A Comprehensive Contamination Investigation of Bohai Bay Seawater: Antibiotics Occurrence, Distribution, Ecological Risks and Their Interactive Factors
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.2.1. Sample Collection
2.2.2. Sample Preparation
2.2.3. Sample Analysis
2.3. Environmental Factors and Trace Element Analysis
2.4. Risk Assessment
3. Results and Discussion
3.1. Overall Residual Levels of Different Antibiotic Classes
3.2. Residual Levels of Single Antibiotic Concentration
3.2.1. TCs Levels
3.2.2. QA Levels
3.2.3. SAs Levels
3.2.4. LA and MA Levels
3.3. Residual Levels of Trace Elements
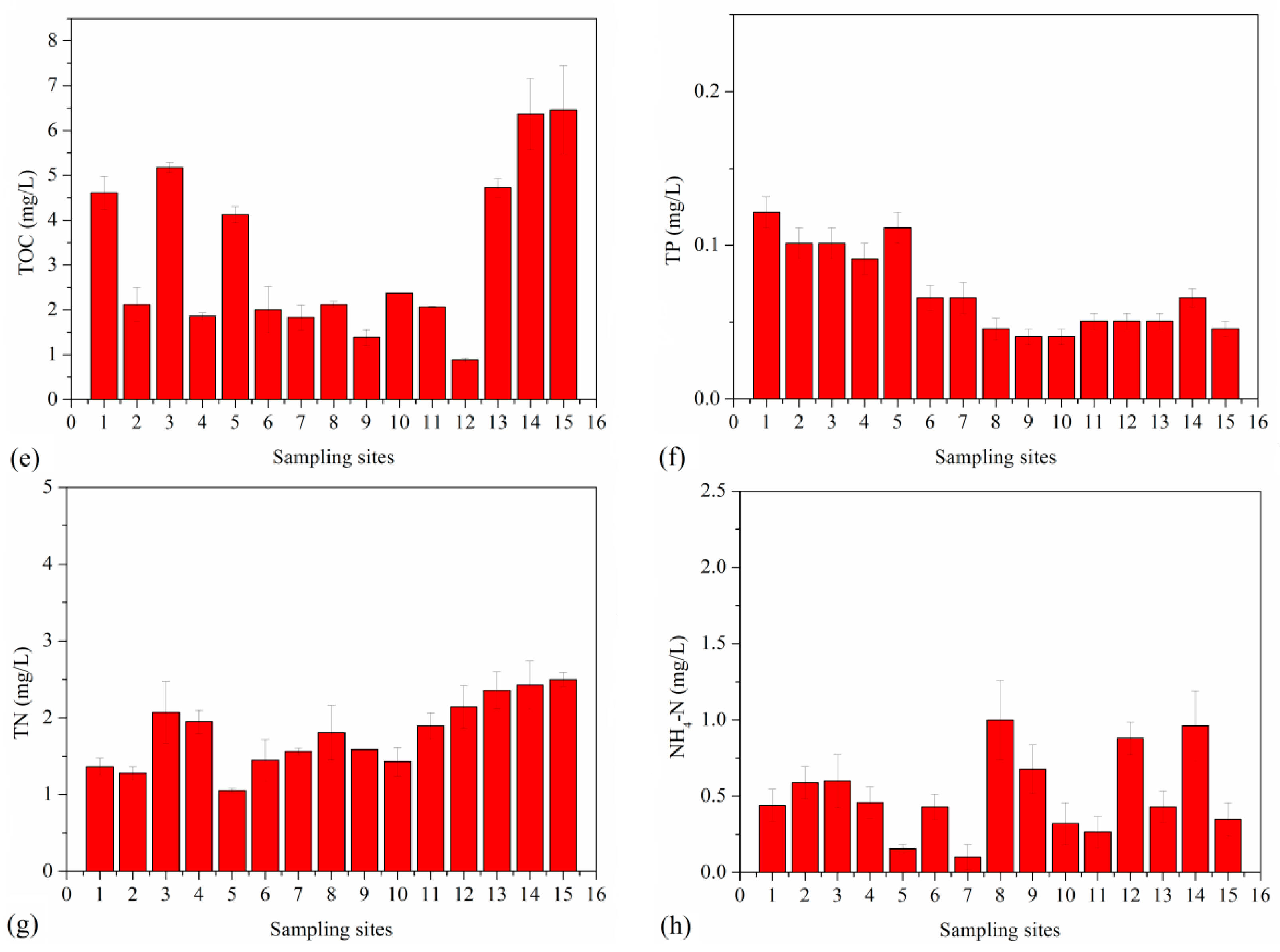
3.4. Variation of Environmental Factors
3.5. Correlation with Antibiotics
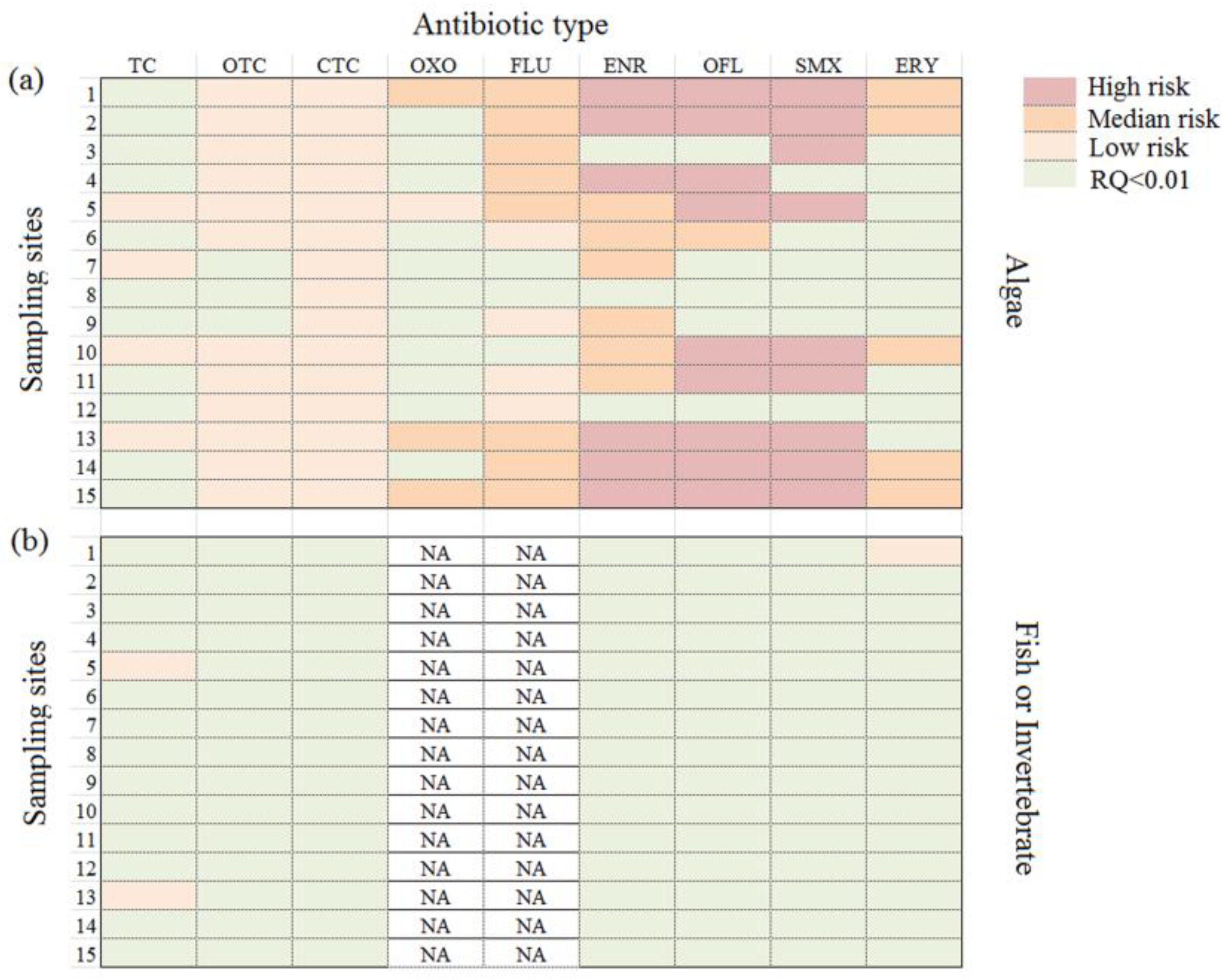
3.6. Ecological Risk
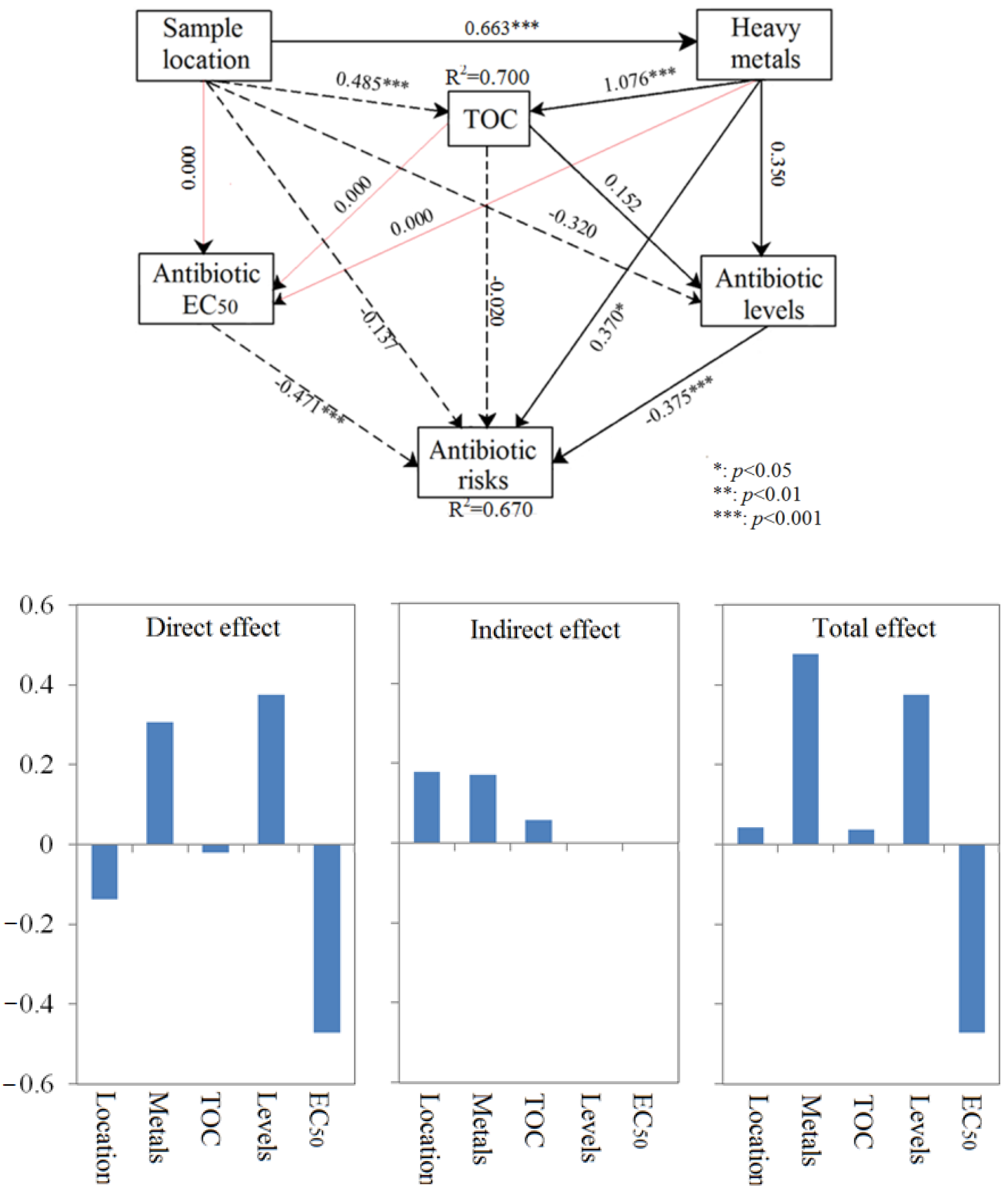
3.7. Direct and Indirect Effects on Ecological Risk
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Du, J.; Zhao, H.; Liu, S.; Xie, H.; Wang, Y.; Chen, J. Antibiotics in the coastal water of the South Yellow Sea in China: Occurrence, distribution and ecological risks. Sci. Total Environ. 2017, 595, 521–527. [Google Scholar] [CrossRef]
- Kümmerer, K. Antibiotics in the aquatic environment-a review-Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef]
- Zhi, S.; Zhou, J.; Yang, F.; Tian, L.; Zhang, K. Systematic analysis of occurrence and variation tendency about 58 typical veterinary antibiotics during animal wastewater disposal processes in Tianjin, China. Ecotox. Environ. Safe. 2018, 165, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.G.; Johnson, T.A.; Su, J.Q.; Qiao, M.; Guo, G.X.; Stedtfeld, R.D.; Hashsham, S.A.; Tiedje, J.M. Diverse and abundant antibiotic resistance genes in Chinese swine farms. Proc. Natl. Acad. Sci. USA 2013, 110, 3435–3440. [Google Scholar] [CrossRef]
