Effects of 12 Weeks of Family and Individual Multi-Disciplinary Intervention in Overweight and Obese Adolescents under Cardiometabolic Risk Parameters: A Clinical Trial
Abstract
:1. Introduction
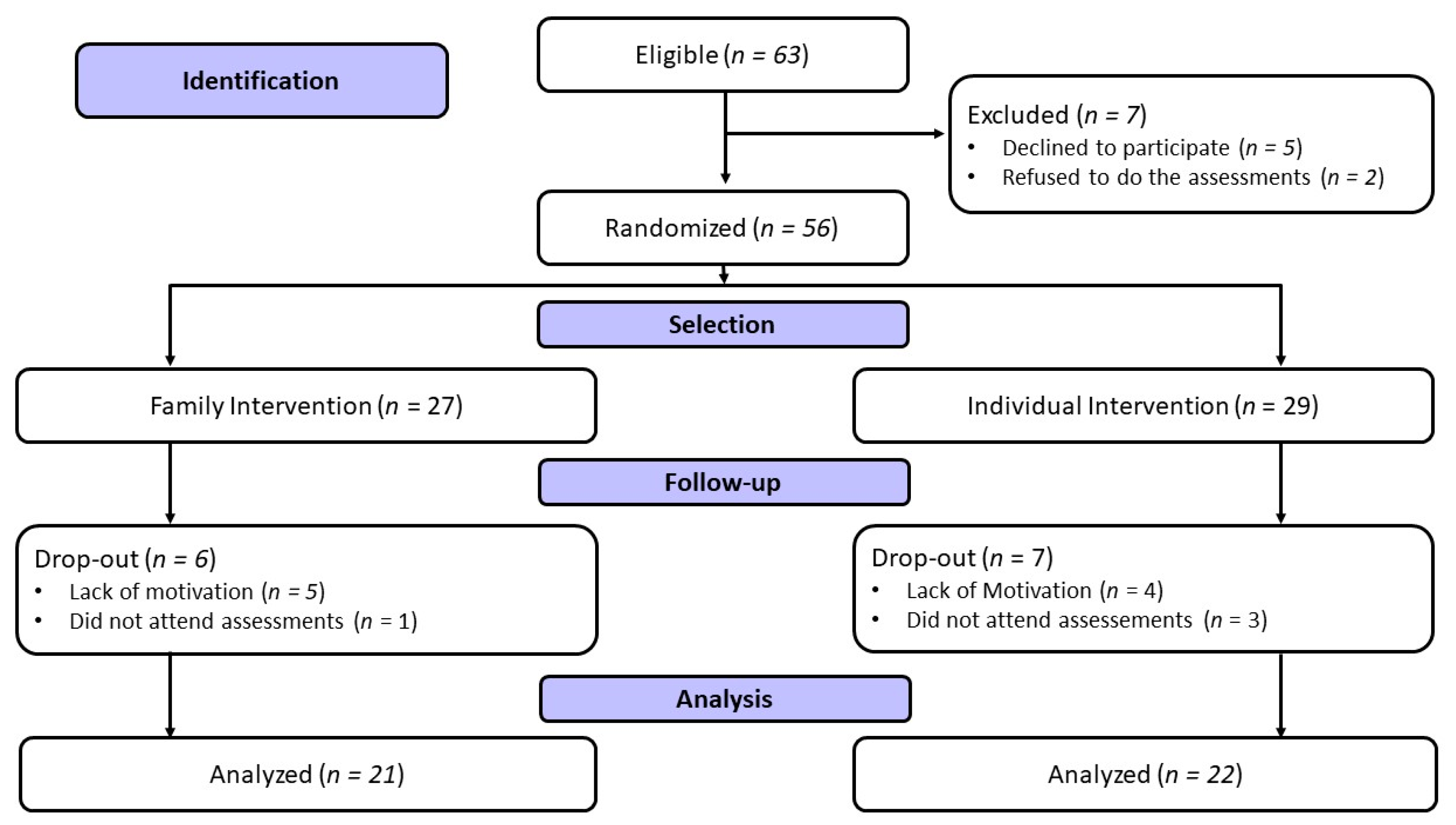
2. Materials and Methods
- (i)
- The interpretation of the body composition assessment in the electrical bioimpedance: to explain how to read and interpret the result of this test. The adolescents were also encouraged to look at body weight and the quality of body composition, such as FM, BF, SMM, FFM, and visceral fat.
- (ii)
- Pre- and post-workout eating: to demonstrate the importance of diet and its relationship with exercise, examples of foods that can help, the quantities needed, and responses time after consumption were considered.
- (iii)
- Healthy eating: the test discusses the food builders, regulators, and energy of different foods; their due quantities; and their position in the food pyramid. Finally, the researchers explained how to assemble a healthy dish in this class.
- (iv)
- Healthy dish: encouraging adolescents to assemble it and explaining the importance of assembling a suitable dish daily.
- (v)
- The micronutrient class: the importance of vitamins and minerals in adolescent health, nutritional interactions, and examples of the nutrients that we consume in food.
- (vi)
- The fiber class: the importance of consuming fiber daily, the amount required, the difference between soluble and insoluble fibers, and where to find the different fibers present in food.
- (vii)
- Food labels: how to read food labels, as well as practical examples, such as juices from sachets, biscuits, and processed foods.
- (viii)
- Food labels in “food for children and adolescents”: to understand kilocalories, macronutrients, micronutrients, preservatives, stabilizers, and dyes in “common” industrialized food.”
- (ix)
- Physical or emotional hunger: explain in detail how to identify the hunger level and whether it is physical or emotional.
