Effect of Vitamin D in Long COVID Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Setting and Participants
2.2. Data Collection
2.3. Data Analyses
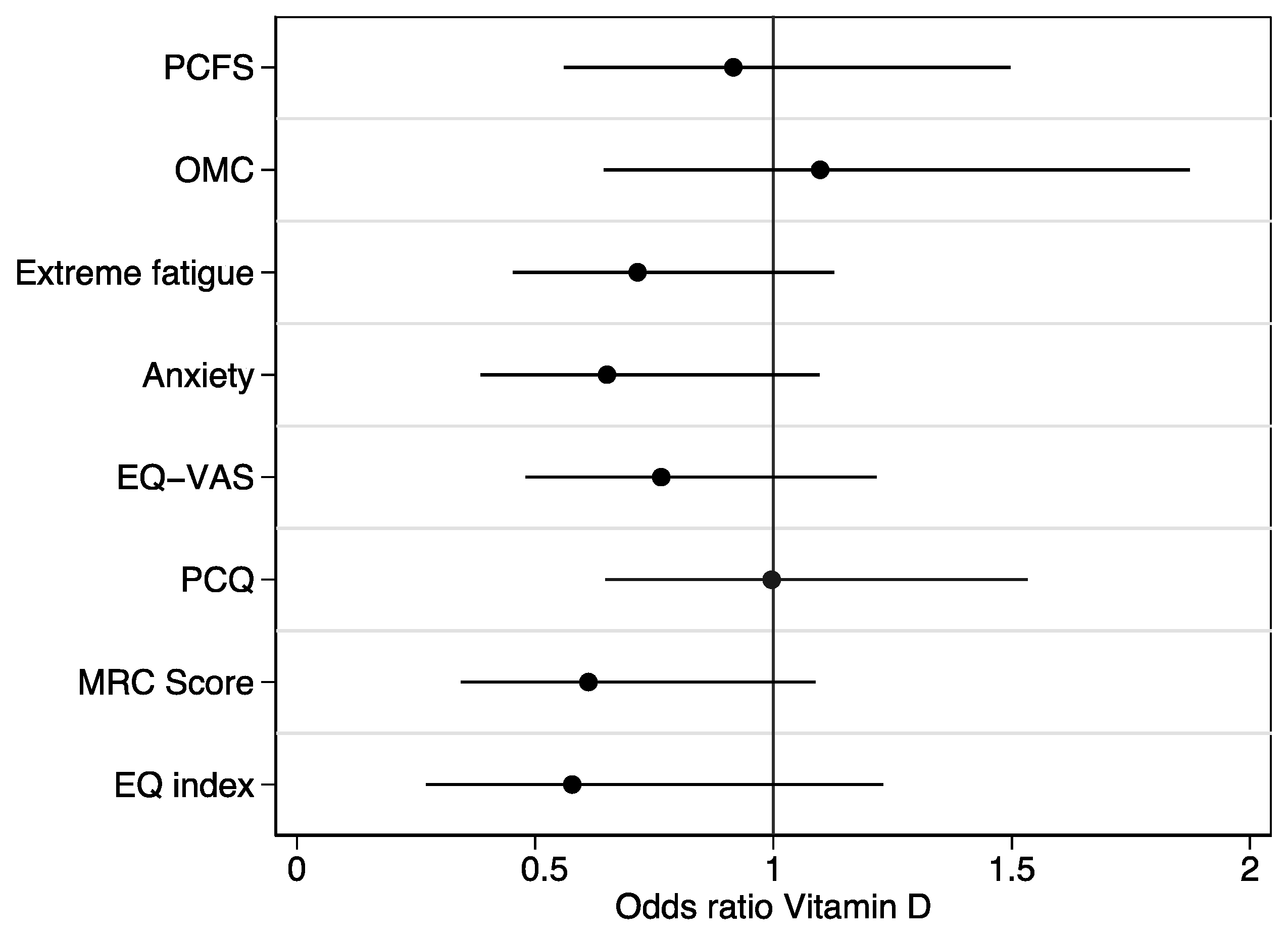
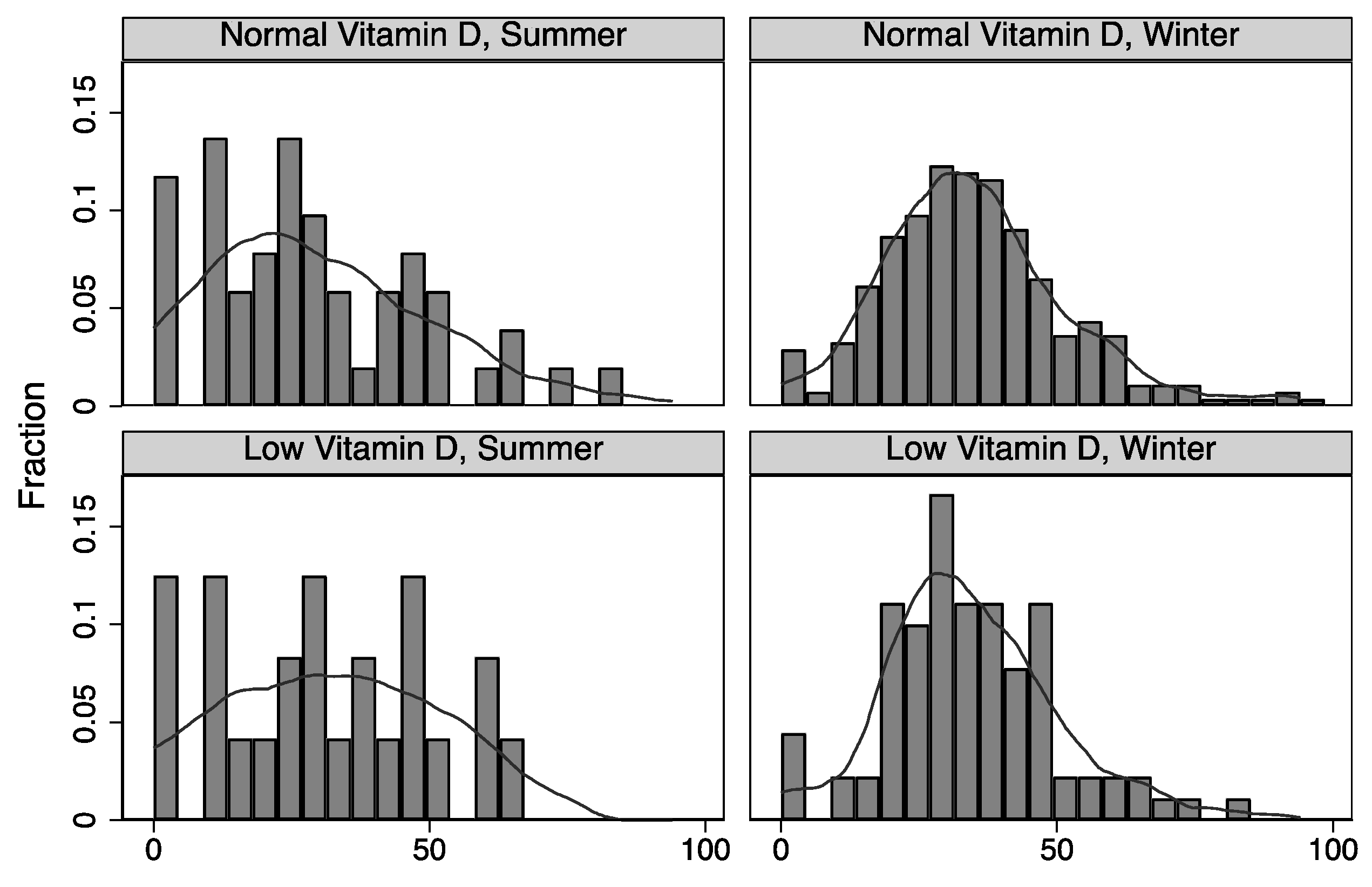
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mølhave, M.; Agergaard, J.; Wejse, C. Clinical Management of COVID-19 Patients—An Update. Semin. Nucl. Med. 2022, 52, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Jamie Gumbrecht, J.H.; Deidre McPhillips, C.N.N. WHO Says COVID-19 Is No Longer a Global Health Emergency. Available online: https://edition.cnn.com/2023/05/05/health/who-ends-covid-health-emergency/index.html (accessed on 22 May 2023).