- Zhang, Q.-Q.; Ying, G.-G.; Pan, C.-G.; Liu, Y.-S.; Zhao, J.-L. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: Source analysis, multimedia modeling, and linkage to bacterial resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [CrossRef] [PubMed]
- Zhi, S.; Zhang, K. Antibiotic residues may stimulate or suppress methane yield and microbial activity during high-solid anaerobic digestion. Chem. Eng. J. 2019, 359, 1303–1315. [Google Scholar] [CrossRef]
- Wang, Z.; Du, Y.; Yang, C.; Liu, X.; Zhang, J.; Li, E.; Zhang, Q.; Wang, X. Occurrence and ecological hazard assessment of selected antibiotics in the surface waters in and around Lake Honghu, China. Sci. Total Environ. 2017, 609, 1423–1432. [Google Scholar] [CrossRef]
- Yao, L.; Wang, Y.; Tong, L.; Li, Y.; Deng, Y.; Guo, W.; Gan, Y. Seasonal variation of antibiotics concentration in the aquatic environment: A case study at Jianghan Plain, central China. Sci. Total Environ. 2015, 527, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Qiao, M.; Ying, G.G.; Singer, A.C.; Zhu, Y.G. Review of antibiotic resistance in China and its environment. Environ. Int. 2017, 10, 160–172. [Google Scholar] [CrossRef]
- Zheng, J.; Zhou, Z.; Wei, Y.; Chen, T.; Feng, W.; Chen, H. High-throughput profiling of seasonal variations of antibiotic resistance gene transport in a peri-urban river. Environ. Int. 2018, 114, 87–94. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, M.; Sui, Q.; Tong, J.; Jiang, C.; Lu, X.; Zhang, Y.; Wei, Y. Impacts of addition of natural zeolite or a nitrification inhibitor on antibiotic resistance genes during sludge composting. Water Res. 2016, 91, 339–349. [Google Scholar] [CrossRef]
- Li, S.; Shi, W.; Li, H.; Xu, N.; Zhang, R.; Chen, X.; Sun, W.; Wen, D.; He, S.; Pan, J.; et al. Antibiotics in water and sediments of rivers and coastal area of Zhuhai City, Pearl River estuary, south China. Sci. Total Environ. 