What Are the Determinants of the Quality of Systematic Reviews in the International Journals of Occupational Medicine? A Methodological Study Review of Published Literature
Abstract
1. Introduction
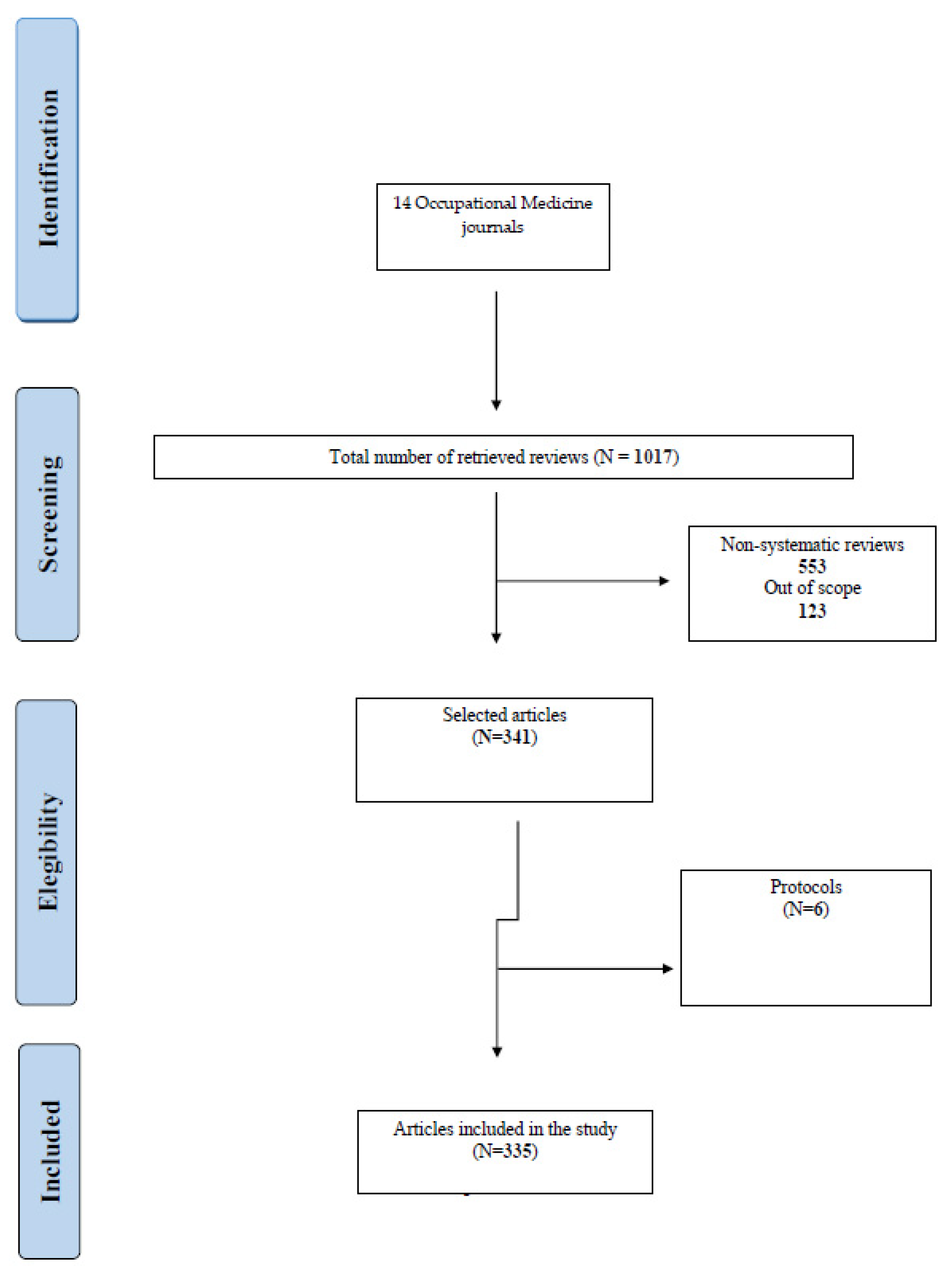
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources
- (1)
- American Journal of Industrial Medicine;
- (2)
- Annals of Occupational Hygiene;
- (3)
- Archives of Environmental and Occupational Health;
- (4)
- Environmental Health Perspectives;
- (5)
- International Journal of Environmental Research and Public Health;
- (6)
- International Journal of Occupational and Environmental Health;
- (7)
- International Journal of Occupational Medicine and Environmental Health;
- (8)
- International Journal of Occupational Safety and Ergonomics;
- (9)
- Journal of Occupational and Environmental Hygiene;
- (10)
- Journal of Occupational and Environmental Medicine;
- (11)
- Journal of Occupational Medicine and Toxicology;
- (12)
- Occupational and Environmental Medicine;
- (13)
- Occupational Medicine;
- (14)
- Scandinavian Journal of Work, Environment & Health.
