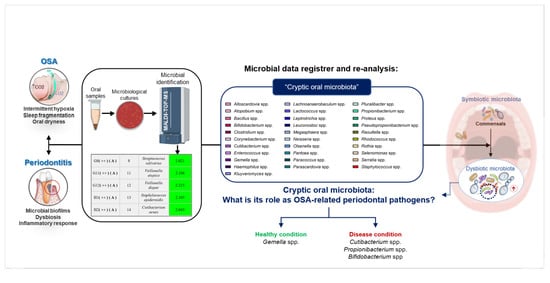
Cryptic Oral Microbiota: What Is Its Role as Obstructive Sleep Apnea-Related Periodontal Pathogens?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Register
2.3. Statistical Analysis
3. Results
3.1. Clinical Data
3.2. Microbiological Data
3.3. Association between Periodontal Parameters and the Cryptic Oral Microorganisms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khodadadi, N.; Khodadadi, M.; Zamani, M. Is Periodontitis Associated with Obstructive Sleep Apnea? A Systematic Review and Meta-Analysis. J. Clin. Exp. Dent. 2022, 14, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Verhelst, A.R.E.; Kosho, M.X.F.; Aarab, G.; Loos, B.G. Screening for the Risk of OSA in Periodontitis Patients. A Pilot Study. Oral Health Prev. Dent. 2022, 20, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Al-Jewair, T.S.; Al-Jasser, R.; Almas, K. Periodontitis and Obstructive Sleep Apnea’s Bidirectional Relationship: A Systematic Review and Meta-Analysis. Sleep Breath 2015, 19, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Seo, W.H.; Cho, E.R.; Thomas, R.J.; An, S.Y.; Ryu, J.J.; Kim, H.; Shin, C. The Association between Periodontitis and Obstructive Sleep Apnea: A Preliminary Study. J. Periodontal Res. 2013, 48, 500–506. [Google Scholar] [CrossRef]
- Nizam, N.; Tasbakan, M.S.; Basoglu, O.K.; Lappin, D.F.; Buduneli, N. Is There an Association between Obstructive Sleep Apnea Syndrome and Periodontal Inflammation? Clin. Oral Investig. 2016, 20, 659–668. [Google Scholar] [CrossRef]
- Wu, B.G.; Sulaiman, I.; Wang, J.; Shen, N.; Clemente, J.C.; Li, Y.; Laumbach, R.J.; Lu, S.; Udasin, I.; Le-hoang, O.; et al. Severe Obstructive Sleep Apnea Is Associated with Alterations in the Nasal Microbiome and an Increase in Inflammation. Am. J. Respir. Crit. Care Med. 2018, 199, 99–109. [Google Scholar] [CrossRef]
- Ko, C.; Hu, A.; Huang, D.C.L.; Su, H.; Yan, F.; Zhang, X.; Zhang, H.; Zeng, Y. Analysis of Oral Microbiota in Patients with Obstructive Sleep Apnea-Associated Hypertension. Hypertens. Res. 2019, 42, 1692–1700. [Google Scholar] [CrossRef]
- Jia, P.; Zou, J.; Yin, S.; Chen, F.; Yi, H.; Zhang, Q. Analysis of the Salivary Microbiome in Obstructive Sleep Apnea Syndrome Patients. Can. J. Infect. Dis. Med. Microbiol. 2020, 2020, 6682020. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Y.; Feng, M.; Huang, X.; Li, C.; Han, F.; Zhang, Q.; Gao, X. Altered Salivary Microbiota in Patients with Obstructive Sleep Apnea Comorbid Hypertension. Nat. Sci. Sleep 2022, 14, 593–607. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, X.; Huang, X.; Duan, Y.; Gao, H.; Gao, X. Analysis of Salivary Microbiome and Its Association With Periodontitis in Patients With Obstructive Sleep Apnea. Front. Cell. Infect. Microbiol. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Tellez Corral, M.A.; Herrera Daza, E.; Cuervo Jimenez, H.K.; Arango Jimenez, N.; Morales Vera, D.Z.; Velosa Porras, J.; Latorre Uriza, C.; Escobar Arregoces, F.M.; Hidalgo Martínez, P.; Cortes, M.E.; et al. Patients with Obstructive Sleep Apnea Can Favor the Predisposing Factors of Periodontitis by the Presence P. Melaninogenica and C. Albicans, Increasing the Severity of the Periodontal Disease. Front. Cell. Infect. Microbiol. 