as mg/g fish muscles | |||||
| C12:0 | 0.12 | 0.04 | dodecanoic | lauric | 0.21 | 0.06 |
| C14:0 | 4.62 | 0.53 | tetradecanoic | myristic | 7.96 | 2.18 |
| C15:0 | 0.37 | 0.08 | pentadecanoic | pentadecylic | 0.63 | 0.19 |
| C16:0 | 19.95 | 1.00 | hexadecanoic | palmitic | 34.40 | 8.51 |
| C17:0 | 0.46 | 0.07 | heptadecanoic | margaric | 0.78 | 0.18 |
| C18:0 | 4.48 | 0.50 | octadecanoic | stearic | 7.70 | 2.00 |
| C20:0 | 0.12 | 0.03 | eicosanoic | arachidic | 0.21 | 0.04 |
| C14:1 | 0.20 | 0.04 | cis-9-tetradecenoic | myristoleic | 0.34 | 0.11 |
| C16:1 | 9.17 | 1.38 | cis-9-hexadecenoic | palmitoleic | 15.78 | 4.61 |
| C18:1 | 37.23 | 3.33 | cis-9-octadecenoic | oleic | 64.05 | 16.22 |
| C20:1 (n-7) | 0.15 | 0.07 | cis-7-eicosenoic | gadoleic | 0.24 | 0.09 |
| C20:1 (n-9) | 1.02 | 0.17 | cis-9-eicosenoic | gadoleic | 1.72 | 0.31 |
| C18:2(n-6) LA | 3.37 | 0.65 | cis,cis-9,12-octadecadienoic | linoleic | 5.74 | 1.53 |
| C18:3γ-lin (n-6) | 0.16 | 0.04 | cis-6,cis-9,cis-12-octadecatrienoic acid | γ-linolenic | 0.27 | 0.08 |
| C20:2(n-6) | 0.55 | 0.22 | cis-11,cis-14- eicosadienoic | eicosadienoic | 0.90 | 0.28 |
| C20:3(n-6) | 0.38 | 0.12 | cis-8,cis-11,cis-14-eicosatrienoic | dihomo-γ-linolenic acid | 0.64 | 0.19 |
| C20:4(n-6) AA | 2.78 | 0.39 | cis-5,cis-8,cis-11,cis-14-eicosatetraenoic | arachidonic | 4.71 | 1.01 |
| C22:5(n-6) | 0.71 | 0.11 | cis-4,cis-7,cis-10,cis-13,cis-16- docosapentaenoicdocosapentaenoic acid | docosapentaenoic | 1.22 | 0.32 |
| C18:3(n-3) ALA | 2.21 | 0.41 | cis-9,cis-12,cis-15-octadecatrienoic | α-linolenic | 3.85 | 1.29 |
| C18:4 (n-3) | 0.30 | 0.08 | cis-6,cis-9,cis-12,cis-15-octadecatetraenoic acid | stearidonic | 0.53 | 0.22 |
| C20:3(n-3) | 0.41 | 0.07 | cis-11,cis-14,cis-17-eicosatrienoic | eicosatrienoic | 0.72 | 0.24 |
| C20:4(n-3) | 1.44 | 0.30 | cis-8,cis-11,cis-14,cis-17-eicosatetraenoic acid | eicosatetraenoic | 2.52 | 0.91 |
| C20:5(n-3) EPA | 2.38 | 0.51 | cis-5,cis-8,cis-11,cis-14,cis-17-eicosapentaenoic | eicosapentaenoic | 4.16 | 1.49 |
| C22:5(n-3)DPA | 2.57 | 0.37 | cis-7,cis-10,cis-13,cis-16,cis-19-docosapentaenoic | docosapentaenoic | 4.40 | 1.13 |
| C22:6(n-3)DHA | 4.84 | 1.12 | cis-4,cis-7,cis-10,cis-13,cis-16,cis-19-docosahexaenoic | docosahexaenoic | 8.51 | 3.25 |
| Mean | SD | |
|---|---|---|
| EPA + DHA | 7.22 | 1.46 |
| n-3/n-6 | 1.83 | 0.39 |
| Σ SFA | 30.12 | 1.21 |
| Σ MUFA | 47.77 | 2.59 |
