Effectiveness and Therapeutic Mechanism of Pharmacopuncture for Pain in Parkinson’s Disease: A Study Protocol for a Pilot Pragmatic Randomized, Assessor-Blinded, Usual Care-Controlled, Three-Arm Parallel Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Aims
- To assess the effectiveness and safety of PA for directly improving PD-related pain and pain-associated motor and non-motor symptoms.
- To investigate, in an exploratory manner, the possibility of neuroimaging and molecular signature indicators as biomarkers of the therapeutic response to PA for PD.
- To explore the pathogenesis of pain subtypes in PD, as well as the corresponding current therapy guidelines for traditional medicine, such as traditional Korean medicine.
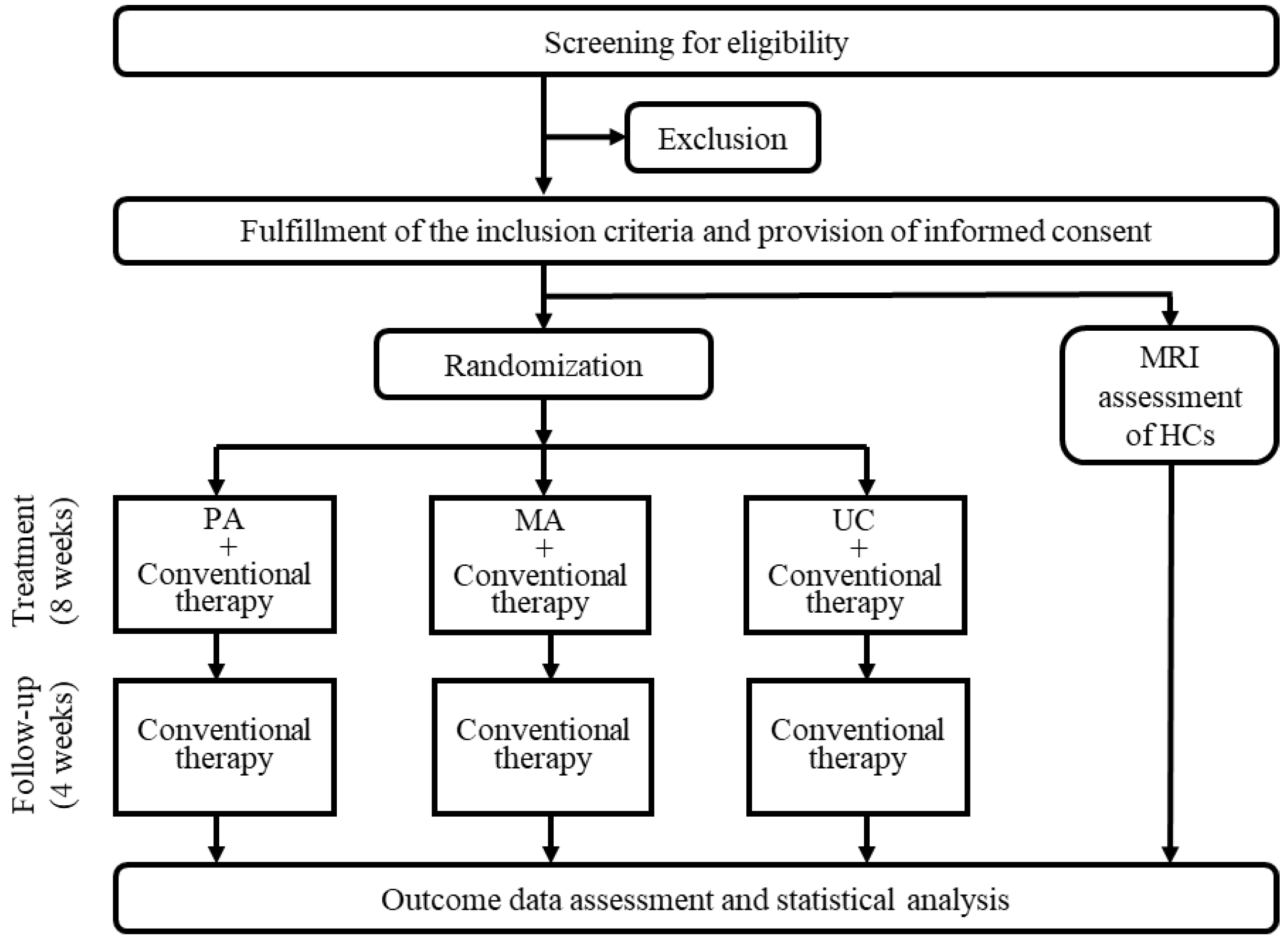
2.2. Study Design and Setting
2.3. Participant Recruitment
2.4. Randomization
2.5. Interventions
2.6. Treatment Group: PA
2.7. Control Group: MA or UC
2.8. Outcome Measures
2.8.1. Primary Outcome: Assessment Scales for PD Pain
2.8.2. Secondary Outcomes: Evaluation Instruments of Pain and Related Symptoms
2.9. Exploratory Outcomes
2.9.1. Investigating Therapeutic Mechanisms and Biomarkers for PA to Treat PD Pain: Molecular Analysis and Neuroimaging Using MRI
2.9.2. Investigation of the Distribution of SD for PD Pain and SD Changes following PA: SD Questionnaire Survey
2.9.3. Objective Evaluation of PA for PD Pain-Related Symptoms: Gait Analysis and Assessment of Facial Expressions in Response to Emotion-Eliciting Stimuli
2.10. Safety Assessment and AEs
2.11. Statistical Methods
2.11.1. Sample Size Calculation
2.11.2. Statistical Analysis
2.12. Data Management and Monitoring
3. Discussion
4. Ethics and Dissemination
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Tommaso, M.; Arendt-Nielsen, L.; Defrin, R.; Kunz, M.; Pickering, G.; Valeriani, M. Pain in neurodegenerative disease: Current knowledge and future perspectives. Behav. Neurol. 2016, 2016, 7576292. [Google Scholar] [CrossRef] [PubMed]
- Buhmann, C.; Kassubek, J.; Jost, W.H. Management of pain in Parkinson’s disease. J. Parkinsons Dis. 2020, 10, S37–S48. [Google Scholar] [CrossRef] [PubMed]
- Ford, B. Pain in Parkinson’s disease. Mov. Disord. 2010, 25, S98–S103. [Google Scholar] [CrossRef] [PubMed]
- Mylius, V.; Perez Lloret, S.; Cury, R.G.; Teixeira, M.J.; Barbosa, V.R.; Barbosa, E.R.; Moreira, L.I.; Listik, C.; Fernandes, A.M.; de Lacerda, D.V.; et al. The Parkinson disease pain classification system: Results from an international mechanism-based classification approach. Pain 2021, 162, 1201–1210. [Google Scholar] [CrossRef]
- Truini, A.; Frontoni, M.; Cruccu, G. Parkinson’s disease related pain: A review of recent findings. J. Neurol. 2013, 260, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Reijnders, J.S.; Ehrt, U.; Weber, W.E.; Aarsland, D.; Leentjens, A.F. A systematic review of prevalence studies of depression in Parkinson’s disease. Mov. Disord. 2008, 23, 183–189. [Google Scholar] [CrossRef]