2022, 12, 1273. [Google Scholar] [CrossRef] [PubMed]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L. Microbial Complexes in Subgingival Plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Caton, J.; Armitage, G.; Berglundh, T.; Chapple, I.L.C.; Jepsen, S.; S. Kornman, K.; L. Mealey, B.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A New Classification Scheme for Periodontal and Peri-Implant Diseases and Conditions – Introduction and Key Changes from the 1999 Classification. J. Clin. Periodontol. 2018, 45, S1–S8. [Google Scholar] [CrossRef]
- Ogawa, Y.; Koizumi, A.; Kasahara, K.; Lee, S.T.; Yamada, Y.; Nakano, R.; Yano, H.; Mikasa, K. Bacteremia Secondary to Alloscardovia Omnicolens Urinary Tract Infection. J. Infect. Chemother. 2016, 22, 424–425. [Google Scholar] [CrossRef] [PubMed]
- Stenfors, L.P.; Mayr, R.; Scherer, S.; Granum, P.E. Pathogenic Potential of Fifty Bacillus Weihenstephanensis Strains. FEMS Microbiol. Lett. 2002, 215, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Sárvári, K.P.; Schoblocher, D. The Antibiotic Susceptibility Pattern of Gas Gangrene-Forming Clostridium Spp. Clinical Isolates from South-Eastern Hungary. Infect. Dis. (Auckl) 2020, 52, 196–201. [Google Scholar] [CrossRef]
- Milano, V.; Biehle, L.; Patel, S.; Hammer, J. Clostridium Tertium Bacteremia and Hepatic Abscess in a Non-Neutropenic Patient. IDCases 2019, 15, e00510. [Google Scholar] [CrossRef]
- Qudeimat, M.A.; Alyahya, A.; Karched, M.; Behbehani, J.; Salako, N.O. Dental Plaque Microbiota Profiles of Children with Caries-Free and Caries-Active Dentition. J. Dent. 2021, 104, 103539. [Google Scholar] [CrossRef]
- Alibi, S.; Ferjani, A.; Boukadida, J.; Cano, M.E.; Fernández-Martínez, M.; Martínez-Martínez, L.; Navas, J. Occurrence of Corynebacterium Striatum as an Emerging Antibiotic-Resistant Nosocomial Pathogen in a Tunisian Hospital. Sci. Rep. 2017, 7, 9704. [Google Scholar] [CrossRef]
- Chong, K.K.L.; Tay, W.H.; Janela, B.; Yong, A.M.H.; Liew, T.H.; Madden, L.; Keogh, D.; Barkham, T.M.S.; Ginhoux, F.; Becker, D.L.; et al. Enterococcus Faecalis Modulates Immune Activation and Slows Healing during Wound Infection. J. Infect. Dis. 2017, 216, 1644–1654. [Google Scholar] [CrossRef]
- Tyrrell, G.J.; Turnbull, L.A.; Teixeira, L.M.; Lefebvre, J.; Carvalho, M.d.G.S.; Facklam, R.R.; Lovgren, M. Enterococcus Gilvus Sp. Nov. and Enterococcus Pallens Sp. Nov. Isolated from Human Clinical Specimens. J. Clin. Microbiol. 2002, 40, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Loke, W.; Girvan, T.; Ingmundson, P.; Verrett, R.; Schoolfield, J.; Mealey, B.L. Investigating the Association Between Obstructive Sleep Apnea and Periodontitis. J. Periodontol. 2015, 86, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Gamsiz-Isik, H.; Kiyan, E.; Bingol, Z.; Baser, U.; Ademoglu, E.; Yalcin, F. Does Obstructive Sleep Apnea Increase the Risk for Periodontal Disease? A Case-Control Study. J. Periodontol. 2017, 88, 443–449. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Zeevi, D.; Levy, M.; Zilberman-Schapira, G.; Suez, J.; Tengeler, A.C.; Abramson, L.; Katz, M.N.; Korem, T.; Zmora, N.; et al. Transkingdom Control of Microbiota Diurnal Oscillations Promotes Metabolic Homeostasis. Cell 2014, 159, 514–529. [Google Scholar] [CrossRef]
