A Novel Adsorbent of Attapulgite & Carbon Composites Derived from Spent Bleaching Earth for Synergistic Removal of Copper and Tetracycline in Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Absorbent
2.2. Characterization of Absorbent
2.3. Adsorption Experiments
2.4. Adsorption Kinetics and Isotherms
3. Results
3.1. Characterization of A&Cs
3.2. Effect of Initial Concentration
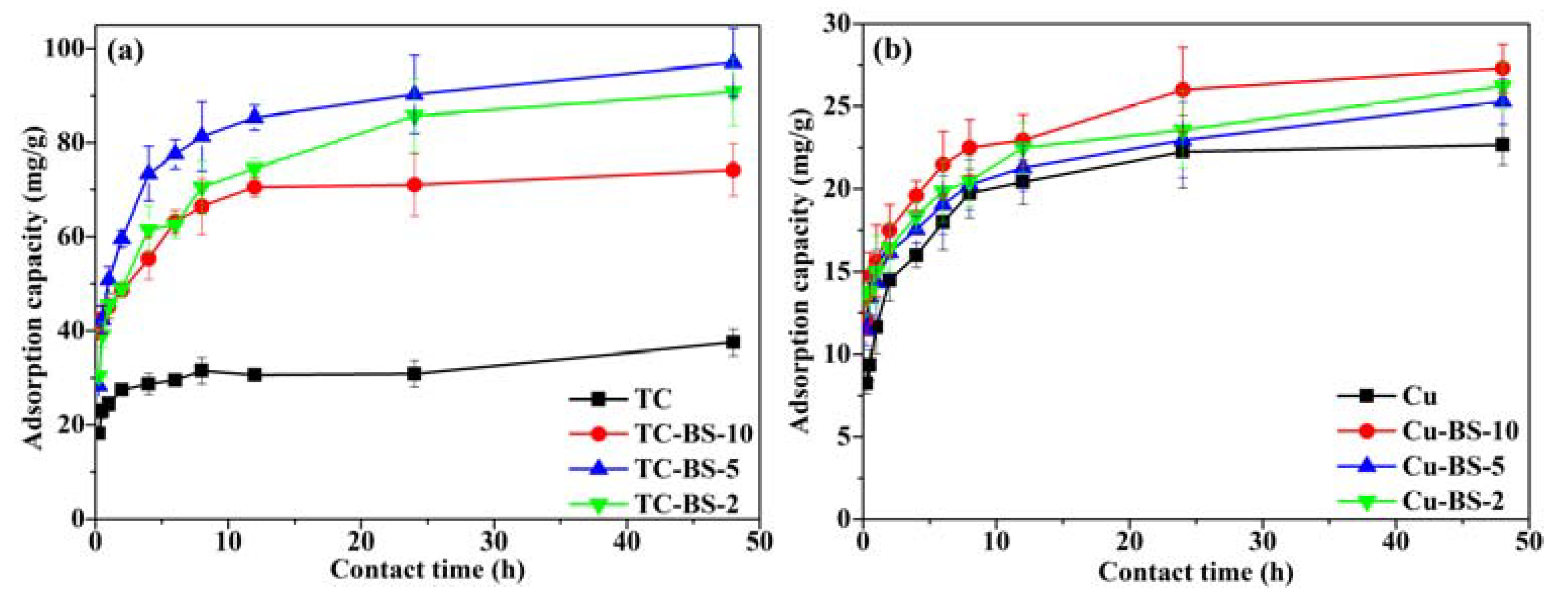
3.3. Effect of Contact Time
3.4. Adsorption Kinetics
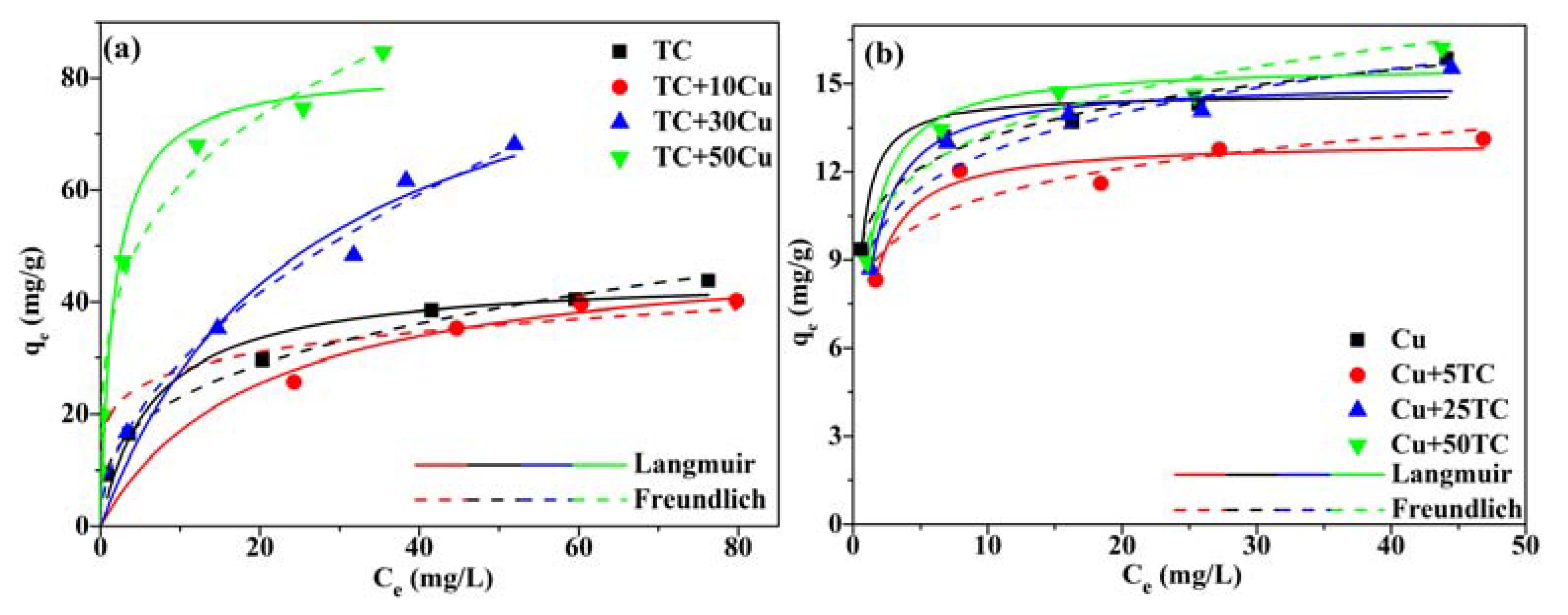
3.5. Adsorption Isotherms
3.6. Competitive Adsorption Analysis
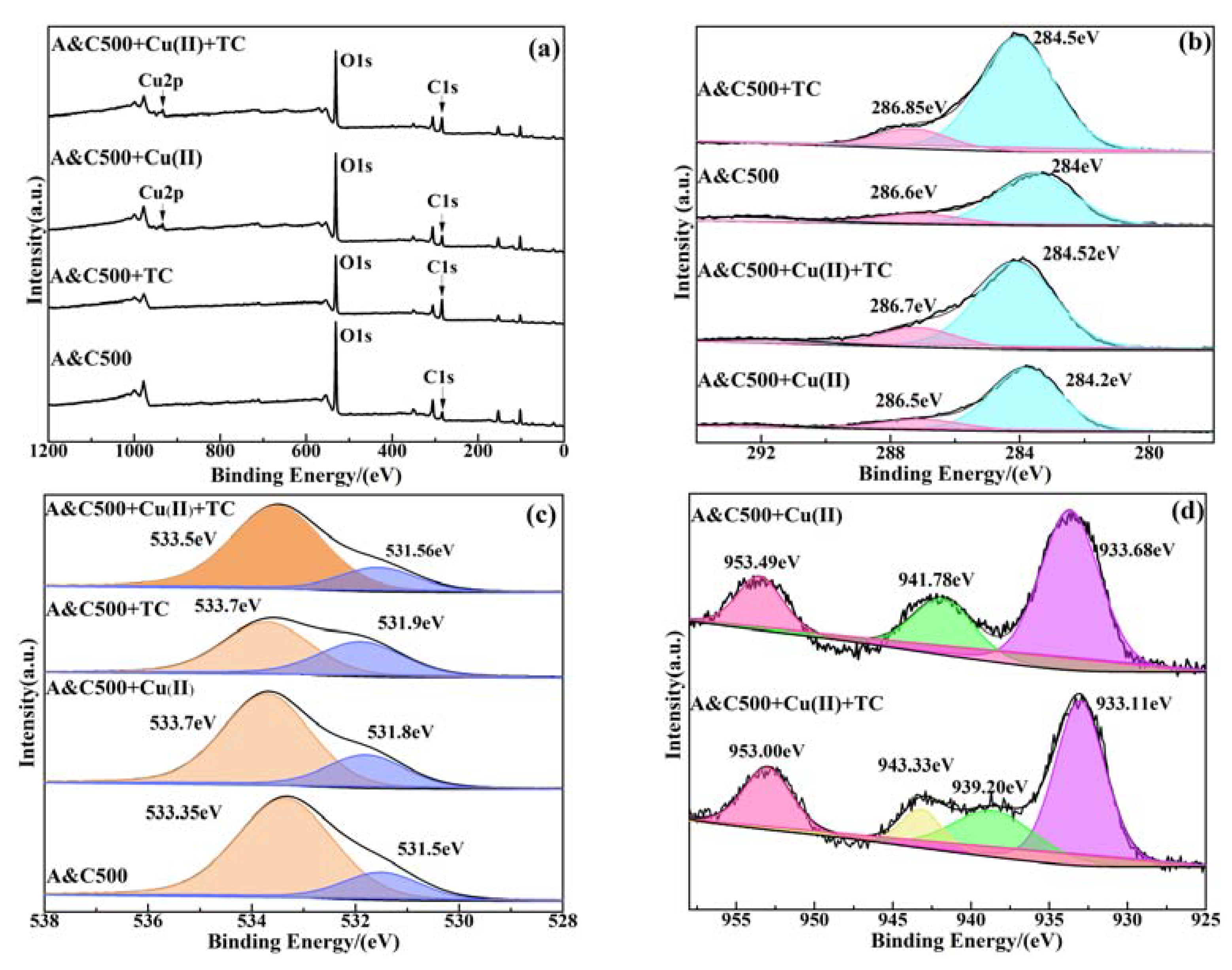
3.7. Adsorption Mechanisms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, D.M.; Feng, Y.; Liu, Y.W.; Li, J.P.; Xue, J.M.; Li, Z.J. Quantitative models for predicting adsorption of oxytetracycline, ciprofloxacin and sulfamerazine to swine manures with contrasting properties. Sci. Total Environ. 2018, 634, 1148–1156. [Google Scholar] [CrossRef]
- Ngigi, A.N.; Ok, Y.S.; Thiele-Bruhn, S. Biochar-mediated sorption of antibiotics in pig manure. J. Hazard. Mater. 2019, 364, 663–670. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Ying, G.G.; Pan, C.G.; Liu, Y.S.; Zhao, J.L. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: Source analysis, multimedia modeling, and linkage to bacterial resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [CrossRef]