- Broen, M.P.; Narayen, N.E.; Kuijf, M.L.; Dissanayaka, N.N.; Leentjens, A.F. Prevalence of anxiety in Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. 2016, 31, 1125–1133. [Google Scholar] [CrossRef]
- Yoon, J.W.; Kim, S.J. A review of clinical study on Jungsongouhyul pharmacopuncture treatment published in Korea. J. Korean Med. Rehabil. 2017, 27, 75–84. [Google Scholar] [CrossRef]
- Lee, S.G.; Won, J.K.; Yeom, S.R.; Lee, S.K.; Song, Y.S.; Kwon, Y.D. The effects of Dokhwalgisaeng-tang (Duhuoqisheng-tang) and Jungsongouhyul pharmacopuncture on pain control and nerve regeneration in the crush-induced sciatic nerve injury of the rat model. J. Korean Rehabil. 2009, 19, 15–32. [Google Scholar]
- Park, J.E.; Kang, S.; Jang, B.H.; Shin, Y.C.; Ko, S.G. Adverse events from pharmacopuncture treatment in Korea: A protocol for systematic review and meta analysis. Medicine 2021, 100, e25107. [Google Scholar] [CrossRef]
- Hwang, J.H.; Ku, J.; Jeong, J.H. Pharmacopuncture for the management of musculoskeletal diseases: A protocol for systematic review. Medicine 2020, 99, e19082. [Google Scholar] [CrossRef]
- Ji, M.J.; Lim, S.C.; Kim, J.; Lee, H.; Lee, Y. The comparative study on effect of Jungsongouhyul pharmacopuncture and electroacupuncture in patients with acute traumatic shoulder pain. J. Acupunct. Res. 2014, 31, 205–211. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, T.R.; Woo, C.H.; Shin, B.C. Comparative effectiveness of Hwangryunhaedok-tang pharmacopuncture, essential bee venom pharmacopuncture and Jungsongouhyul pharmacopuncture for cervical pain caused by traffic accidents: A retrospective observational study. J. Korean Med. Rehabil. 2018, 28, 83–89. [Google Scholar] [CrossRef]
- Jung, M.J.; Lee, J.H.; Yeom, S.R.; Lee, S.K.; Song, Y.S.; Kim, K.B.; Kwon, Y.D. Effects of Ohyaksungi-san (Wuyaoshungi-san) and Jungsongouhyul pharmacopuncture on pain reduction and nerve regeneration after crush injury in rat sciatic nerve. J. Korean Rehabil. 2009, 19, 51–72. [Google Scholar]
- Blanchet, P.J.; Brefel-Courbon, C. Chronic pain and pain processing in Parkinson’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 87, 200–206. [Google Scholar] [CrossRef]
- Engels, G.; McCoy, B.; Vlaar, A.; Theeuwes, J.; Weinstein, H.; Scherder, E.; Douw, L. Clinical pain and functional network topology in Parkinson’s disease: A resting-state fMRI study. J. Neural. Transm. 2018, 125, 1449–1459. [Google Scholar] [CrossRef]
- Mylius, V.; Ciampi de Andrade, D.; Cury, R.G.; Teepker, M.; Ehrt, U.; Eggert, K.M.; Beer, S.; Kesselring, J.; Stamelou, M.; Oertel, W.H. Pain in Parkinson’s disease: Current concepts and a new diagnostic algorithm. Mov. Disord. Clin. Pract. 2015, 2, 357–364. [Google Scholar] [CrossRef]
- Chaudhuri, K.R.; Rizos, A.; Trenkwalder, C.; Rascol, O.; Pal, S.; Martino, D.; Carroll, C.; Paviour, D.; Falup-Pecurariu, C.; Kessel, B.; et al. King’s Parkinson’s disease pain scale, the first scale for pain in PD: An international validation. Mov. Disord. 2015, 30, 1623–1631. [Google Scholar] [CrossRef]
