Abstract
Arthrogryposis multiplex congenita (AMC) and obstetrical brachial plexus palsy (OBPP) are motor disorders with similar symptoms (contractures and the disturbance of upper limb function). Both conditions present as flaccid paresis but differ from each other in the pathogenesis: AMC is a congenital condition, while OBPP results from trauma during childbirth. Despite this difference, these diseases are identical in terms of their manifestations and treatment programmes. We compared the cognitive skills of children with AMC and OBPP diagnoses with those of healthy children; we also compared the motor skills of impaired children with those of healthy ones. The patients in both groups significantly differed from the healthy children with regard to psychological parameters, such as ‘visual memory capacity’ and ‘thinking’. Moreover, the two groups with children with AMC and OBPP significantly differed from each other in motor skill parameters, such as ‘delayed motor development’, ‘general motor development’, and the ‘level of paresis’. Upper limb motor function in the OBPP children was less impaired compared to that of the AMC children. However, we did not find any significant differences in cognitive deficits between the AMC children and the OBPP children. This may indicate that motor impairment is more significant than the underlying cause for the development of cognitive impairment; however, the factors causing this phenomenon require further study (e.g., social environment, treatment, and rehabilitation programme).
1. Introduction
Motor skills and cognitive skills are often discussed together in the context of human development. However, the extent to which one affects the other remains unclear. For example, the question of whether it is worth changing the educational programme for children with motor disabilities has not yet been resolved. In this study, we attempted to find a clearer answer to the following question: what is more important for the child—the diagnosis itself or its manifestation?
Motor development plays a critical role in children’s understanding of the physical and social worlds [1]. However, the extent to which motor development affects cognitive skills remains unclear [2,3,4]. For example, the association between motor planning and working memory performance was shown [5]. In school-age children, a correlation has been found between physical activity and cognitive skills [6]. It can be hypothesised that special school programmes must be developed for children with motor deficits. However, in most countries, educational programmes for children with motor disorders are the same as those for healthy children.
In this study, we focused on diseases associated with impaired functioning of the upper extremities. The upper limbs in humans have a special functional role: unlike the lower limbs, they are mainly involved in the performance of precise voluntary movements. In performing voluntary movements, all levels of the nervous system are involved, especially the highest—the cerebral cortex [7,8,9]. Cognitive skills are also associated with the involvement of higher levels of control in the brain. This may be the critical factor that determines the interaction between the level of development of cognitive skills and motor skills (associated with precise voluntary movements). Cognitive skills and motor skills exhibit a similar developmental timeline, especially between the ages of 5 and 10 years; they also share several important psychophysiological processes, such as sequencing, monitoring, and planning [10,11].
In this study, we assessed the cognitive skills and motor skills of children with upper limb movement disorders, specifically, congenital arthrogryposis multiplex (AMC) and obstetric brachial plexus palsy (OBPP). AMC refers to a group of congenital conditions characterised by joint contractures in two or more areas of the body. While the precise cause may be unknown for some individuals, the causes of AMC are variable and may include genetic, parental, and environmental factors as well as abnormalities that form during foetal development [12]. Individuals with AMC have limited joint movement, with or without muscle weakness, in the affected areas of the body. Contractures vary in distribution and severity and do not progress to previously unaffected joints, but they may change over time due to growth and treatment. The lack of active movement in the joints of the upper extremities is one of the main problems that limit or prevent self-care [13]. AMC is considered a congenital condition, and its pathology is not sufficiently clear. OBPP is an injury to the brachial plexus that occurs during childbirth, usually as a result of strain during a difficult vaginal delivery. This leads to the paralysis of the upper limb; thus, this condition is not congenital, unlike AMC [14]. A factor that was important to this study is that both diseases manifest in the same manner—similar dysfunctions of the upper limbs. Furthermore, surgical interventions and the therapy processes following such interventions are similar. In clinical practice, motor skills are restored through the autotransplantation of muscles from various donor areas [15]. Postoperative therapy involves therapeutic physiotherapy and special physical exercises. Notably, there is no difference between the recovery processes for either of these diseases [14]. Some cognitive skills in children have been shown to be associated with motor illnesses. Compared to healthy children, the capacity for visual and auditory memory is lower in patients with AMC or OBPP. This is particularly evident in children aged 8–11 years [16]. However, the extent to which the degree of movement impairment affects cognitive skills remains unclear.