2018, 636, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, H.; Li, L.; Fu, C.; Tu, C.; Huang, Y.; Wu, L.; Tang, J.; Luo, Y.; Christie, P. Levels, distributions and sources of veterinary antibiotics in the sediments of the Bohai Sea in China and surrounding estuaries. Mar. Pollut. Bull. 2016, 109, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Tamtam, F.; Oort, F.; Le, B.B.; Dinh, T.; Mompelat, S.; Chevreuil, M.; Lamy, I.; Thiry, M. Assessing the fate of antibiotic contaminants in metal contaminated soils four years after cessation of long-term waste water irrigation. Sci. Total Environ. 2011, 409, 540–547. [Google Scholar] [CrossRef]
- Li, W.; Shi, Y.; Gao, L.; Liu, J.; Cai, Y. Investigation of antibiotics in mollusks from coastal waters in the Bohai Sea of China. Environ. Pollut. 2012, 162, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Song, J.; Yuan, H. Persistent organic pollutant residues in the sediments and mollusks from the Bohai Sea coastal areas, North China: An overview. Environ. Int. 2009, 35, 632–646. [Google Scholar] [CrossRef]
- Yang, R.; Liu, Y.; Long, H.; Qiao, L. Spatio-temporal characteristics of rural settlements and land use in the Bohai Rim of China. J. Geogr. Sci. 2015, 25, 559–572. [Google Scholar] [CrossRef]
- Wang, Y.W.; Wang, T.H.; Li, A.; Fu, J.J.; Wang, P.; Zhang, Q.H.; Jiang, G.B. Selection of bioindicators of polybrominated diphenyl ethers, polychlorinated biphenyls, and organochlorine pesticides in mollusks in the Chinese Bohai Sea. Environ. Sci. Technol. 2008, 42, 7159–7165. [Google Scholar] [CrossRef]
- Liang, X.; Tian, C.; Zong, Z.; Wang, X.; Jiang, W.; Chen, Y.; Ma, J.; Luo, Y.; Li, J.; Zhang, G. Flux and source-sink relationship of heavy metals and arsenic in the Bohai Sea, China. Environ. Pollut. 2018, 242, 1353–1361. [Google Scholar] [CrossRef]
- Niu, Z.-G.; Zhang, K.; Zhang, Y. Occurrence and distribution of antibiotic resistance genes in the coastal area of the Bohai Bay, China. Mar. Pollut. Bull. 2016, 107, 245–250. [Google Scholar] [CrossRef]