- Taghizadeh, G.; Joghataei, M.T.; Goudarzi, S.; Bakhsheshi, M.; Habibi, S.A.H.; Mehdizadeh, M. King’s Parkinson’s disease pain scale cut-off points for detection of pain severity levels: A reliability and validity study. Neurosci. Lett. 2021, 745, 135620. [Google Scholar] [CrossRef]
- Beck, A.; Steer, R.; Brown, G. Manual for the BDI-II, the Psychological Corporation; The Psychological Corporation: San Antonio, TX, USA, 1996. [Google Scholar] [CrossRef]
- Sung, H.; Kim, J.; Park, Y.; Bai, D.; Lee, S.; Ahn, H. A study on the reliability and the validity of Korean version of the Beck Depression Inventory—II (BDI-II). J. Korean Soc. Biol. Ther. Psychiatry 2008, 14, 201–212. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A. Manual for the Beck Anxiety Inventory; The Psychological Corporation: San Antonio, TX, USA, 1990. [Google Scholar]
- Lee, H.K.; Kim, J.; Hong, S.; Lee, E.; Hwang, S.T. Psychometric properties of the Beck Anxiety Inventory in the community-dwelling sample of Korean adults. Korean J. Clin. Psychol. 2016, 35, 822–830. [Google Scholar] [CrossRef]
- Cho, S.; Kim, H.Y.; Lee, J.H. Validation of the Korean version of the Pain Catastrophizing Scale in patients with chronic non-cancer pain. Qual. Life Res. 2013, 22, 1767–1772. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, C.; Fitzpatrick, R.; Peto, V.; Greenhall, R.; Hyman, N. The Parkinson’s disease Questionnaire (PDQ-39): Development and validation of a Parkinson’s disease summary index score. Age Ageing 1997, 26, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Krüger, R.; Klucken, J.; Weiss, D.; Tönges, L.; Kolber, P.; Unterecker, S.; Lorrain, M.; Baas, H.; Müller, T.; Riederer, P. Classification of advanced stages of Parkinson’s disease: Translation into stratified treatments. J. Neural. Transm. 2017, 124, 1015–1027. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, Z.; He, J.; Teng, L.; Yuan, C. Traditional Chinese medicine symptom pattern analysis for Parkinson’s disease. J. Tradit. Chin. Med. 2017, 37, 688–694. [Google Scholar] [PubMed]
- Yeo, M.; Park, K.; Bae, K.; Jang, E.; Lee, Y. Development on the questionnaire of cold-heat pattern identification based on usual symptoms for health promotion-focused on reliability study. J. Physiol. Pathol. Korean Med. 2016, 30, 116–123. [Google Scholar] [CrossRef]
- Baek, Y.; Jung, K.; Kim, Y.; Jang, E. Evaluation of validity of deficiency and excess pattern identification questionnaire. J. Physiol. Pathol. Korean Med. 2020, 34, 142–148. [Google Scholar] [CrossRef]
- Sonnby-Borgström, M.; Jönsson, P.; Svensson, O. Emotional empathy as related to mimicry reactions at different levels of information processing. J. Nonverbal. Behav. 2003, 27, 3–23. [Google Scholar] [CrossRef]