In this study, we compared the cognitive skills of children with similar manifestations of upper limb motor dysfunction (including treatment and therapy methods) with the cognitive skills of healthy children of a similar age. The patients with OBPP initially had a higher level of motor skill development. We also compared how this difference in motor skills is reflected in the degree of development of cognitive skills. We also correlated the severity of the motor impairment with the level of cognitive performance.
2. Methods
2.1. Patients and Control Participants
This study had 28 control participants (16 males, 12 females; mean age ± std: 9.95 ± 3.38), 18 amyoplasia participants (10 males, 8 females; mean age ± std: 10.14 ± 2.40), and 11 OBPP participants (6 males, 5 females; mean age ± std: 10.64 ± 2.54).
2.2. Assessment of Cognitive Functions and Motor Functions
Cognitive functions (attention span, auditory memory, visual memory, conceptual development, and thinking) were measured using a battery of diagnostic techniques.
Attention and auditory memory were assessed using the Wechsler Intelligence Scale for Children (WISC). The WISC-IV was used for children over six years of age, and the Wechsler Preschool and Primary Scale of Intelligence (WPPSI) was used for children aged 3–6 years [17]. Attention was reflected in a child’s ability to repeat backward the numbers they had heard, and auditory working memory was reflected in the number of digits the child had memorised.
Intelligence was evaluated using Raven’s progressive matrices (A, B, and C) [18]. We employed two types of tests, namely the CPM/CVS kit and SPM+/MHV, because of the age range of the children in the study. Intelligence was reflected in the number of correct responses to age-appropriate intellectual ability tasks.
Visual memory and conceptual development as an aspect of thinking were measured using Shipitsina’s ‘psychological diagnostics of deviations in the development of children of primary school age’. Visual working memory was reflected in the number of memorised pictures out of the 10 presented. Conceptual development was assessed by the number of points a child scored when composing a story from pictures.
Thinking was assessed using the ‘exclusion of objects’ technique. This technique is designed to study the features of thinking—first, the level of development and second, the qualitative characteristics of the generalisation process of visual materials [19].
During neurology examinations, the patient’s general motor skills (GMD) were estimated. Anamnesis vitae included information about the patient in infancy. A developmental delay was indicated when a child had not reached particular milestones within the expected time period and his motor skills were different from those of a healthy child (DMD). We estimated passive and active movement in the joints, muscles strength, muscle volume, muscle tone, tendon reflexes, and sensation. The paresis level was estimated clinically by the scheme of segmental innervation of upper limb muscles. A lower level of paresis is associated with greater motor impairment in the patient, i.e., greater involvement of the distal muscles [15].
2.3. Statistical Analysis
The Kruskal–Wallis H test was used to test the hypothesis that population median performances in different cognitive tasks are equal among AMC patients, OBPP patients, and control children. Mann–Whitney tests were used to investigate the exact differences between AMC patients, OBPP patients, and control children.
3. Results
The patients were examined during their spare time. They attended the laboratory supervised either by their parents or medical personnel. Their self-care capabilities were significantly different from healthy children. The patients could not take food or perform hygiene actions (wash face and hands) on their own. Rather, they did so under the patronage of their parents or medical personnel. We also noticed that all the movements performed by the patients appeared to be slower compared to healthy children. We plan to quantify these skills. Visual communication did not differ between healthy children and patients.
Comparison of children with AMC, OBPP and healthy children based on the Kruskal–Wallis H-test showed that significant deviations were observed for such parameters as «Visual Memory» and «Thinking» (Table 1).

Table 1.