- Ko, C.Y.; Hu, A.K.; Zhang, L.; Lu, X.L.; Zeng, Y.M. Alterations of Oral Microbiota in Patients with Obstructive Sleep Apnea–Hypopnea Syndrome Treated with Continuous Positive Airway Pressure: A Pilot Study. Sleep Breath. 2022, 26, 811–814. [Google Scholar] [CrossRef] [PubMed]
- Vieira Colombo, A.P.; Magalhães, C.B.; Hartenbach, F.A.R.R.; Martins do Souto, R.; Maciel da Silva-Boghossian, C. Periodontal-Disease-Associated Biofilm: A Reservoir for Pathogens of Medical Importance. Microb. Pathog. 2016, 94, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Kakuta, M.; Hasegawa, T.; Yamaguchi, R.; Uchino, E.; Kobayashi, W.; Sawada, K.; Tamura, Y.; Tokuda, I.; Murashita, K.; et al. Metagenomic Analysis of Bacterial Species in Tongue Microbiome of Current and Never Smokers. NPJ Biofilms Microbiomes 2020, 6, 11. [Google Scholar] [CrossRef]
- Büyükcam, A.; Tuncer, Ö.; Gür, D.; Sancak, B.; Ceyhan, M.; Cengiz, A.B.; Kara, A. Clinical and Microbiological Characteristics of Pantoea Agglomerans Infection in Children. J. Infect. Public Health 2018, 11, 304–309. [Google Scholar] [CrossRef]
- Morinaga, K.; Yoshida, K.; Takahashi, K.; Nomura, N.; Toyofuku, M. Peculiarities of Biofilm Formation by Paracoccus Denitrificans. Appl. Microbiol. Biotechnol. 2020, 104, 2427–2433. [Google Scholar] [CrossRef]
- Freire, M.P.; De Oliveira Garcia, D.; Cury, A.P.; Spadão, F.; Di Gioia, T.S.R.; Francisco, G.R.; Bueno, M.F.C.; Tomaz, M.; De Paula, F.J.; De Faro, L.B.; et al. Outbreak of IMP-Producing Carbapenem-Resistant Enterobacter Gergoviae among Kidney Transplant Recipients. J. Antimicrob. Chemother. 2016, 71, 2577–2585. [Google Scholar] [CrossRef]
- Enigk, K.; Jentsch, H.; Rodloff, A.C.; Eschrich, K.; Stingu, C.S. Activity of Five Antimicrobial Peptides against Periodontal as Well as Non-Periodontal Pathogenic Strains. J. Oral Microbiol. 2020, 12, 1829405. [Google Scholar] [CrossRef] [PubMed]
- Armbruster, C.E.; Mobley, H.L.T.; Pearson, M.M.; Arbor, A.; States, U.; States, U. Pathogenesis of Proteus Mirabilis Infection. EcoSal Plus. 2018, 8, 1–123. [Google Scholar] [CrossRef] [PubMed]
- Spielman, A.F.; Ghumman, A.; Panthaki, Z.; Lessard, A.S. Neisseria Elongata Osteomyelitis: Literature Review and Case Report in a 63-Year-Old Male Presenting with Progressive Right-Handed Redness, Swelling and Pain. Int. J. Surg. Case Rep. 2020, 73, 228–230. [Google Scholar] [CrossRef] [PubMed]
- Baniulyte, G.; Svirpliene, S.; Eccleston, A.; Arjunan, S.; Connor, M. Neisseria Oralis Septicaemia in a Newborn: First Recorded Case. Paediatr. Int. Child Health 2021, 41, 226–227. [Google Scholar] [CrossRef]
- Eribe, E.R.K.; Olsen, I. Leptotrichia Species in Human Infections II. J. Oral Microbiol. 2017, 9, 1368848. [Google Scholar] [CrossRef]
- Spohner, S.C.; Schaum, V.; Quitmann, H.; Czermak, P. Kluyveromyces Lactis: An Emerging Tool in Biotechnology. J. Biotechnol. 2016, 222, 104–116. [Google Scholar] [CrossRef]