- (x)
- Review of class content: to observe if the children and adolescents understand the topics addressed so far, such as (a) how many types of fiber there are, (b) how to assemble a healthy dish, etc.
- (xi)
- Myths and truths of nutrition: to explain some myths commonly commented on between this age, such as “water fasting with slimming lemon”, "sweating makes you lose weight”, “do not eat carbohydrates to lose weight”, etc.
- (xii)
- Final lesson: recommending how to behave on vacation (without the research group) and the ten steps to encourage healthy eating.
- (i)
- The intention was to meet the participants in the project and understand their expectations for the next 12 weeks, as well as inform them how the meetings would occur and what would be developed.
- (ii)
- Health and mental health: adolescents were encouraged to understand what health is and its benefits and assist themselves in understanding the environment and context concerning their senses and emotions.
- (iii)
- Self-image: The identification of the individual by himself can be used to consider elements of the external world, such as parental figures, friends, idols, or even cultural characteristics. Thus, understanding themselves and their reality can help them to control their emotions and behaviors; therefore, a framework was developed for the participants to fill in to explain how they feel about the contexts inserted, such as school, project, work, and home.
- (iv)
- Sleep: a theoretical class to gain an understanding of sleep via an illustrative presentation of these concepts in a participatory model; the goal was to promote a perception of its importance and how it affects their health, as well as to stimulate self-analysis of this aspect in their lives.
- (v)
- Anxiety: theoretical–practical classes provided dynamically and playfully identification of the physical and psychological sensations of anxiety and caused self-perception of these since the theme was about anxiety.
- (vi)
- Pathological anxiety: a theoretical class was provided on the differentiation between normal and pathological anxiety to reflect that, in some cases, it is a natural response of the human being.
- (vii)
- Self-control: following the knowledge previously acquired about anxiety, a more theoretical practical class was held, outlining techniques to assist in anxious moments or crises to promote bodily, psychological, spatial, and temporal self-awareness.
- (viii)
- Communication in the family environment: this class was more theoretical in terms of the relationship between parents and children, but in conversation, the format explained how to improve the quality of the relationship and communication between parents and children and how to deal with daily challenges.
- (ix)
- Emotional intelligence: A theoretical class was used to provoke a self-analysis and discussion about how feelings and communication affect relationships with peers and oneself. For this class, the dynamic proposed was the “Bingo in Emotions,” where the individual drew emotions, and the emotion drawn should be manifested to explain how to act and deal with it in the context into which it was inserted.
- (x)
- Self-sabotage: to lead participants to reflect and self-perceive how their emotions and beliefs can affect the slimming process, promoting self-sabotage.
- (xi)
- Leisure: to ensure that the participants understood the importance of leisure for mental health and their family relationships, as well as how it can increase the lengths of their lives.
- (xii)
- A recap of the content taught throughout the 12 weeks: we recapitulated on all the themes and encouraged adolescents to follow up after completing the multi-disciplinary project.
3. Results
4. Discussion
- (i)
- A decrease in visceral fat, FM, BF, fasting glucose, TC, LDL-c, and TG for both groups, regardless of the type of intervention;
- (ii)
- An increase in LM, FFM, SMM, and HDL-c, regardless of the type of intervention;
- (iii)
- A significant reduction in DBP in the two intervention groups.
4.1. Anthropometric and Body Composition Responses
4.2. Biomarkers Responses
4.3. Blood Pressure Responses
4.4. Health Promotion Considerations
4.5. Limitations, Strengths, and Future Study Possibilities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Skinner, A.C.; Ravanbakht, S.N.; Skelton, J.A.; Perrin, E.M.; Armstrong, S.C. Prevalence of Obesity and Severe Obesity in US Children, 1999–2016. Pediatrics 2018, 141, e20173459. [Google Scholar] [CrossRef] [PubMed]
- Steinbeck, K.S.; Lister, N.B.; Gow, M.L.; Baur, L.A. Treatment of adolescent obesity. Nat. Rev. Endocrinol. 2018, 14, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Kinlen, D.; Cody, D.; O’Shea, D. Complications of obesity. QJM Int. J. Med. 2017, 111, 437–443. [Google Scholar] [CrossRef]
- Dettlaff-Dunowska, M.; Brzeziński, M.; Zagierska, A.; Borkowska, A.; Zagierski, M.; Szlagatys-Sidorkiewicz, A. Changes in Body Composition and Physical Performance in Children with Excessive Body Weight Participating in an Integrated Weight-Loss Programme. Nutrients 2022, 14, 3647. [Google Scholar] [CrossRef] [PubMed]
- WHO. Obesity and Overweight. Available online: https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 9 June 2021).