2.3. Selection of Sources of Evidence
2.4. Data Items
- Name of the first author;
- Name of the journal;
- Title of the article;
- Year of publication;
- Adherence to PRISMA Checklist made by the authors (yes/no);
- Declaration by the authors of the use of PRISMA Checklist (yes/no);
- Journal impact factor;
- Numbers of authors;
- Nationality of the first author;
- Number of investigated databases in the review;
- Multi-country research group;
- Total number of articles included in the systematic review;
- Implementation of meta-analysis;
- Total AMSTAR-2 score: “yes” (all the criteria were met), “no” (none of the criteria were met), “partial yes” (not all the criteria were met).
- AMSTAR-2 checklist.
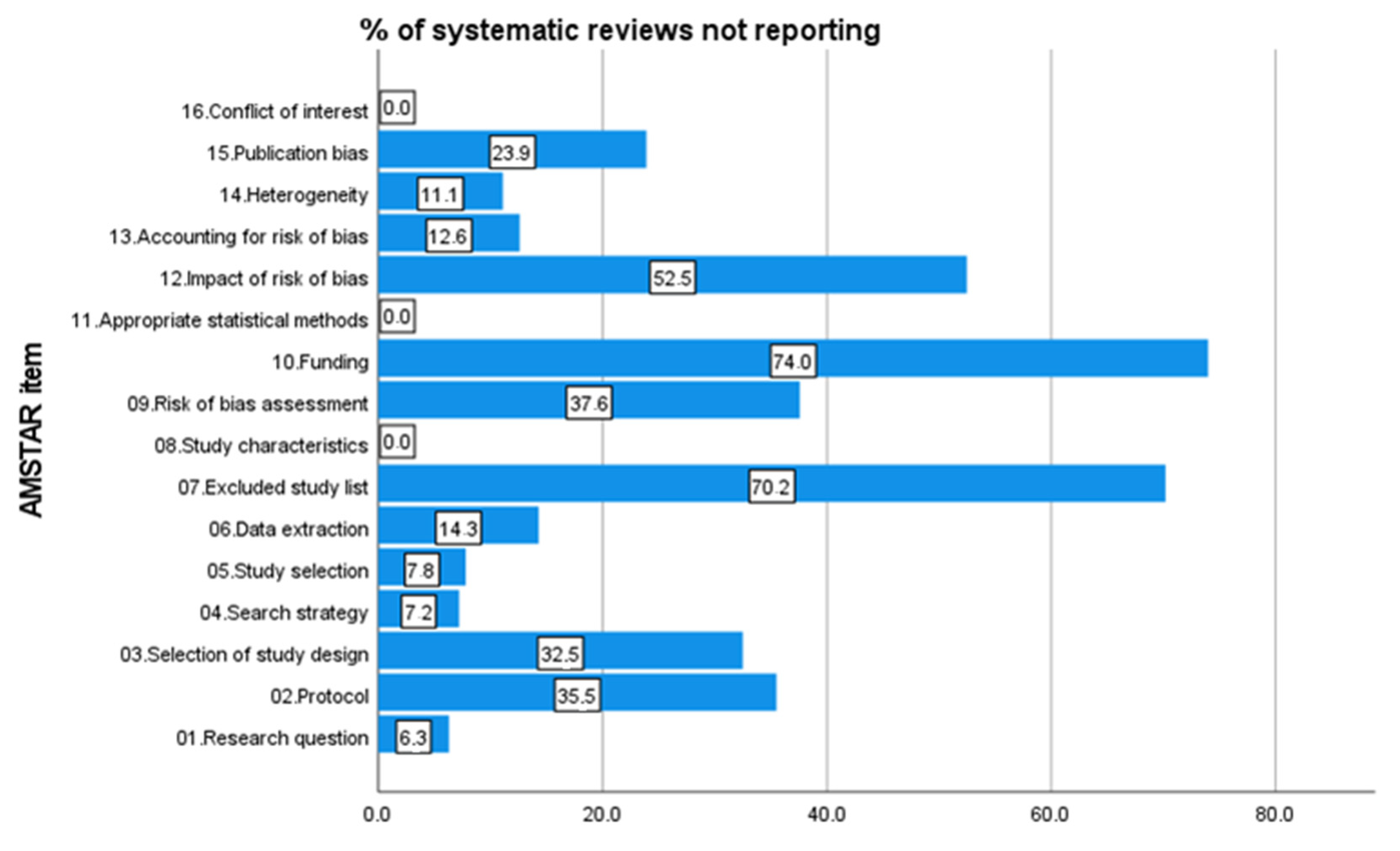
2.5. AMSTAR-2 Checklist
- -
- Did the research questions and inclusion criteria for the review include the components of PICO?
- -
- Did the report of the review contain an explicit statement that the review methods were established prior to the conduct of the review and did the report justify any significant deviations from the protocol?
- -
- Did the review authors explain their selection of the study design for inclusion in the review?
- -
- Did the review authors use a comprehensive literature search strategy?
- -
- Did the review authors perform study selection in duplicate?
- -
- Did the review authors perform data extraction in duplicate?
- -
- Did the review authors provide a list of excluded studies and justify the exclusions?
- -
- Did the review authors describe the included studies in adequate detail?
- -
- Did the review authors use a satisfactory technique for assessing the risk of bias (RoB) in individual studies that were included in the review?
- -
- Did the review authors report on the sources of funding for the studies included in the review?
- -
- If a meta-analysis was performed, did the review authors use appropriate methods for the statistical combination of results?
- -
- If a meta-analysis was performed, did the review authors assess the potential impact of RoB in individual studies on the results of the meta-analysis or other evidence synthesis?
- -
- Did the review authors account for RoB in primary studies when interpreting/discussing the results of the review?
- -
- Did the review authors provide a satisfactory explanation for, and discussion of, any heterogeneity observed in the results of the review?
- -
- If they performed quantitative synthesis, did the review authors carry out an adequate investigation of publication bias (small study bias) and discuss its likely impact on the results of the review?
- -
- Did the review authors report any potential sources of conflict of interest, including any funding they received for conducting the review?
