Leisure-Related Social Work Interventions for Patients with Cognitive Impairment: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Literature Retrieval Strategy
2.3. Literature Screening and Data Extraction
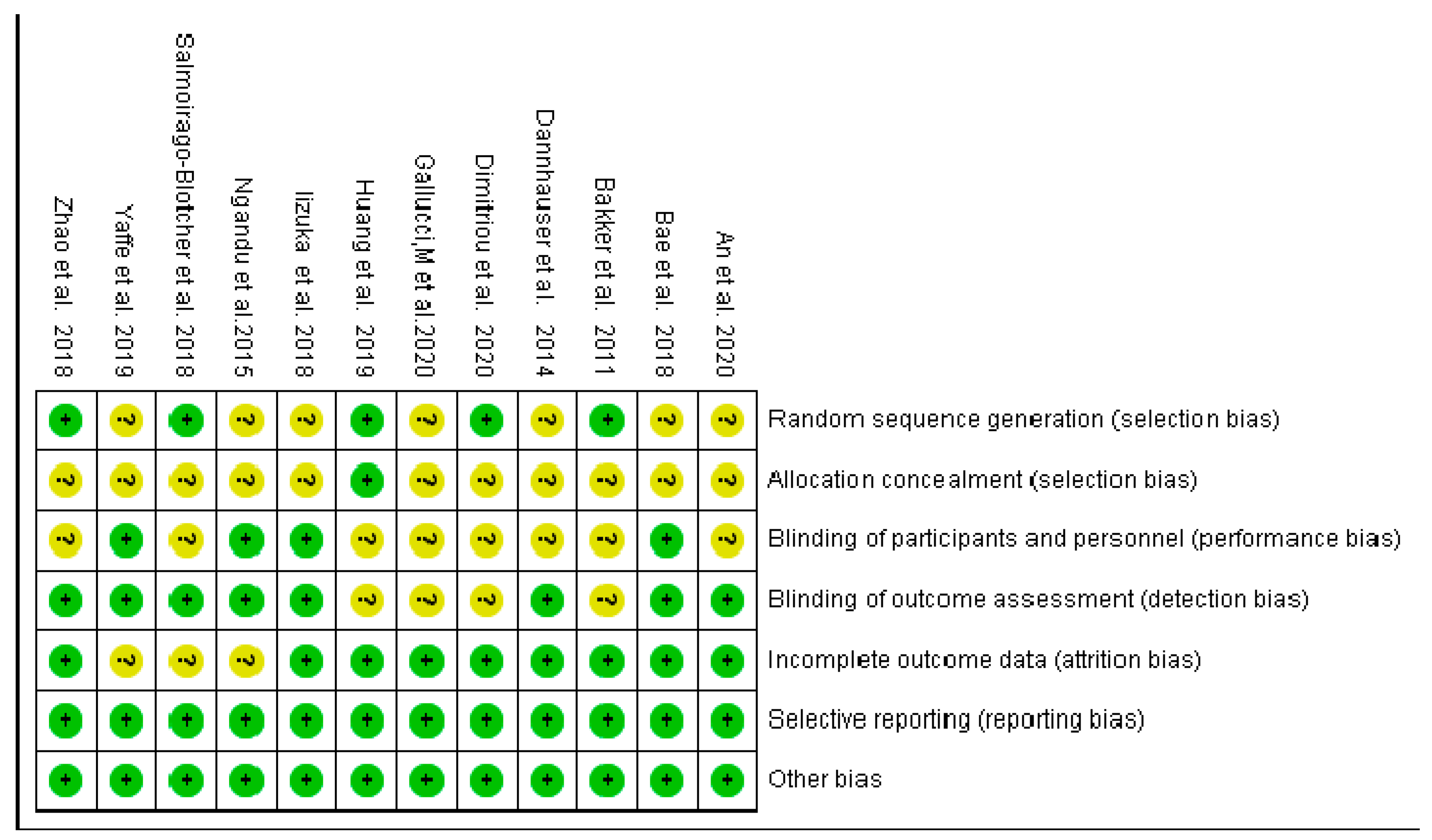
2.4. Risk of Bias Evaluation
2.5. Statistical Analysis
3. Results
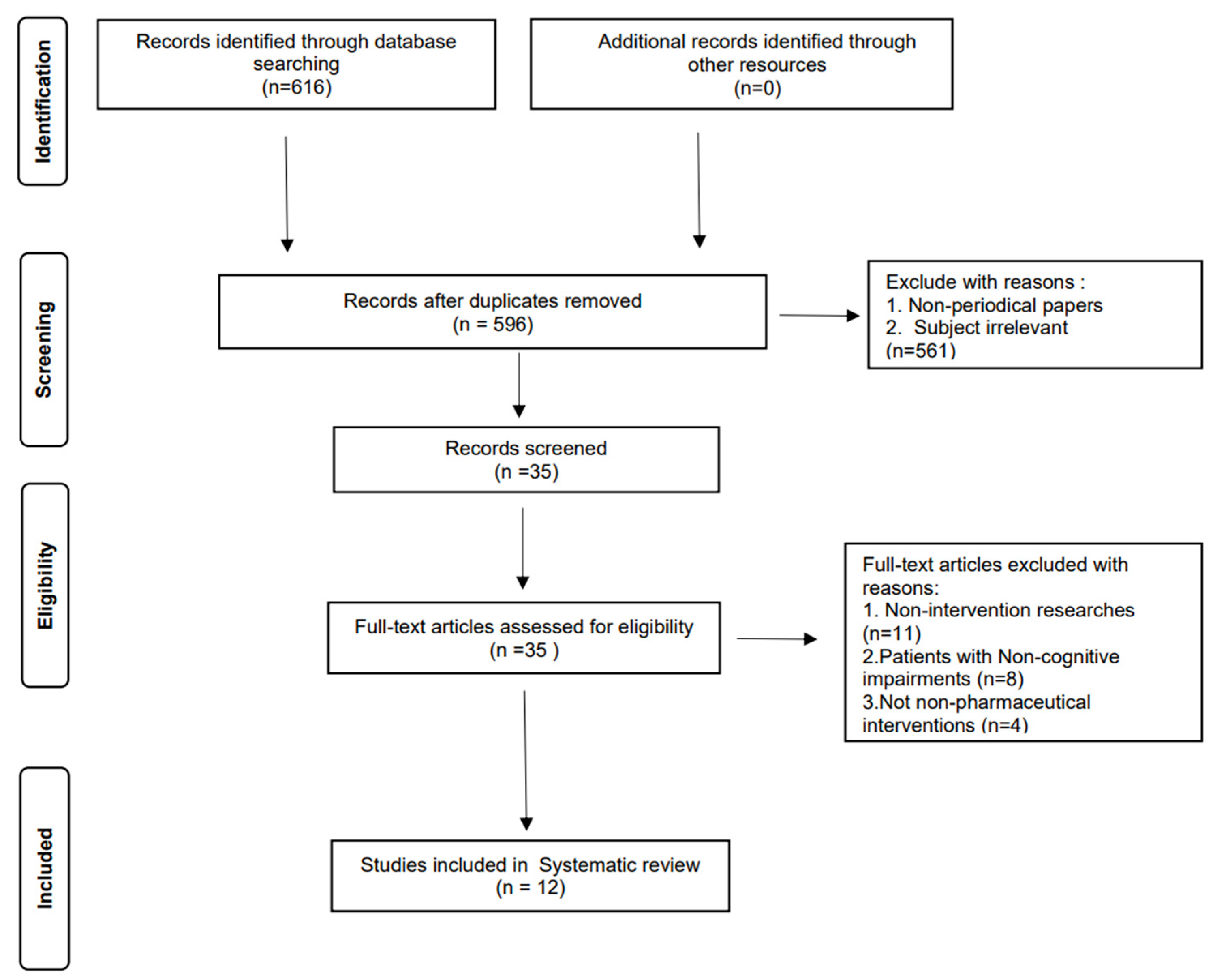
3.1. Literature Screening
3.2. Systematic Review Results
3.2.1. Characteristics of Previous Studies
3.2.2. Research Participant Characteristics
3.2.3. Evaluation Scales
3.2.4. Intervention Content
3.2.5. Intervention Duration and Results
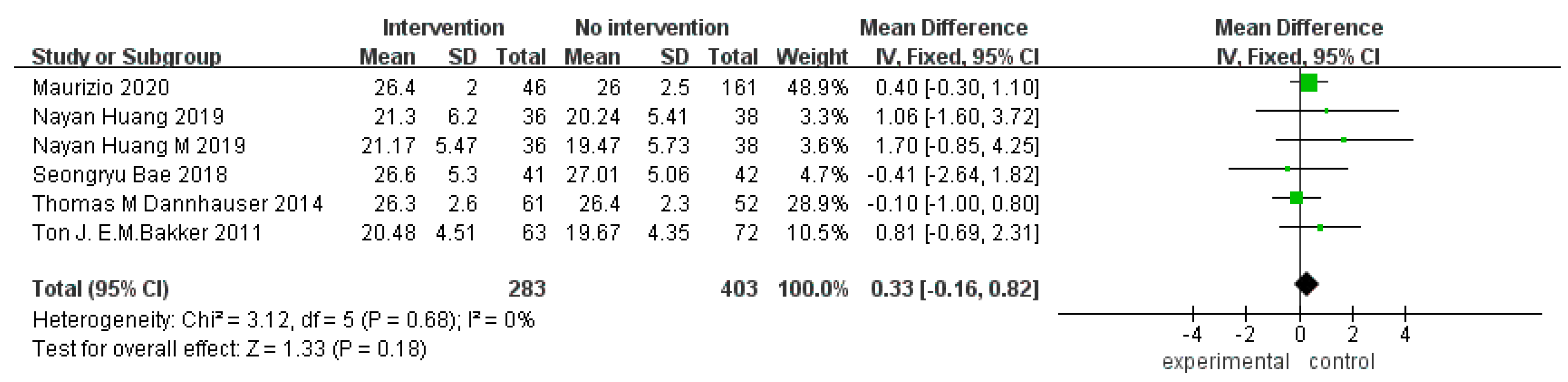
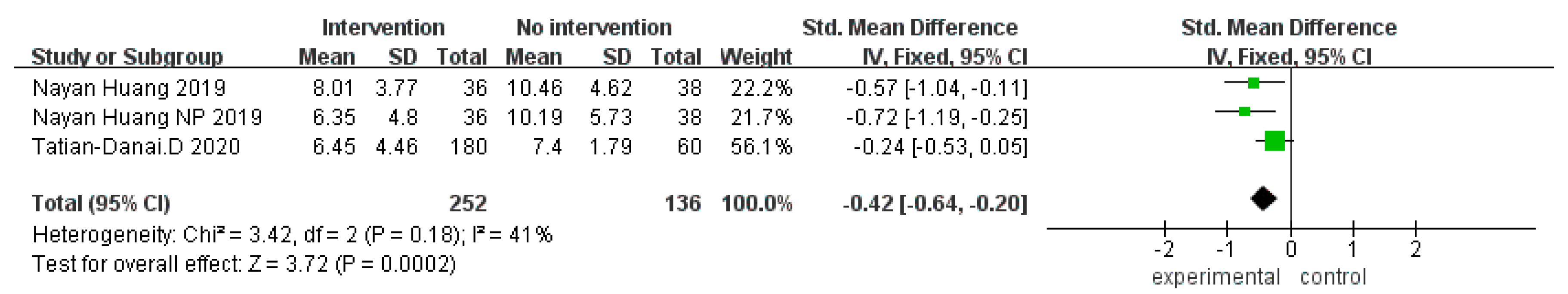
3.3. Meta-Analysis Results
3.3.1. Bias Risk Assessment of Inclusion in the Study
3.3.2. Included Research
3.4. Meta-Analysis Results
3.4.1. Overall Cognitive Function
3.4.2. Neuropsychiatric Questionnaire
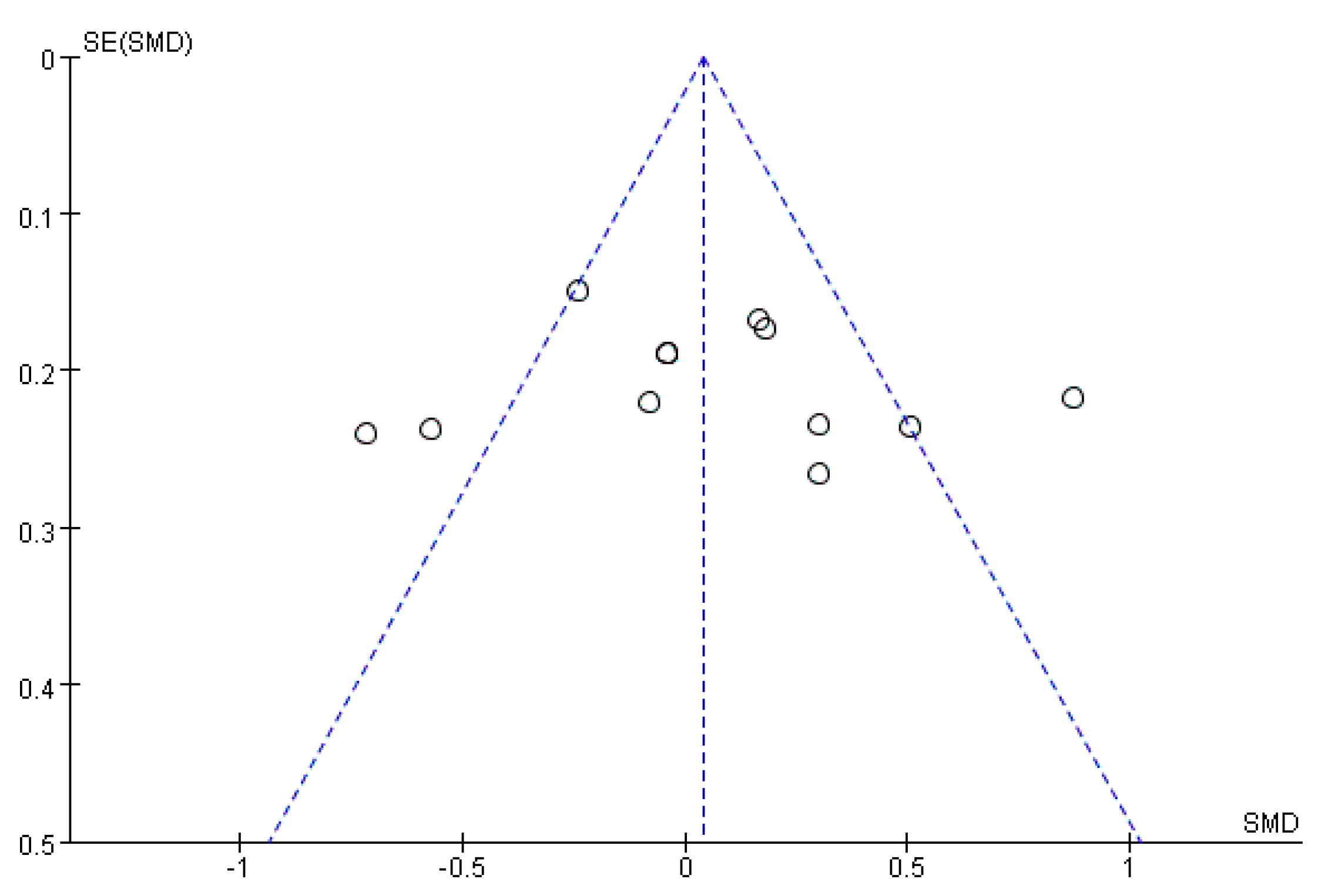
3.5. Publication Bias
4. Conclusions
5. Discussion
6. Limitations and Future Studies
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qin, J.F. Trace elements and vascular dementia. Guangdong Trace Elem. Sci. 2015, 22, 60–70. [Google Scholar]