Results of Kruskal–Wallis H-test series testing the hypothesis that population median performances in different cognitive tasks are equal between AMC patients, OBPP patients, and control children. p-values are adjusted by means of Bonferroni correction (included eta-squared values for the Kruskall–Wallis series with H-values following Murphy and Myors ([20], Appendix A), ‘*’—statistically significant values).
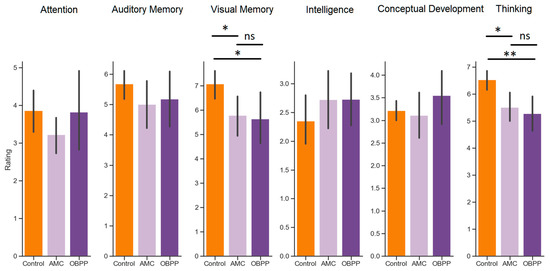
Figure 1.
Performance in cognitive tasks assessed for AMC patients (light purple), OBPP patients (dark purple), and control children (orange). Asterisks between the bars indicate the significant difference shown by post hoc Mann–Whitney comparisons between two groups (* p < 0.05; ** p < 0.01, ns: not significant).
Table 1.
Results of Kruskal–Wallis H-test series testing the hypothesis that population median performances in different cognitive tasks are equal between AMC patients, OBPP patients, and control children. p-values are adjusted by means of Bonferroni correction (included eta-squared values for the Kruskall–Wallis series with H-values following Murphy and Myors ([20], Appendix A), ‘*’—statistically significant values).
| Task | h | η2approximated | p |
|---|---|---|---|
| Attention | 2.38 | 0.04 | 1.000 |
| Auditory Memory | 1.83 | 0.03 | 1.000 |
| Visual Memory | 9.90 | 0.16 | 0.042 * |
| Intelligence | 1.62 | 0.03 | 1.000 |
| Conceptual Development | 2.65 | 0.05 | 1.000 |
| Thinking | 12.76 | 0.19 | 0.010 * |
Mann–Whitney tests were used as post hoc analyses to investigate the exact differences between AMC patients, OBPP patients, and control children. Specifically, with regard to the visual memory performance, both AMC (u = 369.0, p = 0.023) and OBPP (u = 230.5, p = 0.046) differed significantly from their control peers, whereas AMC and OBPP patients showed equal results (u = 102.5, p = 1.00). Similarly, with regard to the thinking task performance, both AMC (u = 353.5, p = 0.012) and OBPP (u = 237.0, p = 0.004) differed significantly from their control peers, whereas AMC and OBPP patients showed equal results (u = 109.0, p = 1.00) (Figure 1).
We also assessed the difference in motor skills between children with AMC and OBPP diagnoses (Figure 2). The series of Mann–Whitney tests revealed that OBPP children showed significantly higher motor performance than AMC children. Specifically, they demonstrated higher DMD scores (u = 24.4, p = 0.001), higher GMD scores (u = 10.5, p < 0.001), and higher level of paresis (u = 21.0, p < 0.001).
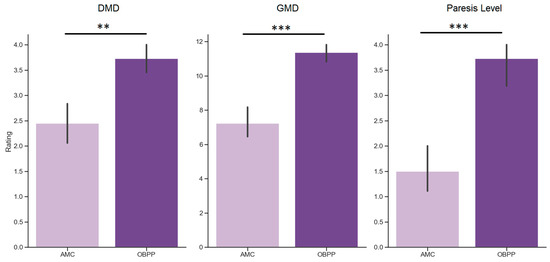
Figure 2.
Motor development in AMC (light purple) and OBPP (dark purple) patients, assessed as delayed motor development (DMD) score, general motor development (GMD) score and the level of paresis (Paresis Level). Asterisks indicate significant difference for hoc Mann–Whitney u-test comparisons between the two groups of patients (** p< 0.01, *** p< 0.001).
4. Discussion
In this study, we evaluated the differences in cognitive skills between children with motor disorders of the upper limbs and healthy children; we then further correlated these factors. This study involved three groups of children (adjusted for age): patients with AMC, patients with OBPP, and healthy children. Despite the similarity of disorders between the patients, the statistical analysis showed a significant difference in the level of motor skills between children diagnosed with AMC and those diagnosed with OBPP. The patients significantly differed in all studied parameters.