- Yang, Q.; Wang, K.; Tian, Q.; Zhang, J.; Qi, L.; Chen, T. Effect of Diet and Exercise-Induced Weight Loss among Metabolically Healthy and Metabolically Unhealthy Obese Children and Adolescents. Int. J. Environ. Res. Public Health 2022, 19, 6120. [Google Scholar] [CrossRef]
- Branco, B.H.M.; Valladares, D.; de Oliveira, F.M.; Carvalho, I.Z.; Marques, D.C.; Coelho, A.A.; de Oliveira, L.P.; Bertolini, S.M.M.G. Effects of the Order of Physical Exercises on Body Composition, Physical Fitness, and Cardiometabolic Risk in Adolescents Participating in an Interdisciplinary Program Focusing on the Treatment of Obesity. Front. Physiol. 2019, 10, 1013. [Google Scholar] [CrossRef]
- Branco, B.H.M.; Carvalho, I.Z.; de Oliveira, H.G.; Fanhani, A.P.; dos Santos, M.C.M.; de Oliveira, L.P.; Boni, S.M.; Nardo, N. Effects of 2 Types of Resistance Training Models on Obese Adolescents’ Body Composition, Cardiometabolic Risk, and Physical Fitness. J. Strength Cond. Res. 2020, 34, 2672–2682. [Google Scholar] [CrossRef]
- Branco, B.H.M.; Mariano, I.R.; de Oliveira, L.P.; Bertolini, S.M.M.G.; de Oliveira, F.M.; Araújo, C.G.A.; Adamo, K. Sports and Functional Training Improve a Subset of Obesity-Related Health Parameters in Adolescents: A Randomized Controlled Trial. Front. Psychol. 2021, 11, 589554. [Google Scholar] [CrossRef]
- Spagnuolo, R.; Montalcini, T.; De Bonis, D.; Ferro, Y.; Cosco, C.; Mazza, E.; Romeo, S.; Doldo, P.; Pujia, A. Weight Gain and Liver Steatosis in Patients with Inflammatory Bowel Diseases. Nutrients 2019, 11, 303. [Google Scholar] [CrossRef]
- Rigamonti, A.E.; Tringali, G.; De Micheli, R.; De Col, A.; Tamini, S.; Saezza, A.; Cella, S.G.; Sartorio, A. Impact of a Three-Week in-Hospital Multidisciplinary Body Weight Reduction Program on Body Composition, Muscle Performance and Fatigue in a Pediatric Obese Population with or without Metabolic Syndrome. Nutrients 2020, 12, 208. [Google Scholar] [CrossRef]
- Gómez, G.; Kovalskys, I.; Leme, A.C.B.; Quesada, D.; Rigotti, A.; Sanabria, L.Y.C.; García, M.C.Y.; Liria-Domínguez, M.R.; Herrera-Cuenca, M.; Fisberg, R.M.; et al. Socioeconomic Status Impact on Diet Quality and Body Mass Index in Eight Latin American Countries: ELANS Study Results. Nutrients 2021, 13, 2404. [Google Scholar] [CrossRef] [PubMed]
- Scaglioni, S.; De Cosmi, V.; Ciappolino, V.; Parazzini, F.; Brambilla, P.; Agostoni, C. Factors Influencing Children’s Eating Behaviours. Nutrients 2018, 10, 706. [Google Scholar] [CrossRef] [PubMed]
- Marques, D.C.S.; Ferreira, W.C.; Santos, I.C.; Ryal, J.J.; Marques, M.G.S.; Oliveira, F.M.; Milani, R.G.; Mota, J.; Valdés-Badilla, P.; Branco, B.H.M. Impacts of a Multi-Professional Family versus Isolated Intervention on Food Level Processing in Overweight Adolescents: A Randomized Trial. Nutrients 2023, 15, 935. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F.; et al. 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society. Circulation 2014, 129 (Suppl. 2), S102–S138. [Google Scholar] [CrossRef]
- Turner, L.; Shamseer, L.; Altman, D.G.; Weeks, L.; Peters, J.; Kober, T.; Dias, S.; Schulz, K.F.; Plint, A.C.; Moher, D. Consolidated standards of reporting trials (CONSORT) and the completeness of reporting of randomised controlled trials (RCTs) published in medical journals. Cochrane Database Syst. Rev. 2012, 2013, MR000030. [Google Scholar] [CrossRef]
- Barroso, W.K.S.; Rodrigues, C.I.S.; Bortolotto, L.A.; Mota-Gomes, M.A.; Brandão, A.A.; Feitosa, A.D.d.M.; Machado, C.A.; Poli-De-Figueiredo, C.E.; Amodeo, C.; Mion, D.; et al. Diretrizes Brasileiras de Hipertensão Arterial 2020. Arq. Bras. Cardiol. 2021, 116, 516–658. [Google Scholar] [CrossRef]
- Tanner, J.M. Growth at Adolescence, 2nd ed.; Blackwell Scientific Publications: Oxford, UK, 1962. [Google Scholar]
- Lohman, T.J.; Roache, A.F.; Martorell, R. Anthropometric Standardization Reference Manual. Med. Sci. Sports Exerc. 1992, 24, 952. [Google Scholar] [CrossRef]
- Branco, B.H.M.; Bernuci, M.P.; Marques, D.C.; Carvalho, I.Z.; Barrero, C.A.L.; de Oliveira, F.M.; Ladeia, G.F.; Júnior, N.N. Proposal of a normative table for body fat percentages of Brazilian young adults through bioimpedanciometry. J. Exerc. Rehabil. 2018, 14, 974–979. [Google Scholar] [CrossRef]
- Sena, R.D.P.; Santos, I.C.; Cerqueira, B.D.S.; de Oliveira, F.M.; Acencio, F.R.; Franco, C.B.; Branco, B.H.M. Establishing a normative table for classifying body fat percentage in adolescents. J. Hum. Growth Dev. 2022, 32, 129–135. [Google Scholar] [CrossRef]
- Onis, M.; Onyango, A.; Borghi, E.; Siyam, A.; Nishida, C.; Siekmann, J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007, 85, 660–667. [Google Scholar] [CrossRef]
- Sociedade Brasileira de Carrdiologia. I Diretriz de Prevenção de Aterosclerose na Infância e Adolescência. Arq. Bras. Cardiol. 2005, 85, 1–36. [Google Scholar] [CrossRef]
- Brasil. Guia Alimentar para a População Brasileira; Ministério da Saúde, Secretaria de Atenção à Saúde, Departamento de Atenção Básica: Brasília, Brazil, 2014; pp. 1–158.