- Saadati, F.; Keramati, N.; Ghazi, M.M. Influence of parameters on the photocatalytic degradation of tetracycline in wastewater: A review. Crit. Rev. Environ. Sci. Technol. 2016, 46, 757–782. [Google Scholar] [CrossRef]
- Xu, X.R.; Li, X.Y. Sorption and desorption of antibiotic tetracycline on marine sediments. Chemosphere 2010, 78, 430–436. [Google Scholar] [CrossRef]
- Ben, W.W.; Qiang, Z.M.; Adams, C.; Zhang, H.Q.; Chen, L.P. Simultaneous determination of sulfonamides, tetracyclines and tiamulin in swine wastewater by solid-phase extraction and liquid chromatography-mass spectrometry. J. Chromatogr. A 2008, 1202, 173–180. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Zhang, C.Q.; Parker, D.B.; Snow, D.D.; Zhou, Z.; Li, X. Occurrence of antimicrobials and antimicrobial resistance genes in beef cattle storage ponds and swine treatment lagoons. Sci. Total Environ. 2013, 463, 631–638. [Google Scholar] [CrossRef]
- Mahesar, S.A.; Sherazi, S.T.H.; Niaz, A.; Bhanger, M.I.; Rauf, A. Simultaneous assessment of zinc, cadmium, lead and copper in poultry feeds by differential pulse anodic stripping voltammetry. Food Chem. Toxicol. 2010, 48, 2357–2360. [Google Scholar] [CrossRef]