- Cadieux, M.A.; Garcia, L.J.; Patrick, J. Needs of people with dementia in long-term care: A systematic review. Am. J. Alzheimers Dis. Other Dement. 2013, 28, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Fahy, M.A.; Livingston, G.A. The needs and mental health of older people in 24-hour care residential placements. Aging Ment. Health 2001, 5, 253–257. [Google Scholar] [CrossRef]
- Hancock, G.A.; Woods, R.; Challis, D.; Orrell, M. The needs of older people with dementia in residential care. Int. J. Geriatr. Psychiatry 2005, 21, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Koca, E.; Taskapilioglu, O.; Bakar, M. Caregiver burden in different stages of alzheimer’s disease. Noro Psikiyatr. Ars. 2017, 54, 82–86. [Google Scholar] [CrossRef]
- Liu-Ambrose, T.Y.; Ashe, M.C.; Graf, P.; Beattie, B.L.; Khan, K.M. Increased risk of falling in older community-dwelling women with mild cognitive impairment. Phys. Ther. 2008, 88, 1482–1491. [Google Scholar] [CrossRef]
- Li, S.W. Diagnosis and treatment of cognitive impairment. Chin. J. Nerv. Ment. Dis. 2006, 2, 189–191. [Google Scholar]
- Li, N.; Liang, W.D.; Zhang, S.H.; Guan, H. Research progress of non-drug intervention in the elderly with mild cognitive impairment. Chin. Nurs. Manag. 2018, 18, 688–692. [Google Scholar]