- Zhang, R.; Tang, J.; Li, J.; Zheng, Q.; Liu, D.; Chen, Y.; Zou, Y.; Chen, X.; Luo, C.; Zhang, G. Antibiotics in the offshore waters of the Bohai Sea and the Yellow Sea in China: Occurrence, distribution and ecological risks. Environ. Pollut. 2013, 174, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Xu, W.; Zhang, R.; Tang, J.; Chen, Y.; Zhang, G. Occurrence and distribution of antibiotics in coastal water of the Bohai Bay, China: Impacts of river discharge and aquaculture activities. Environ. Pollut. 2011, 159, 2913–2920. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Shi, Y.; Lu, Y.; Johnson, A.C.; Sarvajayakesavalu, S.; Liu, Z.; Su, C.; Zhang, Y.; Juergens, M.D.; Jin, X. The relative risk and its distribution of endocrine disrupting chemicals, pharmaceuticals and personal care products to freshwater organisms in the Bohai Rim, China. Sci. Total Environ. 2017, 590–591, 633–642. [Google Scholar] [CrossRef]
- Ding, X.; Ye, S.; Laws, E.A.; Mozdzer, T.J.; Yuan, H.; Zhao, G.; Yang, S.; He, L.; Wang, J. The concentration distribution and pollution assessment of heavy metals in surface sediments of the Bohai Bay, China. Mar. Pollut. Bull. 2019, 149, 110497. [Google Scholar] [CrossRef]
- Park, S.; Choi, K. Hazard assessment of commonly used agricultural antibiotics on aquatic ecosystems. Ecotoxicology 2008, 17, 526–538. [Google Scholar] [CrossRef]
- Xie, H.; Wang, X.; Chen, J.; Li, X.; Jia, G.; Zou, Y.; Zhang, Y.; Cui, Y. Occurrence, distribution and ecological risks of antibiotics and pesticides in coastal waters around Liaodong Peninsula, China. Sci. Total Environ. 2019, 656, 946–951. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yan, W.; Li, X.; Zou, Y.; Chen, X.; Huang, W.; Miao, L.; Zhang, R.; Zhang, G.; Zou, S. Antibiotics in riverine runoff of the Pearl River Delta and Pearl River Estuary, China: Concentrations, mass loading and ecological risks. Environ. Pollut. 