The assessment of cognitive skills showed that, in the tests for visual memory and thinking, the children diagnosed with AMC and those diagnosed with OBPP differed from the healthy children. Our data support the assumption that impairments in motor functions are associated with impairments in individual cognitive functions. This is consistent with data showing that the development of motor skills correlates with the development of cognitive skills [21,22]. However, the sample size did not allow us to statistically estimate the age at which this correlation is the most prominent. There are age periods in which the active formation of voluntary behaviour regulation, reflection, and self-control occurs [23,24,25]. Moreover, voluntary movement skills are mainly formed in childhood. However, it can be argued that, in general, the formation of these skills is sequential; for example, operating with hands is associated with tasks that a child needs to perform at a particular stage of development, such as bringing food to their mouth, handling various objects, and writing; all these movements are associated with cognitive control of voluntary movements. In addition, it is critical for the formation of consistency in all body functions [22]. Thus, it is important to take this factor into account when developing educational programmes for children with motor disorders.
Previous studies have found a link between memory and motor skills [26,27]. A deviation in the capacity of visual memory and auditory memory was also found in children diagnosed with AMC and those diagnosed with OBPP [16].
Taking this into consideration, we suggest that the difference in the development of cognitive skills may be associated with a basic mismatch in the development of motor and cognitive skills. Based on the literature, correlations were found in the cognitive level of children with impaired formation of hand preference [28], posture control [29], and walking [30], among other impairments [1]. Therefore, on the one hand, it can be hypothesised that any motor impairment must directly lead to cognitive impairment. However, our data are partially inconsistent with this claim since they showed selectivity of cognitive impairment in motor diseases and did not show any differences in memory performance between AMC patients and OBPP, patients despite their prominent differences in the motor domain. In this situation, several factors can be identified that affect the impairment of cognitive skills. Cognitive skills are formed in relation to motor skills, and this relationship could be nonlinear. There may be a “ceiling” effect, when, in the case of restraining a specific motor function, the function of the world perception would be discretely violated and, accordingly, cognitive impairments have a similar severity [31,32,33]. Another important factor, in our opinion, would be the social environment, which is also important for the formation of the patient’s cognitive skills. Although, there may be other reasons for the effects we discovered.
Among the various factors that may affect the complex link between the motor and cognitive domains of development, parenting appears to be of particular importance. The parents of children with motor disorders perform most basic activities, such as dressing, feeding, and washing, for their children from birth. Notably, as the child grows, no psychological separation from the parent occurs; they continue employing the same, albeit no longer age-matching, parenting approaches. It is difficult for parents to step back from such a role. They continue to perform actions for the child in the usual routine, thus inhibiting the child’s physical development. Such a manifestation of overprotection in parents who have a child with a serious illness is common [34]. Several studies have shown that such parental behaviour inhibits both the cognitive and mental development of a child [35,36]. Furthermore, parental overprotection increases the level of anxiety in children, which also suppresses the development of cognitive functions [37,38]. Notably, in most countries, the treatment and rehabilitation of AMC patients and OBPP patients are not associated with the severity of disease manifestation. This may also result in the levelling of the factor of individuality.
The involvement of modern neurotechnology may facilitate an understanding of the psychophysiological changes that occur in the brain in children with motor disorders [39]. It has been shown that, in such children, there is a significant decrease in the power of the main EEG rhythms [40]; moreover, specific rearrangements in the brain that are associated with a change in the functional representation of certain muscles of the upper extremities have been found in children with AMC [41]. It is likely that similar changes should occur in children diagnosed with OBPP, but there is no exact data on this issue. Understanding the relationship between such significant reorganisations in the brain and the level of development of cognitive skills may make it possible to reconstruct the mechanisms of compensatory brain activity in children with motor disorders. This will enable the creation of neurotechnology that can help children with motor disorders. Regular clinical and neurophysiological estimation, an assessment of the needs in daily life, and knowledge of the social and family environments are key points for management.