- Sękowska, A. Raoultella Spp.—Clinical Significance, Infections and Susceptibility to Antibiotics. Folia Microbiol. 2017, 62, 221–227. [Google Scholar] [CrossRef]
- Finch, L.C.; Gerdzhikov, S.; Buttery, R. Haemophilus Parainfluenzae Endocarditis Presenting with Symptoms of COVID-19. BMJ Case Rep. 2021, 14, 4–9. [Google Scholar] [CrossRef]
- Modaweb, A.; Mansoor, Z.; Alsarhan, A.; Abuhammour, W. A Case of Successfully Treated Central Line-Associated Bloodstream Infection Due to Vancomycin-Resistant Leuconostoc Citreum in a Child With Biliary Atresia. Cureus 2022, 14, 12–15. [Google Scholar] [CrossRef]
- Omori, R.; Fujiwara, S.; Ishiyama, H.; Kuroda, H.; Kohara, N. Leuconostoc Lactis- A Rare Cause of Bacterial Meningitis in an Immunocompromised Host. Intern. Med. 2020, 59, 2935–2936. [Google Scholar] [CrossRef]
- Menegueti, M.G.; Gaspar, G.G.; Laus, A.M.; Basile-Filho, A.; Bellissimo-Rodrigues, F.; Auxiliadora-Martins, M. Bacteremia by Leuconostoc Mesenteroides in an Immunocompetent Patient with Chronic Chagas Disease: A Case Report. BMC Infect. Dis. 2018, 18, 13–15. [Google Scholar] [CrossRef]
- Grenier, D. Porphyromonas Gingivalis Outer Membrane Vesicles Mediate Coaggregation and Piggybacking of Treponema Denticola and Lachnoanaerobaculum Saburreum. Int. J. Dent. 2013, 2013, 305476. [Google Scholar] [CrossRef] [PubMed]
- Meyburgh, C.M.; Bragg, R.R.; Boucher, C.E. Lactococcus Garvieae: An Emerging Bacterial Pathogen of Fish. Dis. Aquat. Organ. 2017, 123, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Baba, H.; Nada, T.; Ohkusu, K.; Ezaki, T.; Hasegawa, Y.; Paterson, D.L. First Case of Bloodstream Infection Caused by Rhodococcus Erythropolis. J. Clin. Microbiol. 2009, 47, 2667–2669. [Google Scholar] [CrossRef] [PubMed]
- Greve, D.; Moter, A.; Kleinschmidt, M.C.; Pfäfflin, F.; Stegemann, M.S.; Kursawe, L.; Grubitzsch, H.; Falk, V.; Kikhney, J. Rothia Aeria and Rothia Dentocariosa as Biofilm Builders in Infective Endocarditis. Int. J. Med. Microbiol. 2021, 311, 151478. [Google Scholar] [CrossRef] [PubMed]
- Franconieri, F.; Join-Lambert, O.; Creveuil, C.; Auzou, M.; Labombarda, F.; Aouba, A.; Verdon, R.; de La Blanchardière, A. Rothia Spp. Infective Endocarditis: A Systematic Literature Review. Med. Mal. Infect. 2020, 51, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Uranga, C.C.; Arroyo, P.; Gerwick, W.; Edlund, A. Commensal Oral Rothia Mucilaginosa Produces Enterobactin, a Metal-Chelating Siderophore. Host-Microbe Biol. 2020, 5, e00161-20. [Google Scholar] [CrossRef]
- Begrem, S.; Jérôme, M.; Leroi, F.; Delbarre-Ladrat, C.; Grovel, O.; Passerini, D. Genomic Diversity of Serratia Proteamaculans and Serratia Liquefaciens Predominant in Seafood Products and Spoilage Potential Analyses. Int. J. Food Microbiol. 2021, 354, 109326. [Google Scholar] [CrossRef]
- Jiang, B.; You, B.; Tan, L.; Yu, S.; Li, H.; Bai, G.; Li, S.; Rao, X.; Xie, Z.; Shi, X.; et al. Clinical Staphylococcus Argenteus Develops to Small Colony Variants to Promote Persistent Infection. Front. Microbiol. 2018, 9, 1–10. [Google Scholar] [CrossRef]