2.6. Statistical Analysis
3. Results
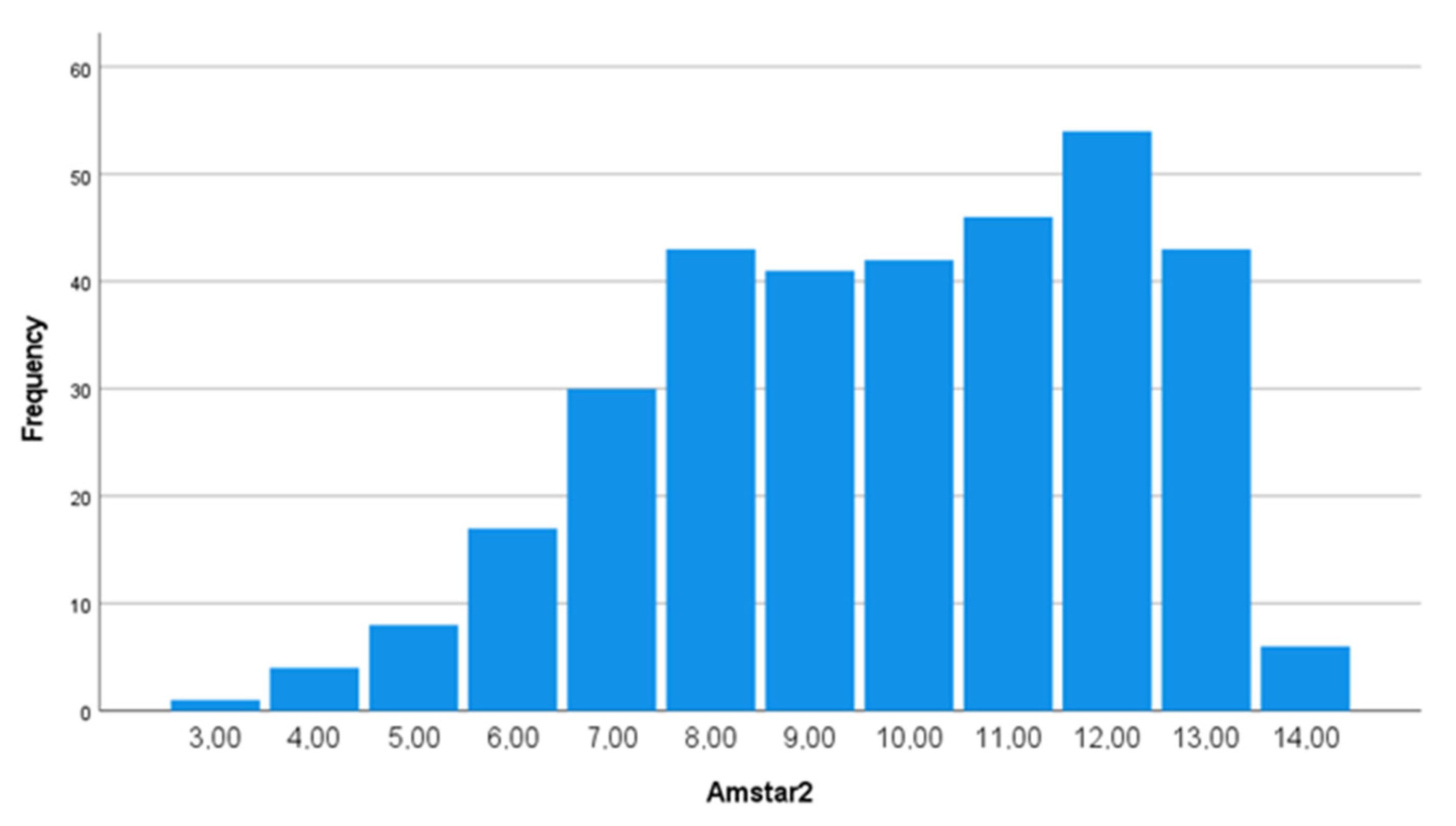
3.1. Univariate and Bivariate Analysis
3.2. Multivariate Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, J.W.; Sward, K.A.; Beck, S.L.; Staggers, N. Quality of Reporting Randomized Controlled Trials in Cancer Nursing Research. Nurs. Res. 2014, 63, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Panic, N.; Leoncini, E.; De Belvis, G.; Ricciardi, W.; Boccia, S. Evaluation of the endorsement of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement on the quality of published systematic review and meta-analyses. PLoS ONE 2013, 26, e83138. [Google Scholar]
- Samaan, Z.; Mbuagbaw, L.; Kosa, D.; Debono, V.B.; Dillenburg, R.; Zhang, S.; Fruci, V.; Dennis, B.; Bawor, M.; Thabane, L. A systematic scoping review of adherence to reporting guidelines in health care literature. J. Multidiscip. Healthc. 2013, 6, 169–188. [Google Scholar]
- Mulrow, C.D. The Medical Review Article: State of the Science. Ann. Intern. Med. 1987, 106, 485–488. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Cook, D.J.; Eastwood, S.; Olkin, I.; Rennie, D.; Stroup, D.F. Improving the quality of reports of meta-analyses of randomised controlled trials: The QUOROM statement. Qual. Report. Meta-Anal. Lancet 1999, 354, 1896–1900. [Google Scholar] [CrossRef]
- Moher, D.; Tetzlaff, J.; Tricco, A.C.; Sampson, M.; Altman, D.G. Epidemiology and reporting characteristics of systematic reviews. PLoS Med. 2007, 4, e78. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef]
- Welch, V.; Petticrew, M.; Tugwell, P.; Moher, D.; O’Neill, J.; Waters, E.; White, H.; the PRISMA-Equity Bellagio group. PRISMA-Equity 2012 Extension: Reporting Guidelines for Systematic Reviews with a Focus on Health Equity. PLoS Med. 2012, 9, e1001333. [Google Scholar] [CrossRef]
- Welch, V.; the PRISMA-Equity Bellagio group; Petticrew, M.; Petkovic, J.; Moher, D.; Waters, E.; White, H.; Tugwell, P. Extending the PRISMA statement to equity-focused systematic reviews (PRISMA-E 2012): Explanation and elaboration. Int. J. Equity Health 2015, 14, 92. [Google Scholar] [CrossRef]
- Welch, V.; Petticrew, M.; Petkovic, J.; Moher, D.; Waters, E.; White, H.; Tugwell, P.; Atun, R.; Awasthi, S.; Barbour, V.; et al. Extending the PRISMA statement to equity-focused systematic reviews (PRISMA-E 2012): Explanation and elaboration. J. Clin. Epidemiol. 2016, 70, 68–89. [Google Scholar] [CrossRef] [PubMed]