- Dimberg, U.; Thunberg, M. Empathy, emotional contagion, and rapid facial reactions to angry and happy facial expressions. PsyCh J. 2012, 1, 118–127. [Google Scholar] [CrossRef]
- Wild, B.; Erb, M.; Eyb, M.; Bartels, M.; Grodd, W. Why are smiles contagious? An fMRI study of the interaction between perception of facial affect and facial movements. Psychiatry Res. 2003, 123, 17–36. [Google Scholar] [CrossRef]
- Allen, N.E.; Wong, C.M.; Canning, C.G.; Moloney, N. The association between Parkinson’s disease motor impairments and pain. Pain Med. 2016, 17, 456–462. [Google Scholar] [CrossRef]
- Yu, S.W.; Lin, S.H.; Tsai, C.C.; Chaudhuri, K.R.; Huang, Y.C.; Chen, Y.S.; Yeh, B.Y.; Wu, Y.R.; Wang, J.J. Acupuncture effect and mechanism for treating pain in patients with Parkinson’s disease. Front. Neurol. 2019, 10, 1114. [Google Scholar] [CrossRef]
- Liu, C.H.; Wang, R.; Jin, Y.B.; Sun, Z.L.; Zhou, X.; He, J.Y. Acupoint injection of kakkonein for early- or mid-stage Parkinson’s disease: A multicenter randomized controlled clinical trial. Zhen Ci Yan Jiu 2015, 40, 56–60. [Google Scholar]
- Cho, S.Y.; Shim, S.R.; Rhee, H.Y.; Park, H.J.; Jung, W.S.; Moon, S.K.; Park, J.M.; Ko, C.N.; Cho, K.H.; Park, S.U. Effectiveness of acupuncture and bee venom acupuncture in idiopathic Parkinson’s disease. Parkinsonism Relat. Disord. 2012, 18, 948–952. [Google Scholar] [CrossRef]
- Løge-Hagen, J.S.; Sæle, A.; Juhl, C.; Bech, P.; Stenager, E.; Mellentin, A.I. Prevalence of depressive disorder among patients with fibromyalgia: Systematic review and meta-analysis. J. Affect. Disord. 2019, 245, 1098–1105. [Google Scholar] [CrossRef]
- Pinheiro, M.B.; Ferreira, M.L.; Refshauge, K.; Maher, C.G.; Ordoñana, J.R.; Andrade, T.B.; Tsathas, A.; Ferreira, P.H. Symptoms of depression as a prognostic factor for low back pain: A systematic review. Spine J. 2016, 16, 105–116. [Google Scholar] [CrossRef]
- Axford, J.; Butt, A.; Heron, C.; Hammond, J.; Morgan, J.; Alavi, A.; Bolton, J.; Bland, M. Prevalence of anxiety and depression in osteoarthritis: Use of the Hospital Anxiety and Depression Scale as a screening tool. Clin. Rheumatol. 2010, 29, 1277–1283. [Google Scholar] [CrossRef]
- Kinugawa, K.; Mano, T.; Yamatani, Y.; Miyasaka, T.; Kataoka, H.; Sugie, K. Pain-related abnormal neuronal synchronization of the nucleus accumbens in Parkinson’s disease. Brain Sci. 2022, 12, 84. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, J.; Peng, J.; Wu, X.; Chen, X.; Liu, J.; Wei, M.; Zou, D.; Han, Y.; Wang, A.; et al. Abnormal connectivity model of raphe nuclei with sensory-associated cortex in Parkinson’s disease with chronic pain. Neurol. Sci. 2022, 43, 3175–3185. [Google Scholar] [CrossRef]
- Polli, A.; Weis, L.; Biundo, R.; Thacker, M.; Turolla, A.; Koutsikos, K.; Chaudhuri, K.R.; Antonini, A. Anatomical and functional correlates of persistent pain in Parkinson’s disease. Mov. Disord. 2016, 31, 1854–1864. [Google Scholar] [CrossRef]