- Li, L.; Ge, Z.X.; Deng, X.L. The effect of reminiscence therapy on patients with Alzheimer′s disease. J. Nurs. Sci. 2015, 30, 1–4. [Google Scholar] [CrossRef]
- Lei, X.X.; Song, L.P. Research progress of non-drug intervention for cognitive dysfunction. Chin. J. Clin. Healthc. 2020, 23, 157–160. [Google Scholar]
- Tang, Y.; Fu, F.; Gao, H.; Shen, L.; Chi, I.; Bai, Z. Art therapy for anxiety, depression, and fatigue in females with breast cancer: A systematic review. J. Psychosoc. Oncol. 2018, 37, 79–95. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Zhao, H.; Tong, F.; Chi, I. A systematic review of psychosocial interventions to cancer caregivers. Front. Psychol. 2017, 8, 834. [Google Scholar] [CrossRef] [PubMed]
- Kanemaru, K.; Kanemaru, A.; Ishii, K. Activation of frontal lobe by music therapy in mild cognitive impairment and Alzheimer’s disease revealed by FDG-PET. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2011, 7, S655. [Google Scholar] [CrossRef]
- Zhai, T. Intervention of social work on students’ anxiety in college entrance examination through music therapy. Arts Technol. 2021, 1, 116–117. [Google Scholar]
- Zhou, B.X. Intervention Study of Music Therapy in Hospice Social Work-Taking—The Case Service of Project A as an Example; Huazhong Agricultural University: Wuhan, China, 2019. [Google Scholar]