- Ahmad-Mansour, N.; Loubet, P.; Pouget, C.; Dunyach-Remy, C.; Sotto, A.; Lavigne, J.P.; Molle, V. Staphylococcus Aureus Toxins: An Update on Their Pathogenic Properties and Potential Treatments. Toxins 2021, 13, 677. [Google Scholar] [CrossRef]
- Cui, B.; Smooker, P.M.; Rouch, D.A.; Daley, A.J.; Deighton, M.A. Differences between Two Clinical Staphylococcus Capitis Subspecies as Revealed by Biofilm, Antibiotic Resistance, and Pulsed-Field Gel Electrophoresis Profiling. J. Clin. Microbiol. 2013, 51, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Brescó, M.S.; Harris, L.G.; Thompson, K.; Stanic, B.; Morgenstern, M.; O’Mahony, L.; Richards, R.G.; Moriarty, T.F. Pathogenic Mechanisms and Host Interactions in Staphylococcus Epidermidis Device-Related Infection. Front. Microbiol. 2017, 8, 1401. [Google Scholar] [CrossRef] [PubMed]
- Szczuka, E.; Krzymińska, S.; Bogucka, N.; Kaznowski, A. Multifactorial Mechanisms of the Pathogenesis of Methicillin-Resistant Staphylococcus Hominis Isolated from Bloodstream Infections. Antonie Van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2018, 111, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, S.; Shah, S.; Raja Sager, A.; Rangan, A.; Durugu, S. Staphylococcus Lugdunensis: Review of Epidemiology, Complications, and Treatment. Cureus 2020, 12, 6–13. [Google Scholar] [CrossRef]
- Wang, P.; Liu, Y.; Xu, Y.; Xu, Z. Staphylococcus Saccharolyticus Infection: Case Series with a PRISMA-Compliant Systemic Review. Medicine 2020, 99, e20686. [Google Scholar] [CrossRef]
- Kanuparthy, A.; Challa, T.; Meegada, S.; Siddamreddy, S.; Muppidi, V. Staphylococcus Warneri: Skin Commensal and a Rare Cause of Urinary Tract Infection. Cureus 2020, 12, e8337. [Google Scholar] [CrossRef]
- Lisowska-Łysiak, K.; Lauterbach, R.; Międzobrodzki, J.; Kosecka-Strojek, M. Epidemiology and Pathogenesis of Staphylococcus Bloodstream Infections in Humans: A Review. Polish J. Microbiol. 2021, 70, 13–23. [Google Scholar] [CrossRef]
- Lima, B.P.; Hu, L.I.; Vreeman, G.W.; Weibel, D.B.; Lux, R. The Oral Bacterium Fusobacterium Nucleatum Binds Staphylococcus Aureus and Alters Expression of the Staphylococcal Accessory Regulator SarA. Microb. Ecol. 2019, 78, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, F.E.; Sammons, R.L.; Chapple, I.L.C. Isolation and Characterization of Subgingival Staphylococci from Periodontitis Patients and Controls. Oral Dis. 2004, 10, 155–162. [Google Scholar] [CrossRef]
- Taimur, S.; Madiha, R.; Samar, F.; Bushra, J. Gemella Morbillorum Endocarditis in a Patient with a Bicuspid Aortic Valve. Hell. J. Cardiol. 2010, 51, 183–186. [Google Scholar] [CrossRef]
- Maraki, S.; Plevritaki, A.; Kofteridis, D.; Scoulica, E.; Eskitzis, A.; Gikas, A.; Panagiotakis, S.H. Bicuspid Aortic Valve Endocarditis Caused by Gemella Sanguinis: Case Report and Literature Review. J. Infect. Public Health 2019, 12, 304–308. [Google Scholar] [CrossRef]
- Collins, M.D. The Genus Gemella. In Prokaryotes; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2006; Volume 6, pp. 197–214. [Google Scholar]