- Ballering, A.V.; Van Zon, S.K.R.; Olde Hartman, T.C.; Rosmalen, J.G.M. Persistence of somatic symptoms after COVID-19 in the Netherlands: An observational cohort study. Lancet 2022, 400, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Wulf Hanson, S.; Abbafati, C.; Aerts, J.G.; Al-Aly, Z.; Ashbaugh, C.; Ballouz, T.; Blyuss, O.; Bobkova, P.; Bonsel, G.; Borzakova, S.; et al. Estimated Global Proportions of Individuals with Persistent Fatigue, Cognitive, and Respiratory Symptom Clusters Following Symptomatic COVID-19 in 2020 and 2021. JAMA 2022, 328, 1604. [Google Scholar] [CrossRef]
- Nikolich, J.Ž.; Rosen, C.J. Toward Comprehensive Care for Long Covid. N. Engl. J. Med. 2023, 388, 2113–2115. [Google Scholar] [CrossRef] [PubMed]
- Blomberg, B.; Mohn, K.G.; Brokstad, K.A.; Zhou, F.; Linchausen, D.W.; Hansen, B.A.; Lartey, S.; Onyango, T.B.; Kuwelker, K.; Saevik, M.; et al. Long COVID in a prospective cohort of home-isolated patients. Nat. Med. 2021, 27, 1607–1613. [Google Scholar] [CrossRef]
- O’Mahoney, L.L.; Routen, A.; Gillies, C.; Ekezie, W.; Welford, A.; Zhang, A.; Karamchandani, U.; Simms-Williams, N.; Cassambai, S.; Ardavani, A.; et al. The prevalence and long-term health effects of Long Covid among hospitalised and non-hospitalised populations: A systematic review and meta-analysis. eClinicalMedicine 2023, 55, 101762. [Google Scholar] [CrossRef]
- Swank, Z.; Senussi, Y.; Manickas-Hill, Z.; Yu, X.G.; Li, J.Z.; Alter, G.; Walt, D.R. Persistent Circulating Severe Acute Respiratory Syndrome Coronavirus 2 Spike Is Associated With Post-acute Coronavirus Disease 2019 Sequelae. Clin. Infect. Dis. 2022, 76, e487–e490. [Google Scholar] [CrossRef]
- Østergaard, L. SARS CoV-2 related microvascular damage and symptoms during and after COVID-19: Consequences of capillary transit-time changes, tissue hypoxia and inflammation. Physiol. Rep. 2021, 9, e14726. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef]
- Nunez-Castilla, J.; Stebliankin, V.; Baral, P.; Balbin, C.A.; Sobhan, M.; Cickovski, T.; Mondal, A.M.; Narasimhan, G.; Chapagain, P.; Mathee, K.; et al. Potential Autoimmunity Resulting from Molecular Mimicry between SARS-CoV-2 Spike and Human Proteins. Viruses 2022, 14, 1415. [Google Scholar] [CrossRef]
- Agergaard, J.; Leth, S.; Pedersen, T.H.; Harbo, T.; Blicher, J.U.; Karlsson, P.; Østergaard, L.; Andersen, H.; Tankisi, H. Myopathic changes in patients with long-term fatigue after COVID-19. Clin. Neurophysiol. 2021, 132, 1974–1981. [Google Scholar] [CrossRef] [PubMed]
- Hejbøl, E.K.; Harbo, T.; Agergaard, J.; Madsen, L.B.; Pedersen, T.H.; Østergaard, L.J.; Andersen, H.; Schrøder, H.D.; Tankisi, H. Myopathy as a cause of fatigue in long-term post-COVID-19 symptoms: Evidence of skeletal muscle histopathology. Eur. J. Neurol. 2022, 29, 2832–2841. [Google Scholar] [CrossRef]