- Beller, E.M.; Glasziou, P.P.; Altman, D.G.; Hopewell, S.; Bastian, H.; Chalmers, I.; Gøtzsche, P.C.; Lasserson, T.; Tovey, D.; For The PRISMA for Abstracts Group. PRISMA for Abstracts: Reporting Systematic Reviews in Journal and Conference Abstracts. PLoS Med. 2013, 10, e1001419. [Google Scholar] [CrossRef] [PubMed]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.A.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-analyses of Health Care Interventions: Checklist and Explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef]
- Stewart, L.A.; Clarke, M.; Rovers, M.; Riley, R.D.; Simmonds, M.; Stewart, G.; Tierney, J.F. Preferred reporting items for systematic review and meta-analyses of individual participant data: The PRISMA-IPD statement. JAMA 2015, 313, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (prisma-p) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 349, g7647. [Google Scholar] [CrossRef] [PubMed]
- Zorzela, L.; Loke, Y.K.; Ioannidis, J.P.; Golder, S.; Santaguida, P.; Altman, D.G.; Moher, B.; Vohra, S. PRISMA harms checklist: Improving harms reporting in systematic reviews. BMJ 2016, 352, i157. [Google Scholar] [CrossRef]
- Guise, J.M.; Butler, M.E.; Chang, C.; Viswanathan, M.; Pigott, T.; Tugwell, P. AHRQ series on complex intervention systematic reviews—Paper 6: PRISMA-CI extension statement and checklist. J. Clin. Epidemiol. 2017, 90, 43–50. [Google Scholar] [CrossRef]
- Guise, J.M.; Butler, M.; Chang, C.; Viswanathan, M.; Pigott, T.; Tugwell, P. AHRQ series on complex intervention systematic reviews—Paper 7: PRISMA-CI elaboration and explanation. J. Clin. Epidemiol. 2017, 90, 51–58. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Des Jarlais, D.C.; Lyles, C.; Crepaz, N.; Trend Group. Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: The TREND statement. Am. J. Public Health 2004, 94, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef] [PubMed]
- Shea, B.; Grimshaw, J.M.; Wells, G.A.; Boers, M.; Andersson, N.; Hamel, C.; Porter, A.; Tugwell, P.; Moher, D. Development of AMSTAR-2: A Measurement Tool to Assess Systematic Reviews. BMC Med. Res. Methodol. 2007, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.; Savović, J.; Higgins, J.P.T.; Caldwell, D.M.; Reeves, B.C.; Shea, B.; Davies, P.; Kleijnen, J.; Churchill, R.; ROBIS group. ROBIS: A new tool to assess risk of bias in systematic reviews was developed. J. Clin. Epidemiol. 2016, 69, 225–234. [Google Scholar] [CrossRef]
- Scottish Intercollegiate Guidelines Network. Methodology Checklist 1: Systematic Reviews and Meta-Analyses. Available online: https://www.sign.ac.uk/what-we-do/methodology/checklists/ (accessed on 12 January 2023).