Accordingly, to eliminate cognitive impairment in children with motor diseases, it is necessary to focus not only on the correction of motor dysfunctions but also on the minimisation of the effects of the children’s social environment.
5. Conclusions
This study has yielded the following conclusions:
- Children with AMC and those with OBPP differ from healthy children in terms of cognitive skills, such as visual memory and thinking.
- Children with AMC and those with OBPP differ in terms of motor skills but not in cognitive tests.
- Presumably, the presence of a motor disease may be a more significant factor than its degree of manifestation in explaining cognitive deficits.
Author Contributions
Conceptualization, E.B., M.K. and O.A.; methodology, M.K., O.A., A.N.S. and E.B.; formal analysis, D.B. and I.P.J.; investigation, M.K., D.K., E.E. and E.B.; data curation, O.A., D.B. and M.K.; writing—original draft preparation, E.B., A.N.S. and I.P.J.; visualization, D.B.; funding acquisition, E.B., O.A., A.N.S. and I.P.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation, (20-65-47016) and has been carried out using HSE unique equipment (Reg. num 354937).
Institutional Review Board Statement
The study was approved by the decision of the local ethics 243 committee No. 19-3 dated 9 December 2019.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to patient confidentiality.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Libertus, K.; Hauf, P. Editorial: Motor skills and their foundational role for perceptual, social, and cognitive development. Front. Psychol. 2017, 8, 301. [Google Scholar] [CrossRef] [PubMed]
- Hauert, C.A. The relationship between motor function and cognition in the developmental perspective. Ital. J. Neurol. Sci. 1986, 5, 101–107. [Google Scholar]
- Iverson, J.M. Developing language in a developing body: The relationship between motor development and language development. J. Child Lang. 2010, 37, 229–261. [Google Scholar] [CrossRef] [PubMed]
- van der Fels, I.M.J.; Te Wierike, S.C.M.; Hartman, E.; Elferink-Gemser, M.T.; Smith, J.; Visscher, C. The relationship between motor skills and cognitive skills in 4-16 year old typically developing children: A systematic review. J. Sci. Med. Sport 2015, 18, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Stöckel, T.; Hughes, C.M.L. The relation between measures of cognitive and motor functioning in 5- to 6-year-old children. Psychol. Res. 2016, 80, 543–554. [Google Scholar] [CrossRef]
- Abdelkarim, O.; Ammar, A.; Chtourou, H.; Wagner, M.; Knisel, E.; Hökelmann, A.; Bös, K. Relationship between motor and cognitive learning abilities among primary school-aged children. Alex. J. Med. 2019, 53, 325–331. [Google Scholar] [CrossRef]
- Blagovechtchenski, E.; Pettersson, L.G.; Perfiliev, S.; Krasnochokova, E.; Lundberg, A. Control of digits via C3-C4 propriospinal neurones in cats; recovery after lesions. Neurosci. Res. 2000, 38, 103–107. [Google Scholar] [CrossRef]
- Lemon, R.N. Descending Pathways in Motor Control. Annu. Rev. Neurosci. 2008, 31, 195–218. [Google Scholar] [CrossRef]
- Pettersson, L.G.; Alstermark, B.; Blagovechtchenski, E.; Isa, T.; Sasaski, S. Skilled digit movements in feline and primate-Recovery after selective spinal cord lesions. Proc. Acta Physiol. 2007, 189, 141–154. [Google Scholar] [CrossRef]