- Saeid, A.; Chojnacka, K.; Korczynski, M.; Korniewicz, D.; Dobrzanski, Z. Biomass of Spirulina maxima enriched by biosorption process as a new feed supplement for swine. J. Appl. Phycol. 2013, 25, 667–675. [Google Scholar] [CrossRef]
- Zhao, W.Y.; Deng, J.B.; Chi, S.L.; Wang, W.Z.; Xu, L.S.; Huang, Q.Z.; Zhang, Y.M.; Yu, X.M.; Xu, J.; Chen, Y.C.; et al. Sustainability assessment of topsoil ecology in Chongqing, China based on the application of livestock and poultry manure. J. Clean. Prod. 2022, 358, 131969. [Google Scholar] [CrossRef]
- Duan, B.L.; Feng, Q. Comparison of the potential ecological and human health risks of heavy metals from sewage sludge and livestock manure for agricultural use. Toxics 2021, 9, 145. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.L.; Luo, W.; Wang, X.X.; Liao, R.; Yu, S.C.; Hong, M.X.; Zhao, C.X.; Yang, B.J.; Liu, Y.; et al. Inhibition of humic acid on copper pollution caused by chalcopyrite biooxidation. Sci. Total Environ. 2022, 851, 158200. [Google Scholar] [CrossRef] [PubMed]
- Bashir, A.; Malik, L.A.; Ahad, S.; Manzoor, T.; Bhat, M.A.; Dar, G.N.; Pandith, A.H. Removal of heavy metal ions from aqueous system by ion-exchange and biosorption methods. Environ. Chem. Lett. 2019, 17, 739–754. [Google Scholar] [CrossRef]