- Pauletto, P.; Polmann, H.; Réus, J.C.; de Oliveira, J.M.D.; Chaves, D.; Lehmkuhl, K.; Massignan, C.; Stefani, C.M.; Martins, C.C.; Flores-Mir, C.; et al. Critical appraisal of systematic reviews of intervention in dentistry published between 2019–2020 using the AMSTAR 2 tool. Evid.-Based Dent. 2022, 1–8. [Google Scholar] [CrossRef]
- Cheung, A.K.L.; Wong, C.H.L.; Ho, L.; Wu, I.X.Y.; Ke, F.Y.T.; Chung, V.C.H. Methodological quality of systematic reviews on Chinese herbal medicine: A methodological survey. BMC Complement. Med. Ther. 2022, 22, 48. [Google Scholar] [CrossRef]
- McGregor, R.H.; Warner, F.M.; Linde, L.D.; Cragg, J.J.; Osborn, J.A.; Varshney, V.P.; Schwarz, S.K.W.; Kramer, J.L.K. Quality of meta-analyses of non-opioid, pharmacological, perioperative interventions for chronic postsurgical pain: A systematic review. Reg. Anesth. Pain Med. 2022, 47, 263–269. [Google Scholar] [CrossRef]
- Yuan, M.; Wu, J.; Austin, R.E.; Hofer, S.O.P.; Lista, F.; Ahmad, J. Evaluating Breast Reconstruction Reviews Using A Measurement Tool to Assess Systematic Reviews (AMSTAR). Plast. Reconstr. Surg. Glob. Open 2021, 9, e3897. [Google Scholar] [CrossRef]
- Chow, R.; Li, A.; Wu, N.; Martin, M.; Wessels, J.M.; Foster, W.G. Quality appraisal of systematic reviews on methods of labour induction: A systematic review. Arch. Gynecol. Obstet. 2021, 304, 1417–1426. [Google Scholar] [CrossRef]
- Yuan, M.; Wu, J.; Austin, R.E.; Lista, F.; Ahmad, J. Evaluating the Quality of Systematic Reviews and Meta-Analyses About Breast Augmentation Using AMSTAR. Aesthetic Surg. J. Open Forum 2021, 3, ojab020. [Google Scholar] [CrossRef]
- Scott, J.; Howard, B.; Sinnett, P.; Schiesel, M.; Baker, J.; Henderson, P.; Vassar, M. Variable methodological quality and use found in systematic reviews referenced in STEMI clinical practice guidelines. Am. J. Emerg. Med. 2017, 35, 1828–1835. [Google Scholar] [CrossRef] [PubMed]
- Nissen, T.; Wayant, C.; Wahlstrom, A.; Sinnett, P.; Fugate, C.; Herrington, J.; Vassar, M. Methodological quality, completeness of reporting and use of systematic reviews as evidence in clinical practice guidelines for paediatric overweight and obesity. Clin. Obes. 2017, 7, 34–45. [Google Scholar] [CrossRef]
- Aran, G.; Hicks, C.; Demand, A.; Johnson, A.L.; Beaman, J.; Bailey, Y.; Haught, M.; Lane, A.; Sinnett, P.; Vassar, M. Treating schizophrenia: The quality of evidence behind treatment recommendations and how it can improve. BMJ Evid.-Based Med. 2020, 25, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.; Rankin, J.; Beaman, J.; Murray, K.; Sinnett, P.; Riddle, R.; Haskins, J.; Vassar, M. Methodological quality of systematic reviews referenced in clinical practice guidelines for the treatment of opioid use disorder. PLoS ONE 2017, 12, e0181927. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Saint, S.; Christakis, D.A. Impact factor: A valid measure of journal quality? J. Med. Libr. Assoc. 2003, 91, 42–46. [Google Scholar]