- Miyoshi, T.; Oge, S.; Nakata, S.; Ueno, Y.; Ukita, H.; Kousaka, R.; Miura, Y.; Yoshinari, N.; Yoshida, A. Gemella Haemolysans Inhibits the Growth of the Periodontal Pathogen Porphyromonas Gingivalis. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Broly, M.; Ruffier d’Epenoux, L.; Guillouzouic, A.; Le Gargasson, G.; Juvin, M.E.; Leroy, A.G.; Bémer, P.; Corvec, S. Propionibacterium/Cutibacterium Species–Related Positive Samples, Identification, Clinical and Resistance Features: A 10-Year Survey in a French Hospital. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Corvec, S. Clinical and Biological Features of Cutibacterium (Formerly Propionibacterium) Avidum, an Underrecognized Microorganism Stéphane. Clin. Microbiol. Rev. 2018, 31, e00064-17. [Google Scholar] [CrossRef]
- Mayslich, C.; Grange, P.A. Cutibacterium Acnes as an Opportunistic Pathogen: An Update of Its Virulence-Associated Factors. Microorganisms 2021, 9, 303. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Teles, F.; Uzel, N.G.; Papas, A. Characterizing Microbiota from Sjögren’s Syndrome Patients. JDR Clin. Transl. Res. 2021, 6, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Emery, D.C.; Shoemark, D.K.; Batstone, T.E.; Waterfall, C.M.; Coghill, J.A.; Cerajewska, T.L.; Davies, M.; West, N.X.; Allen, S.J. 16S RRNA next Generation Sequencing Analysis Shows Bacteria in Alzheimer’s Post-Mortem Brain. Front. Aging Neurosci. 2017, 9, 195. [Google Scholar] [CrossRef]
- Leheste, J.R.; Ruvolo, K.E.; Chrostowski, J.E.; Rivera, K.; Husko, C.; Miceli, A.; Selig, M.K.; Brüggemann, H.; Torres, G.P. Acnes-Driven Disease Pathology: Current Knowledge and Future Directions. Front. Cell. Infect. Microbiol. 2017, 7, 81. [Google Scholar] [CrossRef]
- Lu, X.; Qi, X.; Yi, X.; Jian, Z.; Gao, T. Transcellular Traversal of the Blood-Brain Barrier by the Pathogenic Propionibacterium Acnes. J. Cell. Biochem. 2019, 120, 8457–8465. [Google Scholar] [CrossRef]
- Bernard, C.; Lemoine, V.; Hoogenkamp, M.A.; Girardot, M.; Krom, B.P.; Imbert, C. Candida Albicans Enhances Initial Biofilm Growth of Cutibacterium Acnes under Aerobic Conditions. Biofouling 2019, 35, 350–360. [Google Scholar] [CrossRef]
- Bernard, C.; Renaudeau, N.; Mollichella, M.L.; Quellard, N.; Girardot, M.; Imbert, C. Cutibacterium Acnes Protects Candida Albicans from the Effect of Micafungin in Biofilms. Int. J. Antimicrob. Agents 2018, 52, 942–946. [Google Scholar] [CrossRef] [PubMed]
- McDowell, A.; McLaughlin, J.; Layton, A.M. Is Cutibacterium (Previously Propionibacterium) Acnes a Potential Pathogenic Factor in the Aetiology of the Skin Disease Progressive Macular Hypomelanosis? J. Eur. Acad. Dermatol. Venereol. 2021, 35, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Arshava, E.V.; Ford, B.; Nauseef, W.M. Don’t Let Its Name Fool You: Relapsing Thoracic Actinomycosis Caused by Pseudopropionibacterium Propionicum (Formerly Propionibacterium Propionicum). Am. J. Case Rep. 2019, 20, 1961–1965. [Google Scholar] [CrossRef] [PubMed]
- Clayton, J.J.; Baig, W.; Reynolds, G.W.; Sandoe, J.A.T. Endocarditis Caused by Propionibacterium Species: A Report of Three Cases and a Review of Clinical Features and Diagnostic Difficulties. J. Med. Microbiol. 2006, 55, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Dioguardi, M.; Alovisi, M.; Crincoli, V.; Aiuto, R.; Malagnino, G.; Quarta, C.; Laneve, E.; Sovereto, D.; Russo, L.L.; Troiano, G.; et al. Prevalence of the Genus Propionibacterium in Primary and Persistent Endodontic Lesions: A Systematic Review. J. Clin. Med. 2020, 9, 739. [Google Scholar] [CrossRef]