- Hou, S.Y. Practice of group work of horticulture therapy to alleviate loneliness of elderly empty nesters. In Proceedings of the Labor and Social Security Research Conference (XIII), Beijing, China, 26–28 July 2022; pp. 44–47. [Google Scholar]
- Ban, R. Observation on the effect of horticultural therapy on patients with chronic schizophrenia. J. Nurs. Sci. 2001, 9, 518–520. [Google Scholar]
- Liu, J.T. Internet plus’s painting therapy intervenes in social work of children and adolescents. Motherland 2019, 16, 61–62. [Google Scholar]
- Potash, J.; Ho, R.; Chick, J.; Yeung, F.A. Viewing and engaging in an art therapy exhibit by people living with mental illness: Implications for empathy and social change. Public Health 2013, 127, 735–744. [Google Scholar] [CrossRef]
- Ma, L.J.; Cheng, H.X.; Liu, Y.F.; Liu, Y.F. Action research on elderly health social work under non-pharmaceutical intervention-taking Shenzhen Social Welfare Center’s integrated intervention service for cognitive impairment as an example. China Soc. Work 2021, 27, 4–7. [Google Scholar]
- Li, Q. Research on the Intervention of Memorial Group on Cognitive Ability of the Elderly with Mild Cognitive Impairment; Huazhong University of Science and Technology: Wuhan, China, 2017. [Google Scholar]
- Liu, M.R. Research on intervention of dementia elderly based on horticulture therapy. Think Tank Times 2019, 6, 226–227. [Google Scholar]
- Gambrill, E.E. Evidence-based clinical behavior analysis, evidence-based medicine and the Cochrane collaboration. J. Behav. Ther. Exp. Psychiatry 1999, 30, 153–154. [Google Scholar] [CrossRef]
- Metzdorff, M.T. Evidence-based medicine: What it is, what it isn’t, and are we practicing it? J. Trauma Acute Care Surg. 2013, 75, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Brekke, J.S. Shaping a science of social work. Res. Soc. Work. Pract. 2012, 22, 455–464. [Google Scholar] [CrossRef]
- Zeng, X.T.; Leng, W.D.; Guo, Y.; Liu, J.Y. One of meta-analysis series: Types of meta-analysis. China J. Evid.-Based Cardiovasc. Med. 2012, 4, 3–5. [Google Scholar]
- Rethlefsen, M.L.; Page, M.J. PRISMA 2020 and PRISMA-S: Common questions on tracking records and the flow diagram. J. Med. Libr. Assoc. 2021, 110, 253–257. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions Version 6.3. 2022. Available online: https://www.training.cochrane.org/handbook (accessed on 21 February 2022).