- Bangun, S.R. The Effectiveness of Group Psychotherapy on Reducing Anxiety and Depression Symptoms in Adolescents. J. Psikiatri Surabaya 2022, 11, 119–127. [Google Scholar] [CrossRef]
- Pichon-Rivière, E. O Processo Grupal, 8th ed.; Martins Fontes: São Paulo, Brazil, 2009. [Google Scholar]
- Foster, C.; Florhaug, J.A.; Franklin, J.; Gottschall, L.; Hrovatin, L.A.; Parker, S.; Doleshal, P.; Dodge, C. A New Approach to Monitoring Exercise Training. J. Strength Cond. Res. 2001, 15, 109–115. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Lawrence Erlbaum Associates: Mahwah, NJ, USA, 1988; pp. 1–579. [Google Scholar]
- Ross, R.; Soni, S.; Houle, S.A. Negative energy balance induced by exercise or diet: Effects on visceral adipose tissue and liver fat. Nutrients 2020, 12, 891. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, D.; Hutson, I.; Tycksen, E.; Pietka, T.; Bauerle, K.; Harris, C. Decreased adiposity likely resulted from increased energy expenditure since food intake was not different. Endocrionology 2020, 1612. [Google Scholar] [CrossRef]
- Coffey, V.G.; Hawley, J.A. Concurrent exercise training: Do opposites distract? J. Physiol. 2017, 595, 2883–2896. [Google Scholar] [CrossRef] [PubMed]
- Cota, B.C.; Priore, S.E.; Ribeiro, S.A.V.; Juvanhol, L.L.; Faria, E.R.; Faria, F.R.; Pereira, P.F. Cardiometabolic risk in adolescents with normal weight obesity. Eur. J. Clin. Nutr. 2021, 76, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Capra, M.E.; Pederiva, C.; Banderali, G.; Biasucci, G. Prevention starts from the crib: The pediatric point of view on detection of families at high cardiovascular risk. Ital. J. Pediatr. 2021, 47, 51. [Google Scholar] [CrossRef]
- Zheng, M.; Hesketh, K.D.; McNaughton, S.A.; Salmon, J.; Crawford, D.; Cameron, A.J.; Lioret, S.; Campbell, K.J. Quantifying the overall impact of an early childhood multi-behavioural lifestyle intervention. Pediatr. Obes. 2021, 17, 3. [Google Scholar] [CrossRef]
- Nielsen, T.R.H.; Fonvig, C.E.; Dahl, M.; Mollerup, P.M.; Lausten-Thomsen, U.; Pedersen, O.; Hansen, T.; Holm, J.-C. Childhood obesity treatment; Effects on BMI SDS, body composition, and fasting plasma lipid concentrations. PLoS ONE 2018, 13, e0190576. [Google Scholar] [CrossRef]
- Diabetes Basics. Available online: http://www.diabetes.org/diabetes-basics/?loc=db-slabnav (accessed on 20 March 2021).
- Force, U.P.S.T.; Mangione, C.M.; Barry, M.J.; Nicholson, W.K.; Cabana, M.; Chelmow, D.; Coker, T.R.; Davidson, K.W.; Davis, E.M.; Donahue, K.E.; et al. Screening for Prediabetes and Type 2 Diabetes in Children and Adolescents. JAMA 2022, 328, 963–967. [Google Scholar] [CrossRef]
- Jung, M.K.; Yoo, E.-G. Hypertriglyceridemia in Obese Children and Adolescents. J. Obes. Metab. Syndr. 2018, 27, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.H.; Singh, R.B.; Mehta, V.; Sakshi; Asif, M.; Goyal, K.; Balodhi, A.; Manglik, P.; Sharma, A.; Chahal, A. Impact of exercise training duration on obesity and cardiometabolic biomarkers: A systematic review. J. Diabetes Metab. Disord. 2023, 22, 155–174. [Google Scholar] [CrossRef] [PubMed]
- Purdom, T.; Kravitz, L.; Dokladny, K.; Mermier, C. Understanding the factors that effect maximal fat oxidation. J. Int. Soc. Sports Nutr. 2018, 15, 3. [Google Scholar] [CrossRef] [PubMed]