2013, 182, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Gao, Y.; Ke, J.; Show, P.L.; Ge, Y.; Liu, Y.; Guo, R.; Chen, J. Antibiotics: An overview on the environmental occurrence, toxicity, degradation, and removal methods. Bioengineered 2021, 12, 7376–7416. [Google Scholar] [CrossRef]
- Yang, C.; Song, G.; Lim, W. A review of the toxicity in fish exposed to antibiotics. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 237, 108840. [Google Scholar] [CrossRef]
- Yang, Y.; Owino, A.A.; Gao, Y.; Yan, X.; Xu, C.; Wang, J. Occurrence, composition and risk assessment of antibiotics in soils from Kenya, Africa. Ecotoxicology 2016, 25, 1194–1201. [Google Scholar] [CrossRef]
- Li, N.; Zhang, X.; Wu, W.; Zhao, X. Occurrence, seasonal variation and risk assessment of antibiotics in the reservoirs in North China. Chemosphere 2014, 111, 327–335. [Google Scholar] [CrossRef]
- Zhang, Y.; Niu, Z.; Zhang, Y.; Zhang, K. Occurrence of intracellular and extracellular antibiotic resistance genes in coastal areas of Bohai Bay (China) and the factors affecting them. Environ. Pollut. 2018, 236, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Song, W.; Lin, H.; Wang, W.; Du, L.; Xing, W. Antibiotics and antibiotic resistance genes in global lakes: A review and meta-analysis. Environ. Int. 2018, 116, 60–73. [Google Scholar] [CrossRef]
- Chen, K.; Zhou, J.L. Occurrence and behavior of antibiotics in water and sediments from the Huangpu River, Shanghai, China. Chemosphere 2014, 95, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, Y.; Lu, Y.; Wang, P.; Suriyanarayanan, S.; Meng, J.; Zhou, Y.; Liang, R.; Khan, K. Risk ranking of environmental contaminants in Xiaoqing River, a heavily polluted river along urbanizing Bohai Rim. Chemosphere 2018, 204, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.-J.; Ying, G.-G.; Liu, S.; Zhao, J.L.; Yang, B.; Chen, Z.F.; Lai, H.J. Occurrence and fate of eleven classes of antibiotics in two typical wastewater treatment plants in South China. Sci. Total Environ. 2013, 452–453, 365–376. [Google Scholar] [CrossRef]