- Korona-Glowniak, I.; Piatek, D.; Fornal, E.; Lukowiak, A.; Gerasymchuk, Y.; Kedziora, A.; Bugla-Płoskonska, G.; Grywalska, E.; Bachanek, T.; Malm, A. Patterns of Oral Microbiota in Patients with Apical Periodontitis. J. Clin. Med. 2021, 10, 2707. [Google Scholar] [CrossRef]
- Elfil, M.; Bahbah, E.I.; Attia, M.M.; Eldokmak, M.; Koo, B.B. Impact of Obstructive Sleep Apnea on Cognitive and Motor Functions in Parkinson’s Disease. Mov. Disord. 2021, 36, 570–580. [Google Scholar] [CrossRef]
- Ferini-Strambi, L.; Hensley, M.; Salsone, M. Decoding Causal Links Between Sleep Apnea and Alzheimer’s Disease. J. Alzheimers. Dis. 2021, 80, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Liccardo, D.; Marzano, F.; Carraturo, F.; Guida, M.; Femminella, G.D.; Bencivenga, L.; Agrimi, J.; Addonizio, A.; Melino, I.; Valletta, A.; et al. Potential Bidirectional Relationship Between Periodontitis and Alzheimer’s Disease. Front. Physiol. 2020, 11, 683. [Google Scholar] [CrossRef]
- Manome, A.; Abiko, Y.; Kawashima, J.; Washio, J.; Fukumoto, S.; Takahashi, N. Acidogenic Potential of Oral Bifidobacterium and Its High Fluoride Tolerance. Front. Microbiol. 2019, 10, 1099. [Google Scholar] [CrossRef]
- Ventura, M.; Turroni, F.; Zomer, A.; Foroni, E.; Giubellini, V.; Bottacini, F.; Canchaya, C.; Claesson, M.J.; He, F.; Mantzourani, M.; et al. The Bifidobacterium Dentium Bd1 Genome Sequence Reflects Its Genetic Adaptation to the Human Oral Cavity. PLoS Genet. 2009, 5, e1000785. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, C.; Xu, S.; Xiang, C.; Wang, R.; Yang, D.; Lu, B.; Shi, L.; Tong, R.; Teng, Y.; et al. Fecal Microbiome Alteration May Be a Potential Marker for Gastric Cancer. Dis. Markers 2020, 2020, 3461315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, S.; Xu, H.; Yi, H.; Guan, J.; Yin, S. Metabolomics and Microbiome Profiling as Biomarkers in Obstructive Sleep Apnoea: A Comprehensive Review. Eur. Respir. Rev. 2021, 30, 200–220. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Nagasawa, T.; Uehara, O.; Shimizu, S.; Sugiyama, N.; Hasegawa-Nakamura, K.; Noguchi, K.; Hatae, M.; Kakinoki, H.; Furuichi, Y. Increase in Bifidobacterium Is a Characteristic of the Difference in the Salivary Microbiota of Pregnant and Non-Pregnant Women. BMC Oral Health 2022, 22, 260. [Google Scholar] [CrossRef] [PubMed]
- Galley, J.D.; Mashburn-Warren, L.; Blalock, L.C.; Lauber, C.L.; Carroll, J.E.; Ross, K.M.; Hobel, C.; Coussons-Read, M.; Dunkel Schetter, C.; Gur, T.L. Maternal Anxiety, Depression and Stress Affects Offspring Gut Microbiome Diversity and Bifidobacterial Abundances. Brain. Behav. Immun. 2022, 107, 253–264. [Google Scholar] [CrossRef]
- Yachida, S.; Mizutani, S.; Shiroma, H.; Shiba, S.; Nakajima, T.; Sakamoto, T.; Watanabe, H.; Masuda, K.; Nishimoto, Y.; Kubo, M.; et al. Metagenomic and Metabolomic Analyses Reveal Distinct Stage-Specific Phenotypes of the Gut Microbiota in Colorectal Cancer. Nat. Med. 2019, 25, 968–976. [Google Scholar] [CrossRef]