- He, Y.; May, B.H.; Zhang, A.L.; Guo, X.; Liu, Y.; Qu, Y.; Chang, X.; Lu, C.J.; Xue, C.C.; Zhang, H. Acupuncture for cancer pain: Protocol for a pilot pragmatic randomised controlled trial. BMJ Open 2019, 9, e025564. [Google Scholar] [CrossRef] [PubMed]
- Vickers, A.J.; Linde, K. Acupuncture for chronic pain. JAMA 2014, 311, 955–956. [Google Scholar] [CrossRef] [PubMed]
| Inclusion Criteria |
|---|
| Adult patients (age ≥ 19 years) |
| Diagnosis of idiopathic PD based on the UK Parkinson’s Disease Society Brain Bank criteria |
| Hoehn and Yahr Scale stage 1–4 |
| King’s Parkinson’s Disease Pain Scale score > 0 |
| A stable dose of conventional treatment for at least 4 weeks prior to enrollment |
| Terminated PA or MA treatment 4 weeks before enrollment |
| Provide voluntary written informed consent to participate in this clinical study |
| Exclusion criteria |
| Parkinson-plus syndromes (i.e., multisystem atrophy, progressive supranuclear palsy, corticobasal degeneration, and dementia with Lewy bodies) |
| Pain unrelated to PD (e.g., postoperative pain) |
| History of neuropsychiatric disorder unrelated to PD |
| Moderate or higher cognitive impairment that will interfere with the evaluation of treatment effects |
| Severe acute cardiovascular disease (i.e., heart failure, myocardial infarction, stroke, and hypertension) |
| Serious conditions (i.e., anemia, active pulmonary tuberculosis, thyroid disease, and other infectious and systemic diseases) |
| Active cancer |
| Uncontrolled hypertension (systolic blood pressure > 160 mmHg or diastolic blood pressure > 100 mmHg) |
| History of hypersensitivity reactions to PA |
| Indications for PA treatment being inappropriate or unsafe (i.e., hemorrhagic disease, patients with severe diabetes who have a higher risk of infection) |
| History of taking oral adrenal corticosteroids (steroids), immunosuppressants, or antipsychotic drugs or other drugs that may affect clinical trial results within the last 4 weeks |
| Inability to undergo MRI |
| Pregnant or lactating women or current contraceptive use among women of pregnant potential who are likely to become pregnant (except for women who have undergone sterilization) |
| History of drug or alcohol abuse |
| Unstable medical condition as determined by the research clinician; A patient who shows clinically significant diseases and disorders in physical or clinical examination or is receiving active treatment thereof |
| Participation in another clinical trial within the last 4 weeks |
| History of vaccination within 4 weeks or plans to be vaccinated during the clinical trial period |
| Inappropriate for enrollment due to other reasons as determined by the investigator |
| Screening | MRI | Treatment | MRI | Follow-Up | ||||
|---|---|---|---|---|---|---|---|---|
| Week | −2 to −1 | −1 to −0 | 1 | 4 | 8 | 8 to 9 | 12 | |
| Visit No. | 1 | 2 | 3 (Baseline) | 10 | 18 | 19 | 20 | |
| Provide informed consent | O | |||||||
| Identification of inclusion and exclusion criteria | O | |||||||