- García-Hermoso, A.; Ramírez-Vélez, R.; Ramírez-Campillo, R.; Peterson, M.D.; Martínez-Vizcaíno, V. Concurrent aerobic plus resistance exercise versus aerobic exercise alone to improve health outcomes in paediatric obesity: A systematic review and meta-analysis. Br. J. Sports Med. 2018, 52, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.J.; Deenmamode, A.H.P.; Griffiths, M.; Arnold, O.; Cooper, N.J.; Wiles, J.D.; O’Driscoll, J.M. Exercise training and resting blood pressure: A large-scale pairwise and network meta-analysis of randomised controlled trials. Br. J. Sports Med. 2023, 57, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, E.C.; Abrahin, O.; Ferreira, A.L.L.; Rodrigues, R.P.; Alves, E.A.C.; Vieira, R.P. Resistance training alone reduces systolic and diastolic blood pressure in prehypertensive and hypertensive individuals: Meta-analysis. Hypertens. Res. 2017, 40, 927–931. [Google Scholar] [CrossRef]
- Vlaev, I.; Taylor, M.J.; Taylor, D.; Gately, P.; Gunn, L.H.; Abeles, A.; Kerkadi, A.; Lothian, J.; Jreige, S.K.; Alsaadi, A.; et al. Testing a multicomponent lifestyle intervention for combatting childhood obesity. BMC Public Health 2021, 21, 824. [Google Scholar] [CrossRef]
- Lister, N.B.; Baur, L.A.; Felix, J.F.; Hill, A.J.; Marcus, C.; Reinehr, T.; Summerbell, C.; Wabitsch, M. Child and adolescent obesity. Nat. Rev. Dis. Prim. 2023, 9, 24. [Google Scholar] [CrossRef]
| Order | Exercise (s) | Serie (s) | Repetition (s) | Execution Speed |
|---|---|---|---|---|
| 1 | Warming up—walking, interval, running | 8′ | *** | 1:1 |
| 2 | Step (up and down) | 3x | 30″ | 1:1 |
| 2 | Naval rope | 3x | 30″ | 1:1 |
| 3 | Standing row with squat | 3x | 30″ | 1:1 |
| 4 | Jump trampoline | 3x | 30″ | 1:1 |
| 5 | Hip bridge | 3x | 30″ | 1:1 |
| 6 | Frontal displacement up to the cone with a jump at the end | 3x | 30″ | 1:1 |
| 7 | Squat with biceps curl | 3x | 30″ | 1:1 |
| 8 | Ladder of agility (front) | 3x | 30″ | 1:1 |
| 9 | Rectus abdominals with lying hip adduction | 3x | 30″ | 1:1 |
| 10 | Dislocation with Mini band | 3x | 30″ | 1:1 |
| 11 | Cool-down period | 2′ | *** | 1:1 |
| Stretching | ||||
| Order | Exercise (s) | Serie (s) | Repetition (s) | Execution Speed |
|---|---|---|---|---|
| 1 | Warming up—walking, interval, running | 8′ | *** | 1:1 |
| 2 | Step sideways (Up and down, on one side and the other) | 3x | 30″ | 1:1 |
| 3 | Traction with elastic band | 3x | 30″ | 1:1 |
| 4 | Push-ups | 3x | 30″ | 1:1 |
| 5 | Squat with a ball throw downwards | 3x | 30″ | 1:1 |
| 6 | Agility ladder (side) | 3x | 30″ | 1:1 |
| 7 | Infra-abdominal (feet holding the ball) | 3x | 30″ | 1:1 |
| 8 | Thruster with dumbbells | 3x | 30″ | 1:1 |
| 9 | Zig-zag offset between discs | 3x | 30″ | 1:1 |
| 10 | Jump trampoline | 3x | 30″ | 1:1 |
| 11 | Adapted burpee | 3x | 30″ | 1:1 |
| 12 | Cool-down period | 2′ | *** | 1:1 |
| Stretching | ||||
| Variables | General (F and M) | Female | Male | ||||
|---|---|---|---|---|---|---|---|
| Family Intervention (n = 21) | Individual Intervention (n = 22) | Family Intervention (n = 9) | Individual Intervention (n = 14) | Family Intervention (n = 12) | Individual Intervention (n = 8) | ||
| Health Plan | Yes | 5 (23.80%) | 5 (22.72%) | 6 (66.66%) | 5 (35.71%) | 3 (25.00%) | 0 (0%) |
| No | 12 (57.14%) | 17 (77.27%) | 3 (33.33%) | 9 (64.28%) | 9 (75.00%) | 8 (100%) | |
| Educational Level | Primary School | 12 (57.14%) | 16 (72.72%) | 5 (55.55%) | 12 (85.71%) | 7 (58.33%) | 4 (50.00%) |
| High School | 9 (42.85%) | 6 (27.27%) | 4 (44.44%) | 2 (14.28%) | 5 (41.66%) | 4 (50.00%) | |
| Income | Low | 7 (33.33%) | 3 (13.63%) | 2 (22.22%) | 6 (42.85%) | 5 (41.66%) | 5 (62.50%) |