- Chen, J.; Wu, X.; Zhu, D.; Xu, M.; Yu, Y.; Yu, L.; Zhang, W. Microbiota in Human Periodontal Abscess Revealed by 16S RDNA Sequencing. Front. Microbiol. 2019, 10, 1723. [Google Scholar] [CrossRef]
- Oshima, K.; Hayashi, J.I.; Toh, H.; Nakano, A.; Omori, E.; Hattori, Y.; Morita, H.; Honda, K.; Hattori, M. Complete Genome Sequence of Scardovia Inopinata JCM 12537T, Isolated from Human Dental Caries. Genome Announc. 2015, 3, 5–6. [Google Scholar] [CrossRef]
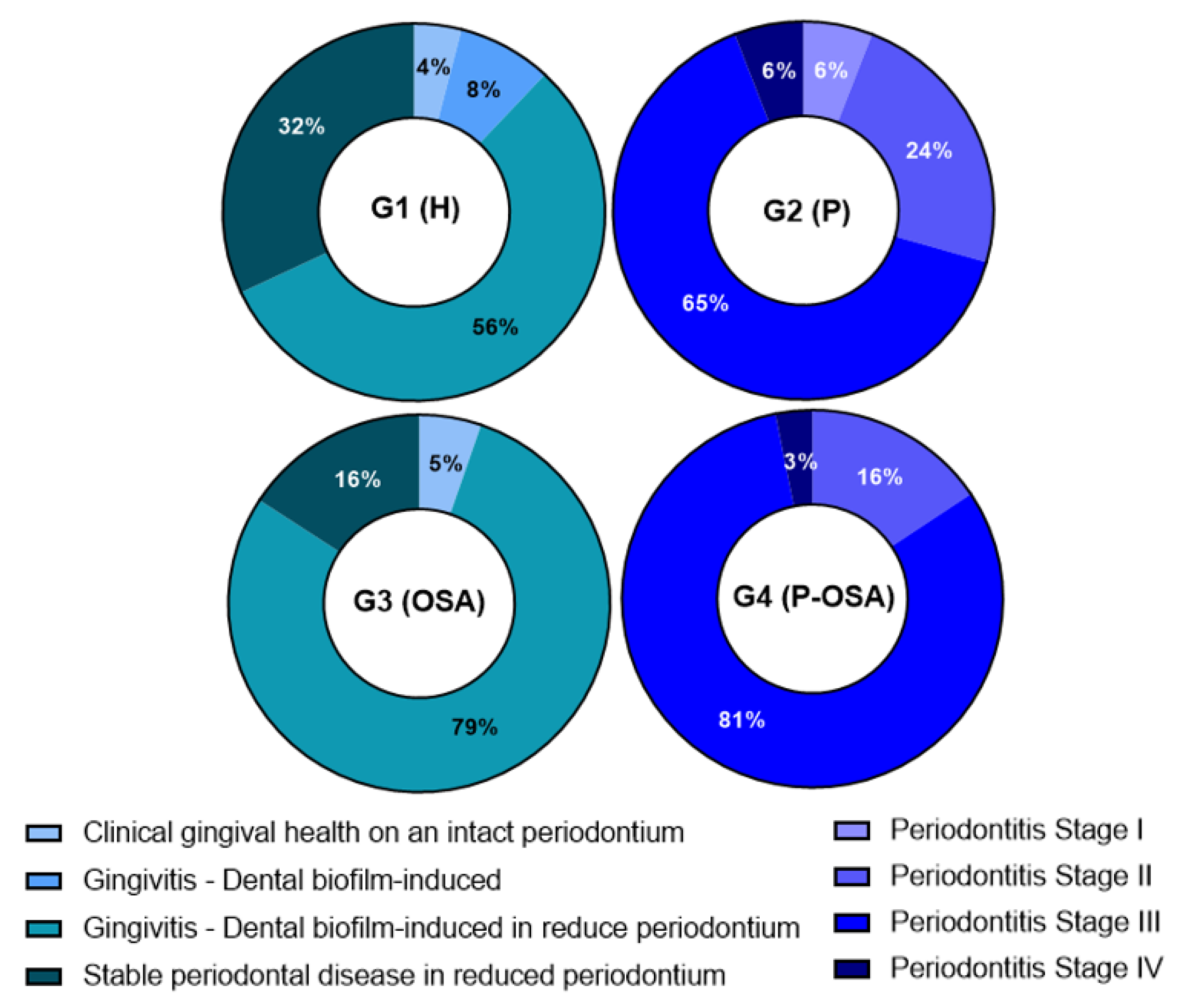
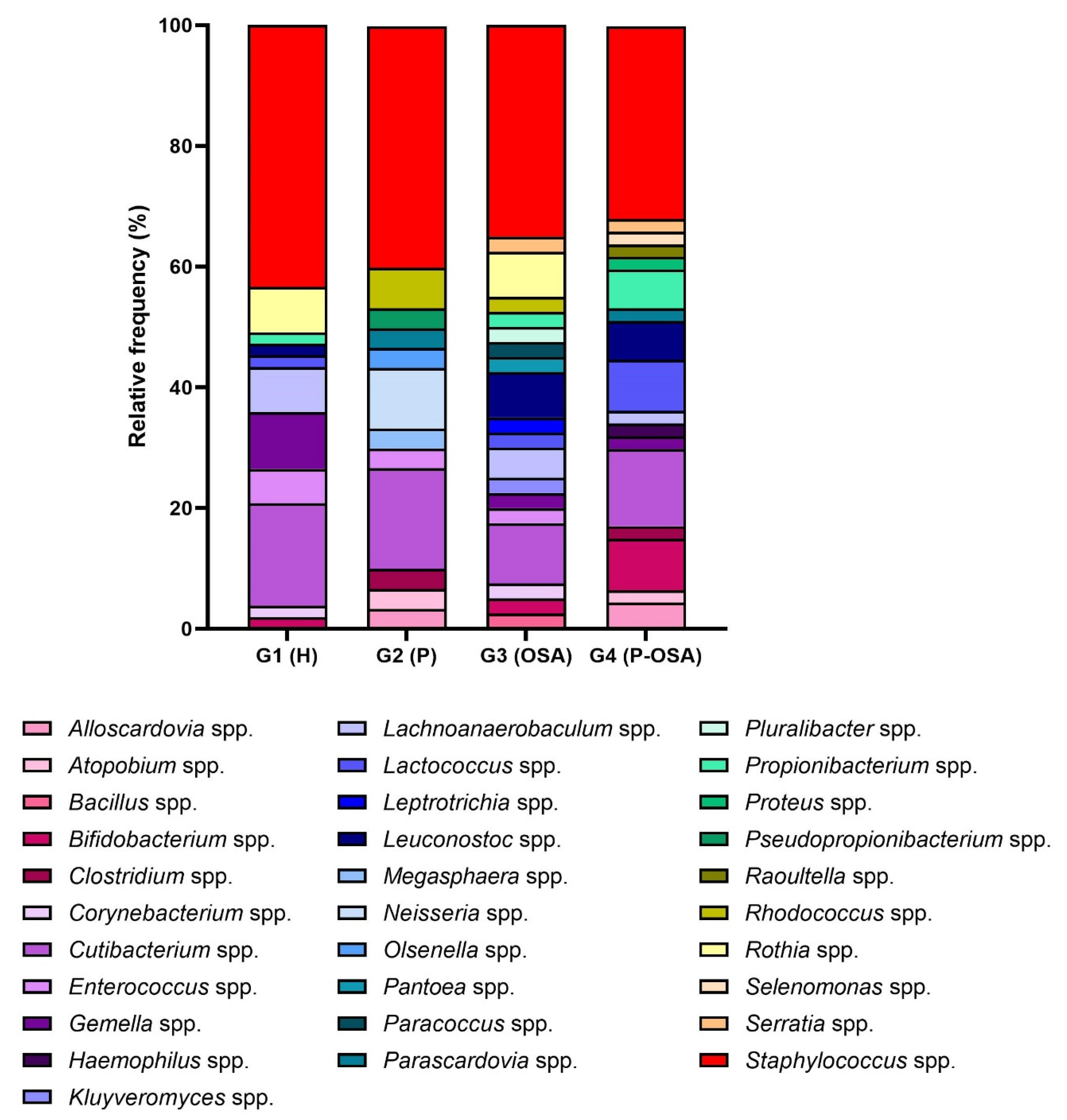


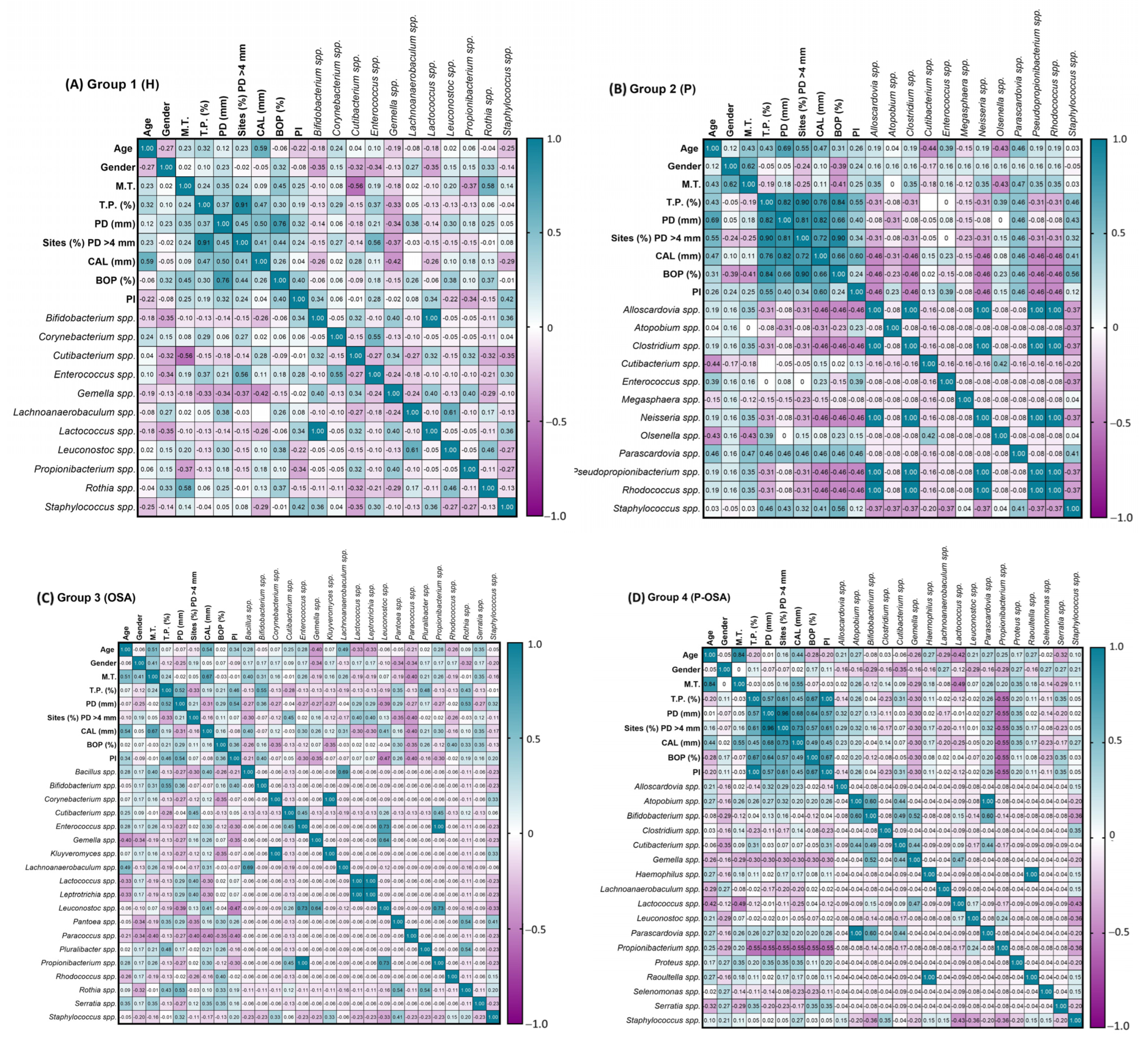
| Clinical Variable | Group 1 (H) (n = 20) | Group 2 (P) (n = 13) | Group 3 (OSA) (n = 18) | Group 4 (P-OSA) (n = 24) |
|---|---|---|---|---|
| Age (years) | 44.35 ± 14.24 | 40.69 ± 10.83 | 50.35 ± 13.09 | 49.33 ± 11.65 |
| Gender (Males) (%) | 32 | 41 | 37 | 69 |
| Missing teeth | 6.21 ± 4.42 | 5.69 ± 2.25 | 8.53 ± 6.53 | 7.08 ± 6.23 |
| Teeth with periodontitis (%) | 2.18 ± 3.99 | 47.91 ± 24.72 * | 1.37 ± 2.98 » | 39.16 ± 19.39 *,§ |
| PD (mm) | 1.81 ± 0.48 | 2.64 ± 0.45 | 2.36 ± 4.82 | 13.83 ± 9.95 |
| Sites (%) PD ≥ 4 mm | 0.29 ± 0.50 | 16.76 ± 10.62 | 2.01 ± 0.15 | 2.64 ± 0.41 |
| CAL (mm) | 1.33 ± 0.78 | 2.15 ± 1.01 | 1.52 ± 0.98 | 2.31 ± 1.14 |
| BOP (%) | 11.59 ± 11.46 | 49.19 ± 26.92 * | 24.02 ± 22.96 » | 48.67 ± 26.69 *,§ |
| PI | 19.30 ± 11.48 | 47.21 ± 26.28 * | 39.65 ± 21.43 * | 39.16 ± 19.39 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Téllez Corral, M.A.; Herrera Daza, E.; Cuervo Jimenez, H.K.; Bravo Becerra, M.d.M.; Villamil, J.C.; Hidalgo Martinez, P.; Roa Molina, N.S.; Otero, L.; Cortés, M.E.; Parra Giraldo, C.M. Cryptic Oral Microbiota: What Is Its Role as Obstructive Sleep Apnea-Related Periodontal Pathogens? Int. J. Environ. Res. Public Health 2023, 20, 1740. https://doi.org/10.3390/ijerph20031740
Téllez Corral MA, Herrera Daza E, Cuervo Jimenez HK, Bravo Becerra MdM, Villamil JC, Hidalgo Martinez P, Roa Molina NS, Otero L, Cortés ME, Parra Giraldo CM. Cryptic Oral Microbiota: What Is Its Role as Obstructive Sleep Apnea-Related Periodontal Pathogens? International Journal of Environmental Research and Public Health. 2023; 20(3):1740. https://doi.org/10.3390/ijerph20031740
Chicago/Turabian StyleTéllez Corral, Mayra A., Eddy Herrera Daza, Hayde K. Cuervo Jimenez, María del Mar Bravo Becerra, Jean Carlos Villamil, Patricia Hidalgo Martinez, Nelly S. Roa Molina, Liliana Otero, María E. Cortés, and Claudia M. Parra Giraldo. 2023. "Cryptic Oral Microbiota: What Is Its Role as Obstructive Sleep Apnea-Related Periodontal Pathogens?" International Journal of Environmental Research and Public Health 20, no. 3: 1740. https://doi.org/10.3390/ijerph20031740
APA StyleTéllez Corral, M. A., Herrera Daza, E., Cuervo Jimenez, H. K., Bravo Becerra, M. d. M., Villamil, J. C., Hidalgo Martinez, P., Roa Molina, N. S., Otero, L., Cortés, M. E., & Parra Giraldo, C. M. (2023). Cryptic Oral Microbiota: What Is Its Role as Obstructive Sleep Apnea-Related Periodontal Pathogens? International Journal of Environmental Research and Public Health, 20(3), 1740. https://doi.org/10.3390/ijerph20031740