- Su, C.; Lu, Y.; Johnson, A.C.; Shi, Y.; Zhang, M.; Zhang, Y.; Juergens, M.D.; Jin, X. Which metal represents the greatest risk to freash water ecosystem in Bohai Region of China? Ecosys Health Sustain. 2017, 3, 1–9. [Google Scholar] [CrossRef]
- Wenk, J.; Gunten, U.; Canonica, S. Effect of dissolved organic matter on the transformation of contaminants induced by excited triplet states and the hydroxyl radical. Environ. Sci. Technol. 2011, 45, 1334–1340. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Lu, S.; Guo, X.; Lu, H.; Qin, P.; Bi, B.; Wan, Z.; Xi, B.; Zhang, T.; et al. Occurrence of typical antibiotics and source analysis based on PCA-MLR model in the East Dongting Lake, China. Ecotox. Environ. Safe. 2018, 163, 145–152. [Google Scholar] [CrossRef]
- Gagne, F.; Blaise, C.; Fournier, M.; Hansen, P. Effects of selected pharmaceutical products on phagocytic activity in Elliptio omplanata mussels. Comp. Biochem. Phys. C 2006, 143, 179–186. [Google Scholar]
- Lützhøft, H.; Halling-Sorensen, B.; Jorgensen, S.E. Algal toxicity of antibacterial agents applied in Danish fish farming. Arch. Environ. Con. Tox. 1999, 36, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. Antibiotics in the aquatic environment-a review-part II. Chemosphere 2009, 75, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wen, Q.; Chen, Z. Impacts of Cu and Zn on the performance, microbial community dynamics and resistance genes variations during mesophilic and thermophilic anaerobic digestion of swine manure. Bioresour. Technol. 2020, 312, 123554. [Google Scholar] [CrossRef] [PubMed]
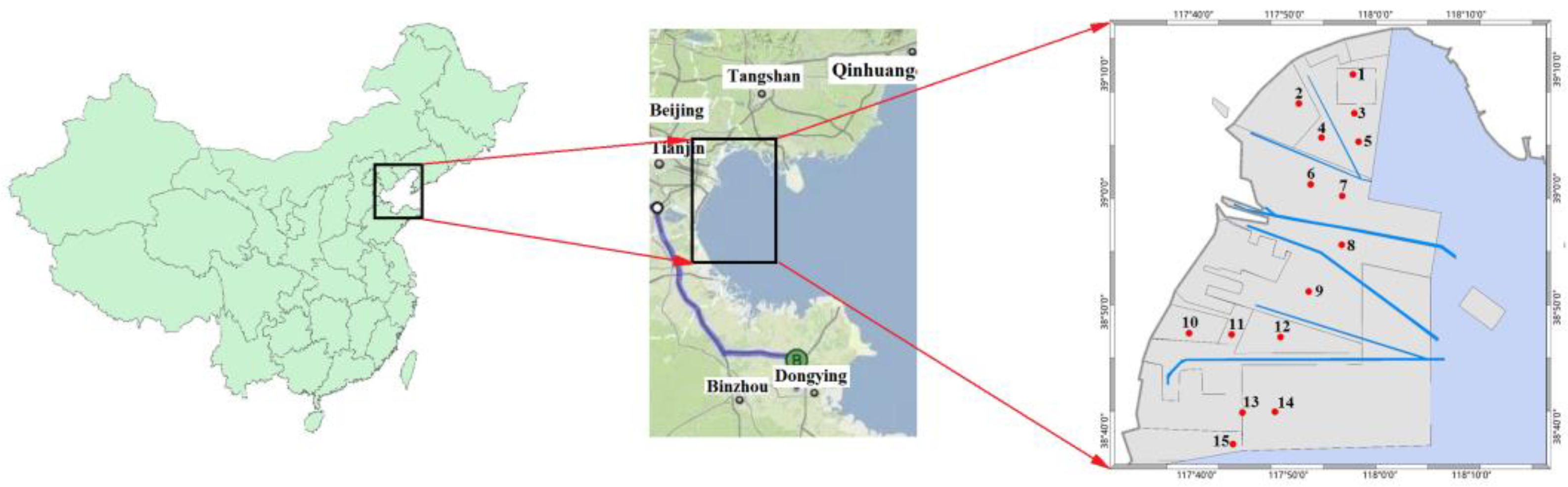
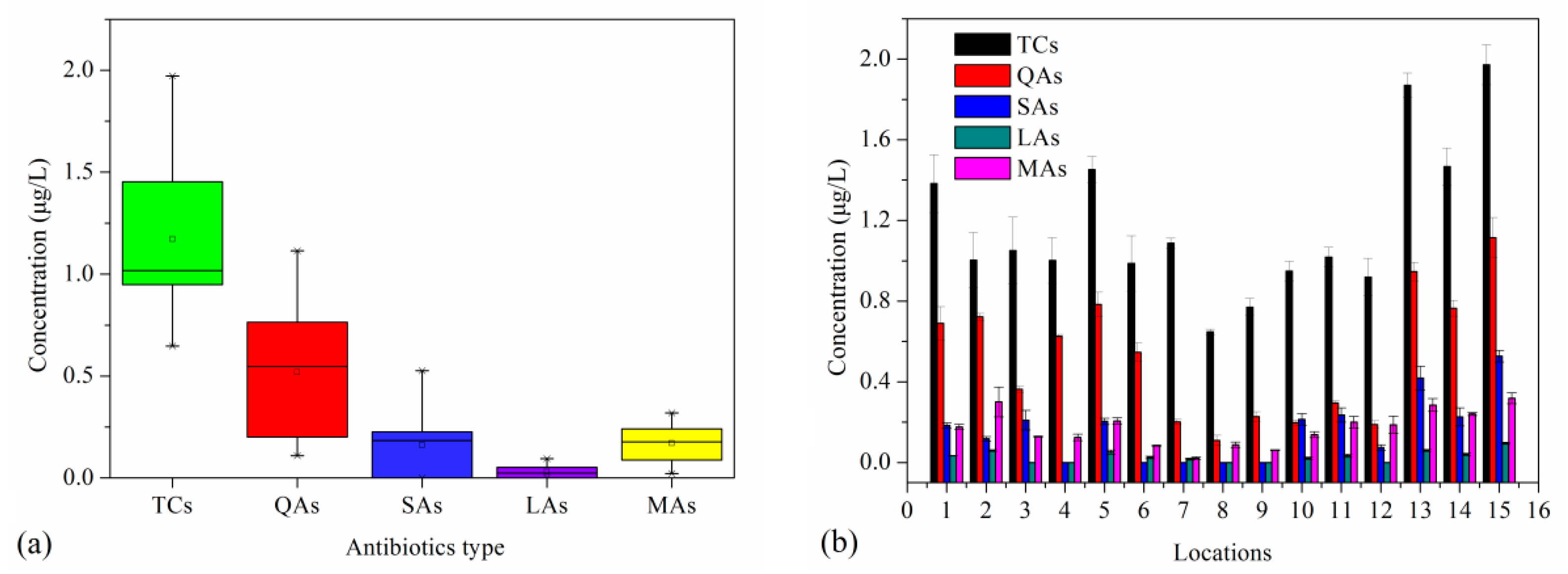
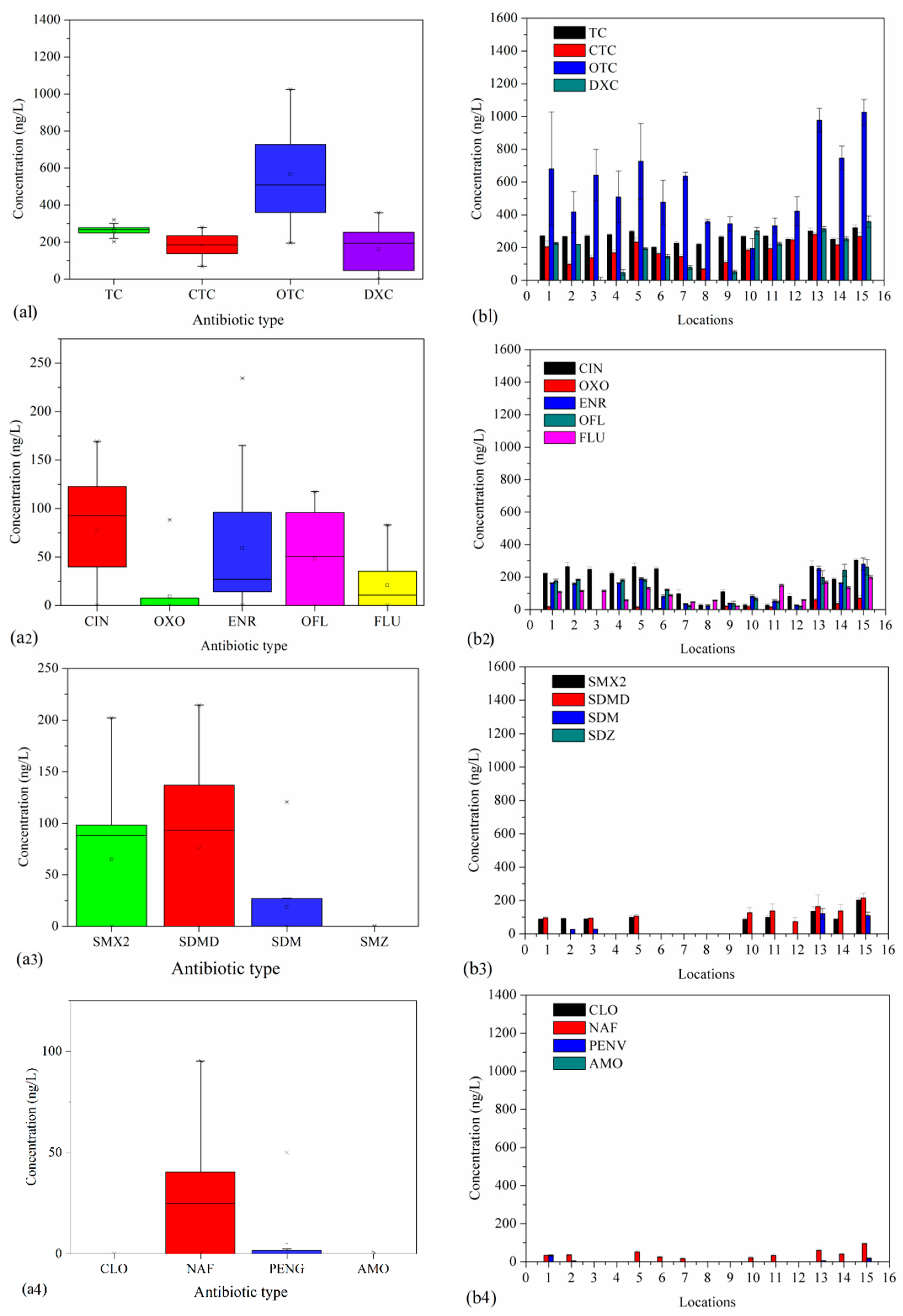