- Tahamtan, I.; Safipour Afshar, A.; Ahamdzadeh, K. Factors affecting number of citations: A comprehensive review of the literature. Scientometrics 2016, 107, 1195–1225. [Google Scholar] [CrossRef]
- Shamseer, L.; Stevens, A.; Skidmore, B.; Turner, L.; Altman, D.G.; Hirst, A.; Hoey, J.; Palepu, A.; Simera, I.; Schulz, K.; et al. Does Journal endorsement of reporting guidelines influence the completeness of reporting of health research? A systematic review protocol. Syst. Rev. 2012, 1, 24. [Google Scholar] [CrossRef]
- Mannocci, A.; Saulle, R.; Colamesta, V.; D’Aguanno, S.; Giraldi, G.; Maffongelli, E.; Meggiolaro, A.; Semyonov, L.; Unim, B.; La Torre, G. What is the impact of reporting guidelines on Public Health journals in Europe? The case of STROBE, CONSORT and PRISMA. J. Public Health 2015, 37, 737–740. [Google Scholar] [CrossRef]
- Banzi, R.; Cinquini, M.; Gonzalez-Lorenzo, M.; Pecoraro, V.; Capobussi, M.; Minozzi, S. Quality assessment versus risk of bias in systematic reviews: AMSTAR-2 and ROBIS had similar reliability but differed in their construct and applicability. J. Clin. Epidemiol. 2018, 99, 24–32. [Google Scholar] [CrossRef]
- Gates, A.; Gates, M.; Duarte, G.; Cary, M.; Becker, M.; Prediger, B.; Vandermeer, B.; Fernandes, R.M.; Pieper, D.; Hartling, L. Evaluation of the reliability, usability, and applicability of AMSTAR-2, AMSTAR-2 2, and ROBIS: Protocol for a descriptive analytic study. Syst. Rev. 2018, 7, 85. [Google Scholar] [CrossRef]
- Pieper, D.; Puljak, L.; González-Lorenzo, M.; Minozzi, S. Minor differences were found between AMSTAR-2 2 and ROBIS in the assessment of systematic reviews including both randomized and nonrandomized studies. J. Clin. Epidemiol. 2019, 108, 26–33. [Google Scholar] [CrossRef] [PubMed]
| Occupational Health Area | N | % |
|---|---|---|
| Toxicology | 44 | 13.2 |
| Health promotion and intervention | 37 | 11.0 |
| Environmental exposure and climate changes | 36 | 10.7 |
| Mental and neurological health | 32 | 9.6 |
| Musculoskeletal disorders | 32 | 9.6 |
| Stress and burnout | 23 | 6.9 |
| Workplace injury and Violence | 21 | 6.3 |
| Non-communicable diseases | 18 | 5.4 |
| Shift work | 14 | 4.2 |
| Return to work | 12 | 3.6 |
| Vaccination and infectious diseases | 11 | 3.3 |
| Healthcare workers | 9 | 2.7 |
| Asbestos | 8 | 2.4 |
| Ergonomy | 7 | 2.1 |
| Respiratory disease | 6 | 1.8 |
| Sickness absence | 6 | 1.8 |
| Tobacco smoking | 5 | 1.5 |
| Physical risk (ionizing and non-ionizing radiations, noise, vibration) | 5 | 1.5 |
| Precarious work and Unemployment | 5 | 1.5 |
| Other | 4 | 1.2 |
| Qualitative Variables | AMSTAR-2 Score Mean (SD) | p Value | |
|---|---|---|---|
| Multi-country Research Team | NO | 9.88 (2.47) | 0.762 |
| YES | 9.79 (1.99) | ||
| Prisma Statement Declared | NO | 9.26 (2.53) | <0.001 |
| YES | 10.35 (2.11) | ||
| Prisma Statement Applied | NO | 9.01 (2.61) | 0.002 |
| YES | 10.08 (2.25) | ||
| Meta-Analysis | NO | 9.38 (2.38) | <0.001 |
| YES | 10.44 (2.23) | ||