| Average | 9 (42.85%) | 8 (36.36%) | 4 (44.44%) | 5 (35.71%) | 5 (41.66%) | 3 (37.50%) | |
| High | 5 (23.80%) | 11 (50%) | 3 (33.33%) | 3 (21.42%) | 2 (16.66%) | 0 (0%) | |
| Medication | Yes | 2 (9.52%) | 4 (18.18%) | 1 (11.11%) | 3 (21.42%) | 1 (8.33%) | 1 (12.50%) |
| No | 19 (90.47%) | 18 (81.81%) | 8 (88.88%) | 11 (78.57%) | 11(91.66%) | 7 (87.50%) | |
| Smoker | Yes | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0(0%) | 0 (0%) |
| No | 21 (100%) | 22 (100%) | 9 (100%) | 14 (100%) | 12 (100%) | 8 (100%) | |
| Alcoholic | Yes | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| No | 21 (100%) | 22 (100%) | 9 (100%) | 14 (100%) | 12 (100%) | 8 (100%) | |
| Variables | Family Intervention (n = 21) | ∆ | Cohen’s d | Individual Intervention (n = 22) | ∆ | Cohen’s d | |||
|---|---|---|---|---|---|---|---|---|---|
| Pre-Intervention | Post-Intervention | Pre-Intervention | Post-Intervention | ||||||
| Age (years old) * | G F M | 14.24 ± 2.61 14.78 ± 2.39 13.83 ± 2.79 | 14.52 ± 2.62 15.00 ± 2.50 14.17 ± 2.76 | 0.29 ± 0.46 0.22 ± 2.50 0.33 ± 0.49 | 0.11 0.09 0.12 | 13.23 ± 2.27 12.79 ± 2.22 14.00 ± 2.27 | 13.45 ± 2.39 13.07 ± 2.37 14.13 ± 2.42 | 0.23 ± 0.43 0.29 ± 0.47 0.13 ± 0.35 | 0.10 0.13 0.06 |
| Body weight (kg) | G F M | 76.95 ± 22.30 67.13 ± 13.35 84.31 ± 25.24 | 77.47 ± 21.79 67.73 ± 12.45 84.77 ± 24.79 | 0.52 ± 2.12 0.60 ± 2.13 0.46 ± 2.20 | 0.02 0.04 0.02 | 83.52 ± 28.76 85.61 ± 30.64 75.15 ± 17.11 | 83.22 ± 26.60 85.35 ± 28.61 75.53 ± 16.18 | −0.30 ± 3.01 −0.26 ± 3.03 0.38 ± 1.74 | −0.01 −0.01 0.07 |
| Height (m²) * | G F M | 1.62 ± 0.13 1.56 ± 0.03 1.67 ± 0.16 | 1.63 ± 0.13 1.57 ± 0.03 1.68 ± 0.16 | 0.01 ± 0.01 0.00 ± 0.01 0.01 ± 0.02 | 0.07 0.11 0.08 | 1.64 ± 0.10 1.62 ± 0.08 1.67 ± 0.14 | 1.65 ± 0.10 1.63 ± 0.08 1.68 ± 0.12 | 0.01 ± 0.01 0.00 ± 0.01 0.01 ± 0.02 | 0.06 0.06 0.07 |
| BMI (kg/m²) | G F M | 28.89 ± 6.11 27.43 ± 5.43 29.98 ± 6.59 | 28.77 ± 5.78 27.54 ± 5.00 29.66 ± 6.31 | −0.12 ± 0.98 0.11 ± 0.80 −0.32 ± 1.12 | −0.02 0.02 −0.05 | 30.77 ± 9.47 32.30 ± 10.58 26.59 ± 3.78 | 30.52 ± 8.84 32.09 ± 9.86 26.50 ± 3.86 | −0.25 ± 1.11 −0.21 ± 1.15 −0.09 ± 0.77 | −0.03 −0.02 −0.02 |
| BMI score Z | G F M | 2.17 ± 1.36 1.54 ± 1.11 2.41 ± 1.99 | 2.12 ± 1.23 1.60 ± 1.03 2.36 ± 1.81 | −0.05 ± 0.26 0.05 ± 0.16 −0.05 ± 0.30 | −0.04 0.05 −0.02 | 2.52 ± 1.63 2.15 ± 1.40 1.68 ± 0.73 | 2.45 ± 1.53 2.11 ± 1.29 1.65 ± 0.75 | −0.07 ± 0.20 −0.04 ± 0.29 −0.04 ± 0.19 | −0.05 −0.03 −0.05 |
| SBP (mmHg) | G F M | 119.05 ± 11.36 118.89 ± 6.01 120.71 ± 9.97 | 118.95 ± 6.09 118.89 ± 14.53 119.29 ±13.85 | −0.10 ± 10.40 0.00 ± 12.25 −1.43 ± 15.12 | 0.02 0.00 −0.14 | 122.73 ± 13.86 119.00 ± 6.41 128.75 ± 12.46 | 119.55 ± 8.99 119.17 ± 9.00 117.50 ± 7.07 | −3.18 ± 15.24 0.17 ± 9.36 −11.25 ± 12.46 | 0.35 0.03 −1.59 |
| DBP (mmHg) * | G F M | 80.00 ± 10.49 83.33 ± 13.23 77.50 ± 7.54 | 76.19 ± 6.69 78.89 ± 6.01 74.17 ± 6.69 | −3.81 ± 9.73 −4.44 ± 10.14 −3.33 ± 9.85 | −0.57 −0.74 −0.50 | 83.64 ± 8.48 82.86 ± 9.14 85.00 ± 7.56 | 79.55 ± 5.75 80.00 ± 6.79 78.75 ± 3.54 | −4.09 ± 9.08 −2.86 ± 9.14 −6.25 ± 9.16 | −0.71 −0.42 −1.77 |
| Anthropometry and Body Composition | Family Intervention (n = 21) | ∆ | Cohen’s d | Individual Intervention (n = 22) | ∆ | Cohen’s d | |||
|---|---|---|---|---|---|---|---|---|---|
| Pre-Intervention | Post-Intervention | Pre-Intervention | Post-Intervention | ||||||