- Agergaard, J.; Yamin Ali Khan, B.; Engell-Sørensen, T.; Schiøttz-Christensen, B.; Østergaard, L.; Hejbøl, E.K.; Schrøder, H.D.; Andersen, H.; Blicher, J.U.; Holm Pedersen, T.; et al. Myopathy as a cause of Long COVID fatigue: Evidence from quantitative and single fiber EMG and muscle histopathology. Clin. Neurophysiol. 2023, 148, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Athanassiou, L.; Mavragani, C.P.; Koutsilieris, M. The Immunomodulatory Properties of Vitamin D. Mediterr. J. Rheumatol. 2022, 33, 7. [Google Scholar] [CrossRef]
- Kim, D.-H.; Meza, C.A.; Clarke, H.; Kim, J.-S.; Hickner, R.C. Vitamin D and Endothelial Function. Nutrients 2020, 12, 575. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef]
- Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef]
- Oscanoa, T.J.; Amado, J.; Vidal, X.; Laird, E.; Ghashut, R.A.; Romero-Ortuno, R. The relationship between the severity and mortality of SARS-CoV-2 infection and 25-hydroxyvitamin D concentration—A metaanalysis. Adv. Respir. Med. 2021, 89, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Razdan, K.; Singh, K.; Singh, D. Vitamin D Levels and COVID-19 Susceptibility: Is there any Correlation? Med. Drug Discov. 2020, 7, 100051. [Google Scholar] [CrossRef]
- Barrea, L.; Verde, L.; Grant, W.B.; Frias-Toral, E.; Sarno, G.; Vetrani, C.; Ceriani, F.; Garcia-Velasquez, E.; Contreras-Briceno, J.; Savastano, S.; et al. Vitamin D: A Role Also in Long COVID-19? Nutrients 2022, 14, 1625. [Google Scholar] [CrossRef]
- Nielsen, N.M.; Junker, T.G.; Boelt, S.G.; Cohen, A.S.; Munger, K.L.; Stenager, E.; Ascherio, A.; Boding, L.; Hviid, A. Vitamin D status and severity of COVID-19. Sci. Rep. 2022, 12, 19823. [Google Scholar] [CrossRef]
- Konikowska, K.; Kiliś-Pstrusińska, K.; Matera-Witkiewicz, A.; Kujawa, K.; Adamik, B.; Doroszko, A.; Kaliszewski, K.; Pomorski, M.; Protasiewicz, M.; Sokołowski, J.; et al. Association of serum vitamin D concentration with the final course of hospitalization in patients with COVID-19. Front. Immunol. 2023, 14, 1231813. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Fogacci, F.; Borghi, C. Vitamin D Supplementation and COVID-19 Outcomes: Mounting Evidence and Fewer Doubts. Nutrients 2022, 14, 3584. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Li, X.; Liu, W.; Xiao, Y.; Tang, H.; Wu, Y.; Xiong, Y.; Gao, S. The Role of Vitamin D in the Prevention and Treatment of SARS-CoV-2 Infection: A Meta-analysis of Randomized Controlled Trials. Clin. Nutr. 2023, 2, 2198–2206. [Google Scholar] [CrossRef]
- Agergaard, J.; Ullahammer, W.M.; Gunst, J.D.; Østergaard, L.; Schiøttz-Christensen, B. Characteristics of a Danish Post-COVID Cohort Referred for Examination due to Persistent Symptoms Six Months after Mild Acute COVID-19. J. Clin. Med. 2022, 11, 7338. [Google Scholar] [CrossRef] [PubMed]
- Sundhedsstyrrelsen. Senfølger efter COVID-19: Anbefalinger. Available online: https://www.sst.dk/da/Udgivelser/2020/Senfoelger-efter-COVID-19 (accessed on 8 June 2022).
- World Population Review. Western Countries 2023. Available online: https://worldpopulationreview.com/country-rankings/western-countries (accessed on 27 September 2023).