| Σ n-6 PUFA | 7.94 | 1.25 |
| Σ n-3 PUFA | 14.16 | 2.30 |
| Σ PUFA | 22.11 | 2.51 |
| OFA | 24.69 | 1.25 |
| DFA | 74.36 | 1.27 |
| AI | 0.55 | 0.04 |
| TI | 0.41 | 0.04 |
| FLQ | 7.23 | 1.46 |
| PUFA/SFA | 0.74 | 0.09 |
| HH | 2.26 | 0.18 |
| NVI | 2.10 | 0.24 |
| HPI | 1.82 | 0.15 |
| Mean | SD | |
|---|---|---|
| EPA + DHA | 12.68 | 4.60 |
| Σ SFA | 51.88 | 12.61 |
| Σ MUFA | 82.13 | 19.65 |
| Σ n-6 PUFA | 13.48 | 2.99 |
| Σ n-3 PUFA | 24.71 | 8.09 |
| Σ PUFA | 38.19 | 10.52 |
| Ratio n-3/n-6 | 1.83 | 0.39 |
| Ratio EPA/Hg | 12.40 | 6.74 |
| Ratio DHA/Hg | 23.96 | 11.90 |
| Ratio EPA + DHA/Hg | 36.36 | 18.22 |
| Ratio PUFA/Hg | 116.21 | 67.55 |
| Ratio n-3 PUFA/Hg | 72.66 | 38.78 |
| Ratio n-6 PUFA/Hg | 43.56 | 30.13 |
| de minimis EPA + DHA:Hg | 17.46 | 17.05 |
| r | p | |
|---|---|---|
| C20:5(n-3)EPA | 0.0644 | 0.793 |
| C22:6(n-3)DHA | 0.3645 | 0.125 |
| Σ n-6 PUFA | −0.3250 | 0.175 |
| Σ n-3 PUFA | 0.2106 | 0.387 |
| Σ PUFA | 0.0697 | 0.777 |
| EPA + DHA | 0.2789 | 0.247 |
| Ratio n-3/n-6 | 0.6584 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łuczyńska, J.; Nowosad, J.; Łuczyński, M.J.; Kucharczyk, D. Evaluation of Chemical Elements, Lipid Profiles, Nutritional Indices and Health Risk Assessment of European Eel (Anguilla anguilla L.). Int. J. Environ. Res. Public Health 2023, 20, 2257. https://doi.org/10.3390/ijerph20032257
Łuczyńska J, Nowosad J, Łuczyński MJ, Kucharczyk D. Evaluation of Chemical Elements, Lipid Profiles, Nutritional Indices and Health Risk Assessment of European Eel (Anguilla anguilla L.). International Journal of Environmental Research and Public Health. 2023; 20(3):2257. https://doi.org/10.3390/ijerph20032257
Chicago/Turabian StyleŁuczyńska, Joanna, Joanna Nowosad, Marek Jan Łuczyński, and Dariusz Kucharczyk. 2023. "Evaluation of Chemical Elements, Lipid Profiles, Nutritional Indices and Health Risk Assessment of European Eel (Anguilla anguilla L.)" International Journal of Environmental Research and Public Health 20, no. 3: 2257. https://doi.org/10.3390/ijerph20032257
APA StyleŁuczyńska, J., Nowosad, J., Łuczyński, M. J., & Kucharczyk, D. (2023). Evaluation of Chemical Elements, Lipid Profiles, Nutritional Indices and Health Risk Assessment of European Eel (Anguilla anguilla L.). International Journal of Environmental Research and Public Health, 20(3), 2257. https://doi.org/10.3390/ijerph20032257