- Ngandu, T.; Lehtisalo, J.; Solomon, A.; Levälahti, E.; Ahtiluoto, S.; Antikainen, R.; Bäckman, L.; Hänninen, T.; Jula, A.; Laatikainen, T.; et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. Lancet 2015, 385, 2255–2263. [Google Scholar] [CrossRef]
- Dannhauser, T.M.; Cleverley, M.; Whitfield, T.J.; Fletcher, B.C.; Stevens, T.; Walker, Z. A complex multimodal activity intervention to reduce the risk of dementia in mild cognitive impairment–ThinkingFit: Pilot and feasibility study for a randomized controlled trial. BMC Psychiatry 2014, 14, 129. [Google Scholar] [CrossRef]
- Iizuka, A.; Suzuki, H.; Ogawa, S.; Kobayashi-Cuya, K.E.; Kobayashi, M.; Inagaki, H.; Sugiyama, M.; Awata, S.; Takebayashi, T.; Fujiwara, Y. Does social interaction influence the effect of cognitive intervention program? A randomized controlled trial using Go game. Int. J. Geriatr. Psychiatry 2018, 34, 324–332. [Google Scholar] [CrossRef]
- Huang, N.; Li, W.; Rong, X.; Champ, M.; Wei, L.; Li, M.; Mu, H.; Hu, Y.; Ma, Z.; Lyu, J. Effects of a modified Tai Chi program on older people with mild dementia: A randomized controlled trial. J. Alzheimer’s Dis. 2019, 72, 947–956. [Google Scholar] [CrossRef]
- Zhao, J.; Li, H.; Lin, R.; Wei, Y.; Yang, A. Effects of creative expression therapy for older adults with mild cognitive impairment at risk of Alzheimer’s disease: A randomized controlled clinical trial. Clin. Interv. Aging 2018, 13, 1313–1320. [Google Scholar] [CrossRef]
- Salmoirago-Blotcher, E.; DeCosta, J.; Harris, K.; Breault, C.; Dunsiger, S.; Santos, C.; Snyder, P. Exploring synergistic effects of aerobic exercise and mindfulness training on cognitive function in older adults: Protocol for a pilot randomized controlled trial. Medicine 2018, 97, e10626. [Google Scholar] [CrossRef]
- Bakker, T.J.; Duivenvoorden, H.J.; van der Lee, J.; Rikkert, M.G.O.; Beekman, A.T.F.; Ribbe, M.W. Integrative psychotherapeutic nursing home program to reduce multiple psychiatric symptoms of cognitively impaired patients and caregiver burden: Randomized controlled trial. Am. J. Geriatr. Psychiatry 2011, 19, 507–520. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, T.-D.; Verykouki, E.; Papatriantafyllou, J.; Konsta, A.; Kazis, D.; Tsolaki, M. Non-Pharmacological interventions for the anxiety in patients with dementia. A cross-over randomised controlled trial. Behav. Brain Res. 2020, 390, 112617. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Lee, S.; Lee, S.; Jung, S.; Makino, K.; Harada, K.; Harada, K.; Shinkai, Y.; Chiba, I.; Shimada, H. The effect of a multicomponent intervention to promote community activity on cognitive function in older adults with mild cognitive impairment: A randomized controlled trial. Complement. Ther. Med. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- An, Q.; Wang, K.; Sun, F.; Zhang, A. The effectiveness of modified, group-based CBT for dementia worry among Chinese elders. J. Affect. Disord. 2020, 274, 76–84. [Google Scholar] [CrossRef]
- Yaffe, K.; Barnes, D.E.; Rosenberg, D.; Dublin, S.; Kaup, A.R.; Ludman, E.J.; Vittinghoff, E.; Peltz, C.B.; Renz, A.D.; Adams, K.J.; et al. Systematic Multi-Domain Alzheimer’s Risk Reduction Trial (SMARRT): Study Protocol. J. Alzheimer’s Dis. 2019, 70, S207–S220. [Google Scholar] [CrossRef]