| WC (cm) | G FM | 85.08 ± 12.84 80.14 ± 10.78 89.50 ± 18.72 | 82.44 ± 19.45 72.82 ± 17.90 89.60 ± 17.84 | −2.64 ± 12.87 −7.32 ± 17.63 0.10 ± 3.15 | −0.21 −0.68 0.01 | 88.92 ± 17.62 88.78 ± 13.43 87.90 ± 16.72 | 88.57 ± 16.27 89.65 ± 17.96 86.76 ± 14.04 | −0.35 ± 3.20 0.88 ± 6.60 −1.14 ± 3.36 | −0.02 0.07 −0.07 |
| AC (cm) | G FM | 93.32 ± 14.90 86.70 ± 10.21 98.29 ± 16.27 | 91.64 ± 14.84 84.79 ± 9.86 96.78 ± 16.20 | −1.68 ± 5.21 −1.91 ± 3.40 −1.51 ± 6.39 | −0.11 −0.19 −0.09 | 97.24 ± 18.56 98.14 ± 20.03 95.66 ± 16.85 | 97.11 ± 17.91 98.12 ± 19.86 95.35 ± 14.99 | −0.12 ± 4.95 −0.01 ± 5.39 −0.31 ± 4.42 | −0.01 0.001 −0.02 |
| HC (cm) | G FM | 106.12 ± 13.60 103.44 ± 11.80 110.16 ± 17.78 | 105.10 ± 12.68 101.52 ± 8.05 110.39 ± 16.30 | −1.02 ± 4.27 −1.92 ± 6.21 0.23 ± 3.03 | −0.07 −0.16 0.01 | 108.27 ± 17.10 108.13 ± 14.98 104.96 ± 16.45 | 107.45 ± 16.64 107.78 ± 15.07 102.33 ± 17.05 | −0.81 ± 3.43 −0.34 ± 1.97 −2.64 ± 3.49 | −0.05 −0.02 −0.16 |
| WHR (cm) | G FM | 0.80 ± 0.05 0.77 ± 0.05 0.82 ± 0.04 | 0.78 ± 0.13 0.72 ± 0.17 0.83 ± 0.08 | −0.02 ± 0.12 −0.06 ± 0.16 0.01 ± 0.06 | −0.37 −1.03 0.18 | 0.82 ± 0.06 0.81 ± 0.06 0.84 ± 0.07 | 0.82 ± 0.07 0.81 ± 0.05 0.85 ± 0.08 | 0.01 ± 0.04 0.01 ± 0.03 0.02 ± 0.05 | 0.08 −0.02 0.25 |
| LM (kg) * | G FM | 42.87 ± 10.54 36.54 ± 3.89 47.62 ± 11.56 | 44.22 ± 10.45 37.59 ± 3.66 49.19 ± 11.23 | 1.35 ± 1.44 1.01 ± 1.49 1.58 ± 1.42 | 0.13 0.27 0.14 | 44.73 ± 11.35 42.83 ± 9.88 48.05 ± 13.62 | 45.57 ± 10.85 43.62 ± 9.65 48.99 ± 12.63 | 0.85 ± 1.58 0.79 ± 1.18 0.94 ± 2.20 | 0.07 0.08 0.07 |
| FFM (kg) * | G FM | 45.52 ± 11.22 38.83 ± 4.04 50.53 ± 12.37 | 47.00 ± 11.15 39.94 ± 3.79 52.28 ± 12.02 | 1.48 ± 1.54 1.11 ± 1.59 1.75 ± 1.50 | 0.13 0.28 0.14 | 47.54 ± 11.97 45.51 ± 10.34 51.10 ± 14.45 | 48.47 ± 11.46 46.39 ± 10.12 52.11 ± 13.42 | 0.93 ± 1.67 0.89 ± 1.29 1.01 ± 2.29 | 0.08 0.09 0.07 |
| SMM (kg) * | G FM | 24.95 ± 6.59 21.00 ± 2.47 24.77 ± 6.18 | 25.87 ± 6.57 21.70 ± 2.36 25.34 ± 6.06 | 0.91 ± 0.89 0.70 ± 0.90 0.56 ± 0.78 | 0.14 0.28 0.09 | 26.06 ± 7.21 27.92 ± 7.22 28.31 ± 8.71 | 26.67 ± 6.92 28.99 ± 7.04 29.01 ± 8.10 | 0.61 ± 1.03 1.08 ± 0.89 0.70 ± 1.42 | 0.09 0.15 0.08 |
| FM (kg) * | G FM | 31.43 ± 13.29 28.30 ± 9.96 40.10 ± 21.36 | 30.49 ± 12.96 27.79 ± 9.81 38.96 ± 19.79 | −0.94 ± 1.50 −0.51 ± 0.85 −1.14 ± 2.63 | −0.07 −0.05 −0.05 | 35.98 ± 20.70 33.78 ± 15.33 28.78 ± 18.57 | 34.75 ± 19.31 32.51 ± 15.00 27.38 ± 17.12 | −1.24 ± 2.44 −1.27 ± 1.82 −1.40 ± 2.23 | −0.06 −0.08 −0.08 |
| BF (%) * | G FM | 39.70 ± 8.12 41.03 ± 6.94 43.89 ± 10.23 | 38.20 ± 8.25 39.94 ± 7.58 42.83 ± 10.10 | −1.50 ± 1.83 −1.09 ± 0.95 −1.06 ± 1.88 | −0.19 −0.16 −0.10 | 40.50 ± 11.46 38.70 ± 9.08 34.56 ± 11.67 | 39.25 ± 11.52 36.88 ± 8.80 32.98 ± 11.76 | −1.25 ± 1.99 −1.82 ± 2.28 −1.59 ± 2.27 | −0.11 −0.20 −0.14 |
| Visceral Fat (%) * | G FM | 14.33 ± 5.48 14.00 ± 4.97 15.43 ± 5.65 | 13.81 ± 5.88 13.44 ± 5.27 15.21 ± 5.67 | −0.52 ± 0.93 −0.56 ± 0.53 −0.21 ± 1.37 | −0.10 −0.11 −0.04 | 14.00 ± 5.81 14.58 ± 6.04 11.50 ± 5.53 | 13.64 ± 6.03 14.08 ± 6.52 10.88 ± 5.96 | −0.36 ± 1.22 −0.50 ± 1.17 −0.63 ± 0.92 | −0.06 −0.08 −0.11 |
| Variables | Family Intervention (n = 21) | ∆ | Cohen’s d | Individual Intervention (n = 22) | ∆ | Cohen’s d | |||
|---|---|---|---|---|---|---|---|---|---|
| Pre-Intervention | Post-Intervention | Pre-Intervention | Post-Intervention | ||||||
| Fasting glucose (mg/dL) * | G F M | 99.71 ± 9.24 96.76 ± 8.84 101.93 ± 9.27 | 94.76 ± 10.21 91.56 ± 11.84 97.17 ± 8.54 | −4.95 ± 9.26 −5.20 ± 12.11 −4.76 ± 7.02 | −0.54 −0.59 −0.51 | 99.64 ± 8.02 96.36 ± 6.54 105.38 ± 7.37 † | 94.95 ± 9.33 91.29 ± 6.58 101.38 ± 10.34 | −4.68 ± 7.69 −5.07 ± 7.44 −4.00 ± 8.59 | −0.58 −0.78 −0.54 |