- Anderson, V.A.; Anderson, P.; Northam, E.; Jacobs, R.; Catroppa, C. Development of executive functions through late childhood and adolescence in an Australian sample. Dev. Neuropsychol. 2001, 20, 385–406. [Google Scholar] [CrossRef]
- Roebers, C.M.; Kauer, M. Motor and cognitive control in a normative sample of 7-year-olds. Dev. Sci. 2009, 12, 175–181. [Google Scholar] [CrossRef]
- Dahan-Oliel, N.; Cachecho, S.; Barnes, D.; Bedard, T.; Davison, A.M.; Dieterich, K.; Donohoe, M.; Fąfara, A.; Hamdy, R.; Hjartarson, H.T.; et al. International multidisciplinary collaboration toward an annotated definition of arthrogryposis multiplex congenita. Am. J. Med. Genet. C. Semin. Med. Genet. 2019, 181, 288–299. [Google Scholar] [CrossRef]
- Hall, J.G. Arthrogryposis multiplex congenita: Etiology, genetics, classification, diagnostic approach, and general aspects. J. Pediatr. Orthop. B 1997, 6, 159–166. [Google Scholar] [CrossRef]
- Komolkin, I.; Ulrich, E.V.; Agranovich, O.E.; van Bosse, H.J.P. Treatment of Scoliosis Associated With Arthrogryposis Multiplex Congenita. J. Pediatr. Orthop. 2017, 37 (Suppl. S1), S24–S26. [Google Scholar] [CrossRef]
- Trofimova, S.I.; Agranovich, O.E. Restoration of active forearm flexion in children with arthrogryposis:results of transfer of long head of triceps. Pediatr. Traumatol. Orthop. Reconstr. Surg. 2015, 3, 15. [Google Scholar] [CrossRef]
- Koriakina, M.; Agranovich, O.; Petrova, E.; Kadieva, D.; Kopytin, G.; Ermolovich, E.; Moiseenko, O.; Alekseeva, M.; Bredikhin, D.; Bermúdez-Margaretto, B.; et al. Aberrant Auditory and Visual Memory Development of Children with Upper Limb Motor Disorders. Brain Sci. 2021, 11, 1650. [Google Scholar] [CrossRef]
- Lin, Y.J.; Kao, T.W.; Chen, W.L. Relationship between peripheral neuropathy and cognitive performance in the elderly population. Medicine 2021, 100, e26071. [Google Scholar] [CrossRef]
- Piek, J.P.; Dawson, L.; Smith, L.M.; Gasson, N. The role of early fine and gross motor development on later motor and cognitive ability. Hum. Mov. Sci. 2008, 27, 668–681. [Google Scholar] [CrossRef]
- Belopolskaya, N.L. Exclusion of Objects (The Fourth Extra): A Modified Psychodiagnostic Technique: A Manual for Use, 3rd ed.; Stereotyped: Moscow, Russia, 2009. [Google Scholar]
- Murphy, K.R.; Myors, B. Statistical Power Analysis: A Simple and General Model for Traditional and Modern Hypothesis Tests: Second Edition; Routledge: London, UK, 2003; pp. 1–139. [Google Scholar] [CrossRef]
- Higashionna, T.; Iwanaga, R.; Tokunaga, A.; Nakai, A.; Tanaka, K.; Nakane, H.; Tanaka, G. Relationship between motor coordination, cognitive abilities, and academic achievement in Japanese children with neurodevelopmental disorders. Hong Kong J. Occup. Ther. 2017, 30, 49–55. [Google Scholar] [CrossRef]
- Zeng, N.; Ayyub, M.; Sun, H.; Wen, X.; Xiang, P.; Gao, Z. Effects of physical activity on motor skills and cognitive development in early childhood: A systematic review. Biomed Res. Int. 2017, 2017, 2760716. [Google Scholar] [CrossRef]
- Vygotsky, L.; Luria, A. Tool and Symbol in Child Development; Blackwell Publishers: Oxford, UK, 1978. [Google Scholar]
- Jenni, O.G.; Chaouch, A.; Caflisch, J.; Rousson, V. Correlations between motor and intellectual functions in normally developing children between 7 and 18 years. Dev. Neuropsychol. 2013, 38, 98–113. [Google Scholar] [CrossRef] [PubMed]