- Gallucci, M.; Mazzarolo, A.P.; Focella, L.; Piovesan, C.; Mazzetto, M.; Ramigni, M.; Marzetti, E. “CamminandoeLeggendo … Ricordo” (Walking and Reading … I Remember): Prevention of Frailty Through the Promotion of Physical Activity and Reading in People with Mild Cognitive Impairment. Results from the TREDEM Registry. J. Alzheimer’s Dis. 2020, 77, 689–699. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-Mental State”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Cummings, J.L.; Mega, M.; Gray, K.; Rosenberg-Thompson, S.; Carusi, D.A.; Gornbein, J. The neuropsychiatric inventory: Comprehensive assessment of psychopathology in dementia. Neurology 1994, 44, 2308. [Google Scholar] [CrossRef]
- Zeng, Z.; Deng, Y.-H.; Shuai, T.; Zhang, H.; Wang, Y.; Song, G.-M. Effect of physical activity training on dementia patients: A systematic review with a meta-analysis. Chin. Nurs. Res. 2016, 3, 168–175. [Google Scholar] [CrossRef]
- Franco-Marina, F.; García-González, J.J.; Wagner-Echeagaray, F.; Gallo, J.; Ugalde, O.; Sánchez-García, S.; Espinel-Bermúdez, C.; Juárez-Cedillo, T.; Rodríguez, M.V.; García-Peña, C. The Mini-mental State Examination revisited: Ceiling and floor effects after score adjustment for educational level in an aging Mexican population. Int. Psychogeriatr. 2009, 22, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Pei, F.; Meng, T.; Zhang, K.X.; Mi, X.T.; Wang, J.; Yuan, J.; Li, L.; Ma, C.G.; Wei, J.Z. Comparison of the Mini-mental State Examination Scale and Montreal Cognitive Assessment Scale in the screening of cognitive dysfunction in the elderly. Chin. Med. Clin. 2020, 20, 1771–1774. [Google Scholar]
- Hoang, C.; Wang, F.; Shen, W.Y. Research progress of Chinese and Western medicine on mild cognitive impairment. Sichuan Tradit. Chin. Med. 2013, 31, 137–139. [Google Scholar]
- Jiang, Y.; Wang, L.Y.; Zhang, Y.H.; Guo, C.; Geng, T.T.; Sha, L.Y. Research progress of non-pharmaceuticals intervention on depression of the elderly with mild cognitive impairment. Gen. Nurs. 2022, 20, 1473–1477. [Google Scholar]
- Richard, E.; Schmand, B.; Eikelenboom, P.; Yang, S.C.; Ligthart, S.; Van Charante, E.P.M.; Van Gool, W.A. Symptoms of apathy are associated with progression from mild cognitive impairment to Alzheimer’s Disease in Non-depressed subjects. Dement. Geriatr. Cogn. Disord. 2012, 33, 204–209. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, X.R.; Guo, Y.; Zhang, M.; Qin, H.Y.; Fu, W.Z.; Zhang, Y.X. Effect of health education on awareness rate of mild cognitive impairment among community residents. Shanghai Med. Sci. 2018, 41, 355–358. [Google Scholar]
- Yang, H.; Liu, Y.; Chen, H.; Yin, Y. Integrated nonpharmacological intervention for patients with MCI—A preliminary study in Shanghai, China. Int. J. Integr. Care 2022, 22, 21. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xiao, M.; Lin, Y.; Hämäläinen, J.; Hagedon, A.; Yin, Y. Current development of a nonpharmacological intervention approach for mild cognitive impairment patients and a clinical trial in China. J. Transl. Intern. Med. 2022, 10, 5–8. [Google Scholar] [CrossRef]
- Li, J.; Yu, J.; Niu, Y. Characteristics of neuropsychological damage of mild cognitive impairment and its early recognition and intervention—Prevention and delay of Alzheimer’s disease. Adv. Biochem. Biophys. 2012, 39, 804–810. [Google Scholar]
| Study | Site | Experimental Method | Basic Feature of Research Participants (n, Sex, Age) | Scale | Content | Duration and Frequency | Results | ||
|---|---|---|---|---|---|---|---|---|---|
| Experimental | Control | Disease Type | |||||||
| Ngandu et al. (2015) [29] | Finland | Double-blind | 591, 45% female, 69.5 | 599, 47% female, 69.2 | Dementia | MMSE | Online training, diet, exercise, cognitive training | 2 years | Notable |