| Total cholesterol (mg/dL) * | G F M | 174.05 ± 33.41 174.00 ± 35.33 174.04 ± 33.48 | 158.27 ± 36.45 169.53 ± 36.55 149.82 ± 35.53 | −15.78 ± 27.13 −4.47 ± 23.99 −24.27 ± 27.14 | −0.47 −0.13 −0.72 | 174.82 ± 30.64 165.43 ± 30.76 191.25 ± 23.97 | 155.27 ± 23.18 150.00 ± 22.55 164.50 ± 22.70 | −19.55 ± 26.03 −15.43 ± 29.51 −26.75 ± 17.93 | −0.64 −0.50 −1.12 |
| HDL-c (mg/dL) * | G F M | 37.13 ± 3.85 38.67 ± 4.61 35.98 ± 2.84 | 46.33 8.02 48.06 ± 6.81 45.03 ± 8.89 | 9.20 ± 6.44 9.39 ± 6.83 9.06 ± 6.44 | 2.39 2.04 3.19 | 38.20 ± 6.13 37.37 ± 4.25 39.66 ± 8.68 | 46.21 ± 8.74 46.37 ± 9.19 45.94 ± 8.48 | 8.01 ± 7.75 9.00 ± 6.49 6.28 ± 9.82 | 1.31 2.12 0.76 |
| LDL-c (mg/dL) * | G F M | 134.20 ± 30.06 133.67 ± 29.19 134.60 ± 31.99 | 95.65 ± 32.01 105.13 ± 27.71 88.54 ± 34.30 | −38.55 ± 22.77 −28.54 ± 15.71 −46.06 ± 24.89 | −1.28 −0.98 −1.44 | 133.68 ± 26.92 127.69 ± 28.12 144.18 ± 22.55 | 92.35 ± 21.40 88.81 ± 24.01 98.53 ± 15.32 | −41.34 ± 19.24 −38.87 ± 18.88 −45.65 ± 20.37 | −1.54 −1.38 −2.02 |
| Triglycerides (mg/dL) * | G F M | 98.29 ± 51.15 86.89 ± 28.69 106.83 ± 63.02 | 89.90 ± 49.77 80.89 ± 27.41 96.67 ± 61.97 | −8.38 ± 28.63 −6.00 ± 12.56 −10.17 ± 36.98 | −0.16 −0.21 −0.16 | 94.82 ± 44.75 103.93 ± 49.50 78.88 ± 31.62 | 81.86 ± 43.79 87.71 ± 47.60 71.63 ± 36.81 | −12.95 ± 17.64 −16.21 ± 17.21 −7.25 ± 18.02 | −0.29 −0.33 −0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza Marques, D.C.; dos Santos Moraes, L.R.; de Souza Marques, M.G.; Ryal, J.J.; Santos, I.C.; De Paula Silva Lalucci, M.P.; Mota, J.; Valdés-Badilla, P.; Westphal Nardo, G.; Magnani Branco, B.H. Effects of 12 Weeks of Family and Individual Multi-Disciplinary Intervention in Overweight and Obese Adolescents under Cardiometabolic Risk Parameters: A Clinical Trial. Int. J. Environ. Res. Public Health 2023, 20, 6954. https://doi.org/10.3390/ijerph20206954
de Souza Marques DC, dos Santos Moraes LR, de Souza Marques MG, Ryal JJ, Santos IC, De Paula Silva Lalucci MP, Mota J, Valdés-Badilla P, Westphal Nardo G, Magnani Branco BH. Effects of 12 Weeks of Family and Individual Multi-Disciplinary Intervention in Overweight and Obese Adolescents under Cardiometabolic Risk Parameters: A Clinical Trial. International Journal of Environmental Research and Public Health. 2023; 20(20):6954. https://doi.org/10.3390/ijerph20206954
Chicago/Turabian Stylede Souza Marques, Déborah Cristina, Lilian Rosana dos Santos Moraes, Marilene Ghiraldi de Souza Marques, Joed Jacinto Ryal, Isabella Caroline Santos, Marielle Priscila De Paula Silva Lalucci, Jorge Mota, Pablo Valdés-Badilla, Greice Westphal Nardo, and Braulio Henrique Magnani Branco. 2023. "Effects of 12 Weeks of Family and Individual Multi-Disciplinary Intervention in Overweight and Obese Adolescents under Cardiometabolic Risk Parameters: A Clinical Trial" International Journal of Environmental Research and Public Health 20, no. 20: 6954. https://doi.org/10.3390/ijerph20206954
APA Stylede Souza Marques, D. C., dos Santos Moraes, L. R., de Souza Marques, M. G., Ryal, J. J., Santos, I. C., De Paula Silva Lalucci, M. P., Mota, J., Valdés-Badilla, P., Westphal Nardo, G., & Magnani Branco, B. H. (2023). Effects of 12 Weeks of Family and Individual Multi-Disciplinary Intervention in Overweight and Obese Adolescents under Cardiometabolic Risk Parameters: A Clinical Trial. International Journal of Environmental Research and Public Health, 20(20), 6954. https://doi.org/10.3390/ijerph20206954