| Demographics | O | |||||||
| Medical and disease history | O | |||||||
| Vital signs | O | O | O (every visit) | O (every visit) | O | O | O | |
| Clinical laboratory examination | O | O | ||||||
| Identification of eligibility criteria for MRI measurement | O | |||||||
| Randomization | O | |||||||
| Outcome measures | KPPS | O | O | O | O | O | ||
| NRS | O | O | O | O | ||||
| UPDRS II, III | O | O | O | O | ||||
| Pain catastrophizing score | O | O | ||||||
| EQ-5D | O | O | ||||||
| PDSS-2 | O | O | ||||||
| Facial expression analysis | O | O | ||||||
| Syndrome differentiation | O | O | ||||||
| BDI II | O | O | ||||||
| BAI | O | O | ||||||
| Gait analysis | O | O | O | |||||
| MRI measurement | O | O | ||||||
| Blood collection for molecular analysis | O | O | ||||||
| PA or MA or UC | O (every visit) | O (every visit) | O | |||||
| Treatment compliance | O | |||||||
| Identification of concomitant drug change | O | O | O (every visit) | O (every visit) | O | O | O | |
| Identification of adverse reaction(s) | O (every visit) | O | O | O | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, J.-H.; Kim, J.; Kwon, O.; Jung, S.Y.; Lee, H.-J.; Cho, S.-Y.; Park, J.-M.; Ko, C.-N.; Park, S.-U.; Kim, H. Effectiveness and Therapeutic Mechanism of Pharmacopuncture for Pain in Parkinson’s Disease: A Study Protocol for a Pilot Pragmatic Randomized, Assessor-Blinded, Usual Care-Controlled, Three-Arm Parallel Trial. Int. J. Environ. Res. Public Health 2023, 20, 1776. https://doi.org/10.3390/ijerph20031776
Jang J-H, Kim J, Kwon O, Jung SY, Lee H-J, Cho S-Y, Park J-M, Ko C-N, Park S-U, Kim H. Effectiveness and Therapeutic Mechanism of Pharmacopuncture for Pain in Parkinson’s Disease: A Study Protocol for a Pilot Pragmatic Randomized, Assessor-Blinded, Usual Care-Controlled, Three-Arm Parallel Trial. International Journal of Environmental Research and Public Health. 2023; 20(3):1776. https://doi.org/10.3390/ijerph20031776
Chicago/Turabian StyleJang, Jung-Hee, Jieun Kim, Ojin Kwon, So Young Jung, Hye-Jin Lee, Seung-Yeon Cho, Jung-Mi Park, Chang-Nam Ko, Seong-Uk Park, and Hyungjun Kim. 2023. "Effectiveness and Therapeutic Mechanism of Pharmacopuncture for Pain in Parkinson’s Disease: A Study Protocol for a Pilot Pragmatic Randomized, Assessor-Blinded, Usual Care-Controlled, Three-Arm Parallel Trial" International Journal of Environmental Research and Public Health 20, no. 3: 1776. https://doi.org/10.3390/ijerph20031776
APA StyleJang, J.-H., Kim, J., Kwon, O., Jung, S. Y., Lee, H.-J., Cho, S.-Y., Park, J.-M., Ko, C.-N., Park, S.-U., & Kim, H. (2023). Effectiveness and Therapeutic Mechanism of Pharmacopuncture for Pain in Parkinson’s Disease: A Study Protocol for a Pilot Pragmatic Randomized, Assessor-Blinded, Usual Care-Controlled, Three-Arm Parallel Trial. International Journal of Environmental Research and Public Health, 20(3), 1776. https://doi.org/10.3390/ijerph20031776