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Tmava Berisha, A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef]
- Casseb, G.A.S.; Kaster, M.P.; Rodrigues, A.L.S. Potential Role of Vitamin D for the Management of Depression and Anxiety. CNS Drugs 2019, 33, 619–637. [Google Scholar] [CrossRef] [PubMed]
- Hansen, L.; Tjønneland, A.; Køster, B.; Brot, C.; Andersen, R.; Cohen, A.S.; Frederiksen, K.; Olsen, A. Vitamin D Status and Seasonal Variation among Danish Children and Adults: A Descriptive Study. Nutrients 2018, 10, 1801. [Google Scholar] [CrossRef]
- Mejborn, H.; Andersen, R.; Bredsdorff, L.; Brot, C.; Jakobsen, J.; Krogholm, K.S.; Mosekilde, L.; Mølgaard, C.; Olsen, A.; Rejnmark, L.; et al. D-Vitamin. Opdatering af Videnskabelig Evidens for Sygdomsforebyggelse Og Anbefalinger; National Food Institute: Doncaster, Australia, 2010. [Google Scholar]
- Madsen, K.H.; Rasmussen, L.B.; Mejborn, H.; Andersen, E.W.; Mølgaard, C.; Nissen, J.; Tetens, I.; Andersen, R. Vitamin D status and its determinants in children and adults among families in late summer in Denmark. Br. J. Nutr. 2014, 112, 776–784. [Google Scholar] [CrossRef]
- Madsen, K.H.; Rasmussen, L.B.; Andersen, R.; Mølgaard, C.; Jakobsen, J.; Bjerrum, P.J.; Andersen, E.W.; Mejborn, H.; Tetens, I. Randomized controlled trial of the effects of vitamin D–fortified milk and bread on serum 25-hydroxyvitamin D concentrations in families in Denmark during winter: The VitmaD study. Am. J. Clin. Nutr. 2013, 98, 374–382. [Google Scholar] [CrossRef]
- Mohamed Hussein, A.A.R.; Galal, I.; Amin, M.T.; Moshnib, A.A.; Makhlouf, N.A.; Makhlouf, H.A.; Abd-Elaal, H.K.; Kholief, K.M.S.; Abdel Tawab, D.A.; Kamal Eldin, K.A.; et al. Prevalence of vitamin D deficiency among patients attending Post COVID-19 follow-up clinic: A cross-sectional study. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 3038–3045. [Google Scholar] [CrossRef]
- Botros, R.M.; Sabry, I.M.; Abdelbaky, R.S.; Eid, Y.M.; Nasr, M.S.; Hendawy, L.M. Vitamin D deficiency among healthy Egyptian females. Endocrinol. Nutr. 2015, 62, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Galal, I.; Hussein, A.A.R.M.; Amin, M.T.; Saad, M.M.; Zayan, H.E.E.; Abdelsayed, M.Z.; Moustafa, M.M.; Ezzat, A.R.; Helmy, R.E.D.; Abd_Elaal, H.K.; et al. Determinants of persistent post-COVID-19 symptoms: Value of a novel COVID-19 symptom score. Egypt. J. Bronchol. 2021, 15, 1–8. [Google Scholar] [CrossRef]
- di Filippo, L.; Frara, S.; Nannipieri, F.; Cotellessa, A.; Locatelli, M.; Rovere Querini, P.; Giustina, A. Low vitamin D levels are associated with Long COVID syndrome in COVID-19 survivors. J. Clin. Endocrinol. Metab. 2023, 108, e1106–e1116. [Google Scholar] [CrossRef]
- Goodwill, A.M.; Szoeke, C. A Systematic Review and Meta-Analysis of The Effect of Low Vitamin D on Cognition. J. Am. Geriatr. Soc. 2017, 65, 2161–2168. [Google Scholar] [CrossRef]
- Shea, M.K.; Barger, K.; Dawson-Hughes, B.; Leurgans, S.E.; Fu, X.; James, B.D.; Holland, T.M.; Agarwal, P.; Wang, J.; Matuszek, G.; et al. Brain vitamin D forms, cognitive decline, and neuropathology in community-dwelling older adults. Alzheimer’s Dement. 2022, 19, 2389–2396. [Google Scholar] [CrossRef]
- Andersen, R.; Mølgaard, C.; Skovgaard, L.T.; Brot, C.; Cashman, K.D.; Jakobsen, J.; Lamberg-Allardt, C.; Ovesen, L. Pakistani immigrant children and adults in Denmark have severely low vitamin D status. Eur. J. Clin. Nutr. 2008, 62, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.H.; Kruse, A.; Frederiksen, H.W.; Ahmadi, A.; Norredam, M. Health status of refugees newly resettled in Denmark. Dan. Med. J. 2020, 67, A08200567. [Google Scholar] [PubMed]
- Statens Serum Institut. Status på COVID-19-Smitte Blandt Etniske Minoriteter I Danmark. Available online: https://www.ssi.dk/aktuelt/nyheder/2020/status-pa-covid-19-smitte-blandt-etniske-minoriteter-i-danmark (accessed on 27 September 2023).