- Koziol, L.F.; Lutz, J.T. From movement to thought: The development of executive function. Appl. Neuropsychol. Child 2013, 2, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hady, S.S.; Abd El-Azim, F.H.; El-Talawy, H.A.E.A.M. Correlation between cognitive function, gross motor skills and health–Related quality of life in children with Down syndrome. Egypt. J. Med. Hum. Genet. 2018, 19, 97–101. [Google Scholar] [CrossRef]
- Diamond, A. Close interrelation of motor development and cognitive development and of the cerebellum and prefrontal cortex. Child Dev. 2000, 71, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Michel, G.F.; Campbell, J.M.; Marcinowski, E.C.; Nelson, E.L.; Babik, I. Infant Hand Preference and the Development of Cognitive Abilities. Front. Psychol. 2016, 7, 410. [Google Scholar] [CrossRef]
- Morse, A.F.; Benitez, V.L.; Belpaeme, T.; Cangelosi, A.; Smith, L.B. Posture Affects How Robots and Infants Map Words to Objects. PLoS ONE 2015, 10, e0116012. [Google Scholar] [CrossRef]
- Walle, E.A. Infant social development across the transition from crawling to walking. Front. Psychol. 2016, 7, 960. [Google Scholar] [CrossRef]
- Kenny, L.; Hill, E.; Hamilton, A.F. The Relationship between Social and Motor Cognition in Primary School Age-Children. Front. Psychol. 2016, 7, 228. [Google Scholar] [CrossRef]
- Anderson, D.I.; Lohse, K.R.; Lopes, T.C.V.; Williams, A.M. Individual differences in motor skill learning: Past, present and future. Hum. Mov. Sci. 2021, 78, 102818. [Google Scholar] [CrossRef]
- Magallón, S.; Narbona, J.; Crespo-Eguílaz, N. Acquisition of motor and cognitive skills through repetition in typically developing children. PLoS ONE 2016, 11, e0158684. [Google Scholar] [CrossRef]
- Sanders, K.Y. Overprotection and lowered expectations of persons with disabilities: The unforeseen consequences. Work 2006, 27, 181–188. [Google Scholar]
- Gere, M.K.; Villabø, M.A.; Torgersen, S.; Kendall, P.C. Overprotective parenting and child anxiety: The role of co-occurring child behavior problems. J. Anxiety Disord. 2012, 26, 642–649. [Google Scholar] [CrossRef]
- Kiel, E.J.; Maack, D.J. Maternal BIS Sensitivity, Overprotective Parenting, and Children’s Internalizing Behaviors. Pers. Individ. Dif. 2012, 53, 257–262. [Google Scholar] [CrossRef]
- Clarke, K.; Cooper, P.; Creswell, C. The parental overprotection scale: Associations with child and parental anxiety. J. Affect. Disord. 2013, 151, 618–624. [Google Scholar] [CrossRef]
- Nakamura, C.Y. The relationship between children’s expression of hostility and methods of discipline exercised by dominant overprotective parents. Child Dev. 1959, 30, 109–117. [Google Scholar] [CrossRef]
- Blagovechtchenski, E.; Agranovich, O.; Kononova, Y.; Nazarova, M.; Nikulin, V.V. Perspectives for the Use of Neurotechnologies in Conjunction With Muscle Autotransplantation in Children. Front. Neurosci. 2019, 13, 99. [Google Scholar] [CrossRef]
- Blagoveschenskiy, E.D.; Agranovich, O.E.; Kononova, E.L.; Baindurashvili, A.G.; Nazarova, M.A.; Shestokova, A.N.; Gabbasova, E.L.; Nikulin, V.V. Characteristics of electrophysiological activity of the cerebral cortex in children with arthrogryposis. Nervn. Bolezn. 2018, 8, 25–32. [Google Scholar] [CrossRef]
- Golosheykin, S.A.; Blagoveschenskiy, E.D.; Agranovich, O.E.; Nazarova, M.A.; Nikulin, V.V.; Moiseenko, O.E.; Chan, R.W.; Shestakova, A.N. Feasibility and Challenges of Performing Magnetoencephalography Experiments in Children With Arthrogryposis Multiplex Congenita. Front. Pediatr. 2021, 9, 626734. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).