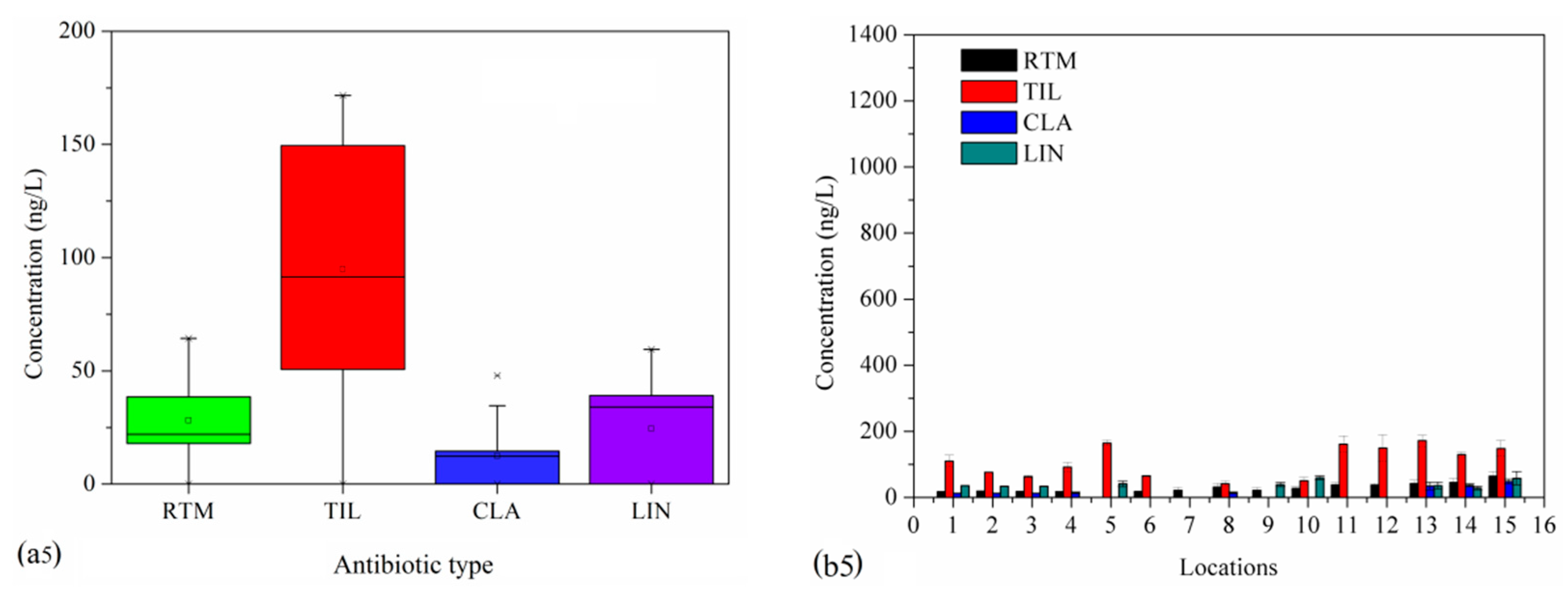


| Full Name | Abbreviation | Full Name | Abbreviation |
|---|---|---|---|
| 19 sulfonamide antibiotics (SAs) | 13 quinolone antibiotics (QAs) | ||
| sulfacetamide | SCM | ofloxacin | OFL |
| sulfisomidin | SIM | enrofloxacin | ENR |
| sulfadiazine | SDZ | difloxacin | DIF |
| sulfathiazole | STZ | danofloxacin | DAN |
| sulfamoxol | SMX1 | nalidixic acid | NAL |
| sulfapyridine | SPD | lomefloxacin | LOM |
| sulfamerazine | SMR | sarafloxacin | SAR |
| sulfamonomethoxine | SMM | ciprofloxacin | CIP |
| sulfadimidine | SDMD | flumequine | FLU |
| sulfamethizole | SMZ | orbifloxacin | ORB |
| sulfadoxin | SDX | norfloxacin | NOR |
| sulfamethoxazole | SMX2 | enoxacin | ENO |
| sulfisoxazole | SIX | oxolinic acid | OXO |
| sulfabenzamide | SB | 6 macrolide antibiotics (MAs) | |
| sulfadimethoxine | SDM | roxithromycin | RTM |
| sulfaquinoxaline | SQX | clarithromycin | CLA |
| Sulfameter | SME | azithromycin | AZI |
| sulfaguanidine | SGN | spiramycin | SPI |
| sulfamethoxypyridazine | SMP | tilmicosin | TIL |
| 5 tetracyclines (TCs) | Lincomycin | LIN | |
| chlorotetracycline | CTC | 2 lactam antibiotics (LAs) | |
| tetracycline | TC | penicillin G | PENG |
| oxytetracycline | OTC | oxacillin | OXA |
| doxycycline | DXC | ||
| demeclocycline | DMC | ||
| Site | Zn | Pb | Cd | As | Hg | Cu | Cr |
|---|---|---|---|---|---|---|---|
| 1 | 174.1 ± 4.7 | 23.2 ± 0.0 | 8.6 ± 0.8 | 1.7 ± 0.1 | - | - | - |
| 2 | 163.3 ± 12.5 | 23.2 ± 0.0 | 9.7 ± 0.1 | 1.4 ± 0.1 | - | - | - |
| 3 | 161.5 ± 10.2 | 21.8 ± 0.3 | 10.9 ± 0.6 | 0.7 ± 0.1 | - | - | - |
| 4 | 166.7 ± 17.0 | 33.4 ± 1.0 | 10.0 ± 1.1 | 1.0 ± 0.0 | - | - | - |
| 5 | 120.0 ± 8.2 | 62.8 ± 5.1 | 10.2 ± 1.4 | 2.1 ± 0.0 | - | - | - |
| 6 | 176.7 ± 9.4 | 24.3 ± 0.1 | 6.2 ± 0.2 | 1.6 ± 0.0 | - | - | - |
| 7 | 133.4 ± 11.2 | 24.5 ± 0.6 | 5.7 ± 0.4 | 1.5 ± 0.0 | - | - | - |
| 8 | 140.0 ± 6.0 | 22.0 ± 1.0 | 6.0 ± 0.4 | 1.4 ± 0.0 | - | - | - |