|
Low Vitamin D 26% n = 115/442 |
Normal Vitamin D 74% n = 327/442 |
Unadjusted OR Low Vit D vs. Normal Vit D (95% CI) | |
|---|---|---|---|
| Sex (male) | 37% (42/114) | 25% (81/327) | 1.77 (1.12–2.79) |
| Age, mean (SD) | 41 (12) years | 48 (13) years | 0.96 (0.94–0.97) * |
| Other ethnic origin than Danish | 10% (11/114) | 7% (24/327) | 1.35 (0.64–2.85) |
| Non-western European ethnic origin | 5% (6/114) | 4% (14/327) | 1.24 (0.47–3.31) |
| Familiar dispositions | 34% (39/114) | 44% (145/327) | 0.65 (0.42–1.02) |
| Autoimmunity | 8% (9/114) | 13% (44/327) | 0.55 (0.26–1.17) |
| Metabolic | 21% (24/114) | 25% (81/327) | 0.81 (0.48–1.36) |
| Allergy | 13% (15/114) | 18% (58/327) | 0.70 (0.38–1.30) |
| Transmission of SARS-CoV-2 | |||
| Work | 40% (46/114) | 37% (122/327) | 1.14 (0.73–1.76) |
| Family | 29% (33/114) | 25% (83/327) | 1.20 (0.74–1.93) |
| Travel | <1% | 4% (14/327) | 0.40 (0.09–1.78) |
| Unknown | 18% (21/114) | 18% (60/327) | 1.00 (0.58–1.74) |
| Other | 14% (16/114) | 14% (47/327) | 0.97 (0.53–1.80) |
| Time from symptom onset to baseline, mean (SD), months | 6.9 (3.4) | 7.7 (3.8) | 1.0 (0.996–1.00) |
| SARS-CoV-2 infection (winter) | 77% (84/109) | 81% (227/282) | 0.81 (0.48–1.39) |
| No positive PCR or AB test | <1% | 3% (10/327) | 0.28 (0.04–2.22) |
| Hospitalized 1 | 11% (13/114) | 12% (40/327) | 0.92 (0.47–1.80) |
| Oxygen supplementation | 63% (5/8) | 56% (10/18) | 1.33 (0.24–7.35) |
| Charlson comorbidity index | 0.47 (0.34–0.66) * | ||
| 0 | 77% (88/114) | 50% (163/327) | |
| ≥1 | 23% (26/114) | 50% (164/327) | |
| Diabetes | <1% | 2% (5/327) | 1.74 (0.41–7.40) |
| Asthma | 14% (16/114) | 15% (48/327) | 0.95 (0.52–1.75) |
| COPD | <1% | 2% (7/327) | 0.40 (0.05–3.32) |
| CHD | <1% | <1% | 1.93 (0.32–11.69) |
| CVD | <1% | <1% | 1 |
| Hypertension | 12% (14/114) | 10% (34/327) | 1.21 (0.62–2.34) |
| Malignancy (previous) | <1% | 4% (14/327) | 0.20 (0.02–1.52) |
| Connective tissue disease | <1% | 2% (5/327) | 1 |
| Immunodeficiency | <1% | <1% | 2.88 (0.18–46.5) |
| Previous depression | 9% (10/114) | 14% (47/327) | 0.57 (0.28–1.18) |
| Previous stressful episode | 11% (12/114) | 13% (43/327) | 0.78 (0.40–1.53) |
| Medicine | |||
| ACE or AT2 rec inhibitor | 12% (13/111) | 11% (35/318) | 1.07 (0.55–2.11) |
| Statins | 8% (9/111) | 11% (36/317) | 0.69 (0.32–1.48) |
| Steroids | <1% | 2% (5/316) | 1 |
| Other immunosuppressant | <1% | 3% (10/316) | 1 |
| Current smoker | 12% (13/107) | 6% (17/308) | 2.37 (1.11–5.06) |