| Dannhauser et al. (2014) [30] | Essex | -- | 67, 42% female, 73.8 | 93, 51% female, 76.4 | MCI | MMSE | Online, physical activity, individual and group cognitive stimulation | 12 weeks | Notable |
| Iizuka et al. (2018) [31] | Tokyo | Single-blind | Experiment 1: 25, 72% female, 76.8 Experiment 2: 25, 80% female, 76.5 | 22, 73% female, 77 | MCI | MoCA, MMSE; VMST | Go training | 12 weeks, 12 times | Notable |
| Huang et al. (2019) [32] | China | -- | 40, 70% female, 81.9 | 40, 65% female, 81.9 | MCI | AVLT, MMSE, MoCA, NPI, TMT, WHO-UCLA | Tai chi | 10 months | Notable |
| Zhao et al. (2018) [33] | China | -- | 48, 52.1% female, 70.6 | 45, 51.1% female, 69.5 | MCI | MoCA | Creative expression therapy | 16 weeks | Notable |
| Salmoirago-Blotcher et al. (2018) [34] | United States | -- | 30, --, -- | 10, --, -- | Dementia | MMSE | Aerobic exercise, mindfulness training | 12 weeks | Notable |
| Bakker et al. (2011) [35] | Rotterdam | -- | 54, 66.7% female, 79.8 | 54, 62.1% female, 81.5 | Cognitive disorder | MMSE | Family therapy, life review, cognitive behavioral therapy | 13 weeks | Notable |
| Dimitriou et al. (2020) [36] | Greece | -- | 60, 58.3% female, 74.7 | 60, 58.3% female, 74.7 | Dementia | MMSE, ACE-R, GDS, FRSSD, NPI | Music therapy, transport therapy, aromatherapy massage | 5 days | Notable |
| Bae et al. (2018) [37] | Japan | Single-blind | 41, 43.9% female, 75.5 | 42, 52.1% female, 76.4 | MCI | MMSE, TMT-A | Cognitive training, exercise | 6 months, 24 times | Not notable |
| An et al. (2020) [38] | China | -- | 44;- | 38, --, -- | Dementia | Knowledge of dementia | Cognitive behavioral therapy | 8 weeks, 8 times | Notable |
| Yaffe et al. (2019) [39] | United States | Single-blind | 100;- | 100, --, -- | AD | CASI | Exercise therapy, coach intervention | 2 years | Notable |
| Gallucci et al. (2020) [40] | Italy | -- | 46, 54.3% female, 74.6 | 161, 54% female, 76.7 | MCI | MMSE | Reading therapy, motor therapy | 12 months | Notable |
| Study | Reason for Exclusion |
|---|---|
| Ngandu et al. (2015) [29] | Only provided raw data of baseline MMSE measurement and lacked data after intervention |
| Iizuka et al. (2018) [31] | MMSE and MoCA only used as baseline measurement data, and other scales such as VMST only used in this study |
| Salmoirago-Blotcher et al. (2018) [34] | Missing raw data |
| An et al. (2020) [38] | Intervention focused on improving patients’ worries about their illness, not improving their cognitive status |
| Yaffe et al. (2019) [39] | Missing raw data |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Lv, Z.; Xu, Y.; Chen, H. Leisure-Related Social Work Interventions for Patients with Cognitive Impairment: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2023, 20, 1906. https://doi.org/10.3390/ijerph20031906
Yang H, Lv Z, Xu Y, Chen H. Leisure-Related Social Work Interventions for Patients with Cognitive Impairment: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2023; 20(3):1906. https://doi.org/10.3390/ijerph20031906
Chicago/Turabian StyleYang, Hui, Zhezhen Lv, Yuyue Xu, and Honglin Chen. 2023. "Leisure-Related Social Work Interventions for Patients with Cognitive Impairment: A Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 20, no. 3: 1906. https://doi.org/10.3390/ijerph20031906
APA StyleYang, H., Lv, Z., Xu, Y., & Chen, H. (2023). Leisure-Related Social Work Interventions for Patients with Cognitive Impairment: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 20(3), 1906. https://doi.org/10.3390/ijerph20031906