| Variables | r | p Value |
|---|---|---|
| Impact Factor | 0.176 | 0.001 |
| Publication year | 0.316 | <0.001 |
| N. authors | 0.109 | 0.047 |
| N. of Research Database used | 0.145 | 0.008 |
| N. Articles Included | 0.083 | 0.135 |
| Covariates | β | p |
|---|---|---|
| Impact Factor | 0.154 | 0.003 |
| Year of publication | 0.274 | <0.001 |
| N. of Research Databases | 0.128 | 0.011 |
| Prisma Statement Declared (YES/NO *) | 0.153 | 0.003 |
| Meta-Analysis (YES/NO *) | 0.211 | <0.001 |
| N. of Authors | 0.111 | 0.046 |
| R2 = 0.226 | ||
| Occupational Health Area | ||||||
|---|---|---|---|---|---|---|
| Independent Variable | Toxicology | Health Promotion and Intervention | Environmental Exposure and Climate Changes | Mental and Neurological Health | Musculoskeletal Disorders | Stress and Burnout |
| Impact Factor | x | x | ||||
| Year of publication | x | x | x | |||
| N. of Research Databases | x | x | x | |||
| Prisma Statement Declared (YES/NO *) | x | x | ||||
| Meta-Analysis (YES/NO *) | x | x | x | |||
| N. of Authors | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
La Torre, G.; Bova, R.; Cocchiara, R.A.; Sestili, C.; Tagliaferri, A.; Maggiacomo, S.; Foschi, C.; Zomparelli, W.; Manai, M.V.; Shaholli, D.; et al. What Are the Determinants of the Quality of Systematic Reviews in the International Journals of Occupational Medicine? A Methodological Study Review of Published Literature. Int. J. Environ. Res. Public Health 2023, 20, 1644. https://doi.org/10.3390/ijerph20021644
La Torre G, Bova R, Cocchiara RA, Sestili C, Tagliaferri A, Maggiacomo S, Foschi C, Zomparelli W, Manai MV, Shaholli D, et al. What Are the Determinants of the Quality of Systematic Reviews in the International Journals of Occupational Medicine? A Methodological Study Review of Published Literature. International Journal of Environmental Research and Public Health. 2023; 20(2):1644. https://doi.org/10.3390/ijerph20021644
Chicago/Turabian StyleLa Torre, Giuseppe, Remigio Bova, Rosario Andrea Cocchiara, Cristina Sestili, Anna Tagliaferri, Simona Maggiacomo, Camilla Foschi, William Zomparelli, Maria Vittoria Manai, David Shaholli, and et al. 2023. "What Are the Determinants of the Quality of Systematic Reviews in the International Journals of Occupational Medicine? A Methodological Study Review of Published Literature" International Journal of Environmental Research and Public Health 20, no. 2: 1644. https://doi.org/10.3390/ijerph20021644
APA StyleLa Torre, G., Bova, R., Cocchiara, R. A., Sestili, C., Tagliaferri, A., Maggiacomo, S., Foschi, C., Zomparelli, W., Manai, M. V., Shaholli, D., Barletta, V. I., Moretti, L., Vezza, F., & Mannocci, A. (2023). What Are the Determinants of the Quality of Systematic Reviews in the International Journals of Occupational Medicine? A Methodological Study Review of Published Literature. International Journal of Environmental Research and Public Health, 20(2), 1644. https://doi.org/10.3390/ijerph20021644