| Alcohol, >7 per week | 9% (10/107) | 4% (12/301) | 2.48 (1.04–5.93) |
| BMI ≥ 25 | 66% (73/111) | 63% (200/316) | 1.11 (0.71–1.75) |
| Socioeconomic status | |||
| Education | |||
| Primary school | 12% (14/114) | 8% (26/327) | 1.62 (0.81–3.22) |
| High school | 16% (18/114) | 15% (48/327) | 1.09 (0.60–1.96) |
| Vocational education | 31% (35/114) | 22% (73/327) | 1.54 (0.96–2.48) |
| Medium-term higher education | 32% (36/114) | 44% (143/327) | 0.59 (0.38–0.93) |
| Long-term higher education | 11% (12/114) | 9% (31/327) | 1.12 (0.56–2.27) |
| Master/PhD | <1% | 3% (11/327) | 0.51 (0.11–2.35) |
| Work | |||
| Employed | 82% (93/114) | 81% (264/327) | 1.06 (0.61–1.83) |
| Self employed | 4% (5/114) | 6% (19/327) | 0.74 (0.27–2.04) |
| Student | 9% (10/114) | 4% (12/327) | 2.52 (1.06–6.01) |
| Retired | <1% | 7% (22/327) | 0.50 (0.17–1.50) |
| Not living alone | 90% (84/93) | 80% (220/275) | 1.17 (0.99–1.40) |
| Size of housing, mean (SD) m2 | 146 (67) | 142 (62) | 1.00 (0.997–1.005) |
|
Long COVID Symptoms | Some to Very Much | Odds Ratio (OR) | ||
|---|---|---|---|---|
| During 4 Weeks Previous to Evaluation in a Post-COVID Clinic | Low Vitamin D n = 115/442 | Normal Vitamin D n = 327/442 | Unadjusted OR Low Vit D vs. Normal Vit D | Adjusted 1 OR Low Vit D vs. Normal Vit D |
| Headaches | 62% (66/106) | 62% (196/314) | 0.99 (0.63–1.56) | 0.86 (0.51–1.43) |
| Dizziness | 38% (40/106) | 37% (116/313) | 1.03 (0.65–1.62) | 0.89 (0.54–1.47) |
| Paresthesia | 27% (29/106) | 27% (82/308) | 1.04 (0.63–1.70) | 0.94 (0.55–1.60) |
| Concentration difficulties | 77% (79/102) | 83% (257/308) | 0.68 (0.39–1.19) | 0.60 (0.33–1.09) |
| Short-term memory problems | 59% (63/106) | 65% (205/313) | 0.77 (0.49–1.21) | 0.68 (0.42–1.11) |
| Long-term memory problems | 32% (34/107) | 46% (145/313) | 0.53 (0.33–0.84) | 0.46 (0.28–0.77) |
| Impaired smell | 36% (38/106) | 38% (118/314) | 0.93 (0.59–1.47) | 0.98 (0.60–1.59) |
| Impaired taste | 35% (37/106) | 34% (106/312) | 1.04 (0.66–1.66) | 1.05 (0.64–1.73) |
| Runny nose or nasal congestion | 23% (24/104) | 23% (71/311) | 1.01 (0.60–1.72) | 0.81 (0.45–1.44) |
| Sore throat | 10% (10/105) | 16% (50/308) | 0.54 (0.26–1.11) | 0.39 (0.17–0.89) |
| Cough | 20% (21/105) | 16% (50/307) | 1.29 (0.73–2.26) | 1.14 (0.61–2.11) |
| Expectoration | 14% (15/106) | 11% (34/311) | 1.34 (0.70–2.58) | 0.98 (0.47–2.05) |
| Dyspnea at rest | 31% (33/106) | 29% (90/314) | 1.13 (0.70–1.82) | 1.01 (0.60–1.69) |
| Dyspnea at physical activity | 65% (70/107) | 60% (189/315) | 1.26 (0.80–1.99) | 1.06 (0.65–1.74) |