| 9 | 156.7 ± 9.4 | 22.1 ± 2.8 | 7.2 ± 0.3 | 1.3 ± 0.0 | - | - | - |
| 10 | 153.3 ± 4.7 | 34.0 ± 2.9 | 9.9 ± 0.4 | 1.6 ± 0.0 | - | - | - |
| 11 | 180.0 ± 8.2 | 27.1 ± 0.9 | 8.2 ± 0.1 | 1.7 ± 0.0 | - | - | - |
| 12 | 162.6 ± 17.0 | 23.3 ± 0.9 | 7.5 ± 0.1 | 1.8 ± 0.0 | - | - | - |
| 13 | 226.5 ± 25.0 | 37.7 ± 5.7 | 11.4 ± 0.9 | 15.1 ± 0.1 | - | - | - |
| 14 | 286.9 ± 12.5 | 24.3 ± 0.9 | 7.9 ± 0.1 | 4.3 ± 0.2 | - | - | - |
| 15 | 320.0 ± 35.6 | 46.0 ± 0.1 | 9.8 ± 0.5 | 5.8 ± 0.1 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, L.; Xu, X.; Zhang, Z.; Ding, Y.; Zhang, K.; Zhi, S. A Comprehensive Contamination Investigation of Bohai Bay Seawater: Antibiotics Occurrence, Distribution, Ecological Risks and Their Interactive Factors. Int. J. Environ. Res. Public Health 2023, 20, 1599. https://doi.org/10.3390/ijerph20021599
Tian L, Xu X, Zhang Z, Ding Y, Zhang K, Zhi S. A Comprehensive Contamination Investigation of Bohai Bay Seawater: Antibiotics Occurrence, Distribution, Ecological Risks and Their Interactive Factors. International Journal of Environmental Research and Public Health. 2023; 20(2):1599. https://doi.org/10.3390/ijerph20021599
Chicago/Turabian StyleTian, Liang, Xiaofu Xu, Zulin Zhang, Yongzhen Ding, Keqiang Zhang, and Suli Zhi. 2023. "A Comprehensive Contamination Investigation of Bohai Bay Seawater: Antibiotics Occurrence, Distribution, Ecological Risks and Their Interactive Factors" International Journal of Environmental Research and Public Health 20, no. 2: 1599. https://doi.org/10.3390/ijerph20021599
APA StyleTian, L., Xu, X., Zhang, Z., Ding, Y., Zhang, K., & Zhi, S. (2023). A Comprehensive Contamination Investigation of Bohai Bay Seawater: Antibiotics Occurrence, Distribution, Ecological Risks and Their Interactive Factors. International Journal of Environmental Research and Public Health, 20(2), 1599. https://doi.org/10.3390/ijerph20021599