| Chest pain | 32% (34/107) | 26% (82/312) | 1.31 (0.81–2.11) | 1.02 (0.61–1.72) |
| Palpitations | 36% (39/107) | 28% (86/312) | 1.51 (0.95–2.40) | 1.17 (0.70–1.95) |
| Loss of appetite | 23% (23/98) | 16% (47/288) | 1.57 (0.90–2.76) | 1.09 (0.58–3.8) |
| Nausea | 12% (13/107) | 19% (58/311) | 0.60 (0.32–1.15) | 0.47 (0.23–0.95) |
| Diarrhea | 12% (13/105) | 9% (27/308) | 1.47 (0.73–2.97) | 1.10 (0.51–2.37) |
| Abdominal pain | 10% (10/105) | 15% (45/309) | 0.62 (0.30–1.27) | 0.44 (0.20–0.98) |
| Altered bowel habits | 26% (27/102) | 28% (86/306) | 0.92 (0.56–1.53) | 0.85 (0.49–1.46) |
| Skin rash | 11% (11/104) | 7% (22/301) | 1.50 (0.70–3.21) | 1.39 (0.62–3.13) |
| Itching skin | 14% (15/105) | 15% (47/307) | 0.92 (0.49–1.73) | 0.85 (0.43–1.67) |
| Joint pain | 38% (41/107) | 41% (126/304) | 0.88 (0.56–1.40) | 0.99 (0.61–1.61) |
| Swollen joints | 17% (17/103) | 7% (22/301) | 2.51 (1.27–4.94) | 2.86 (1.36–6.01) |
| Myalgia | 48% (50/105) | 49% (150/308) | 0.96 (0.61–1.49) | 1.05 (0.65–1.69) |
| Muscle exhaustion | 58% (58/100) | 54% (164/302) | 1.17 (0.74–1.85) | 1.14 (0.70–1.86) |
| Physical fatigue | 86% (92/107) | 81% (250/310) | 1.47 (0.80–2.72) | 1.39 (0.72–2.69) |
| Subjective fever | 8% (8/95) | 14% (39/276) | 0.56 (0.25–1.24) | 0.35 (0.14–0.89) |
| Disturbed sleep | 89% (94/106) | 83% (261/314) | 1.59 (0.81–3.11) | 1.39 (0.69–2.80) |
| Problems falling asleep | 37% (40/107) | 42% (132/315) | 0.83 (0.53–1.30) | 0.66 (0.40–1.08) |
| Awakening | 53% (56/106) | 58% (179/311) | 0.84 (0.54–1.31) | 0.85 (0.53–1.39) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hikmet, R.G.; Wejse, C.; Agergaard, J. Effect of Vitamin D in Long COVID Patients. Int. J. Environ. Res. Public Health 2023, 20, 7058. https://doi.org/10.3390/ijerph20227058
Hikmet RG, Wejse C, Agergaard J. Effect of Vitamin D in Long COVID Patients. International Journal of Environmental Research and Public Health. 2023; 20(22):7058. https://doi.org/10.3390/ijerph20227058
Chicago/Turabian StyleHikmet, Ramsen Ghasan, Christian Wejse, and Jane Agergaard. 2023. "Effect of Vitamin D in Long COVID Patients" International Journal of Environmental Research and Public Health 20, no. 22: 7058. https://doi.org/10.3390/ijerph20227058
APA StyleHikmet, R. G., Wejse, C., & Agergaard, J. (2023). Effect of Vitamin D in Long COVID Patients. International Journal of Environmental Research and Public Health, 20(22), 7058. https://doi.org/10.3390/ijerph20227058