- Ji, L.; Wan, Y.; Zheng, S.; Zhu, D. Adsorption of tetracycline and sulfamethoxazole on crop residue-derived ashes: Implication for the relative importance of black carbon to soil sorption. Environ. Sci. Technol. 2011, 45, 5580–5586. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, R.; Patel, A.K.; Nguyen, T.B.; Singhania, R.R.; Chen, C.W.; Dong, C.D. Adsorption of copper (II) in aqueous solution using biochars derived from Ascophyllum nodosum seaweed. Bioresour. Technol. 2021, 328, 124829. [Google Scholar] [CrossRef] [PubMed]
- Leng, L.J.; Wei, L.; Xiong, Q.; Xu, S.Y.; Li, W.T.; Lv, S.; Lu, Q.; Wan, L.P.; Wen, Z.Y.; Zhou, W.G. Use of microalgae based technology for the removal of antibiotics from wastewater: A review. Chemosphere 2020, 238, 124680. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.T.; Wang, X.F.; Xiang, S.; Ding, Y.; Zhao, D.Y.; Hu, M.; Pan, Z.Y.; Varjani, S.; Wong, J.W.C.; Zhao, J. Self-cleaning Mn\\Zn ferrite/biochar adsorbents for effective removal of tetracycline. Sci. Total Environ. 2022, 844, 157202. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.R.; Patel, A.K.; Jaisi, D.P.; Adhikari, S.; Lu, H.; Khanal, S.K. Environmental application of biochar: Current status and perspectives. Bioresour. Technol. 2017, 246, 110–122. [Google Scholar] [CrossRef]
- Zhang, C.; Zeng, G.M.; Huang, D.L.; Lai, C.; Chen, M.; Cheng, M.; Tang, W.W.; Tang, L.; Dong, H.R.; Huang, B.B.; et al. Biochar for environmental management: Mitigating greenhouse gas emissions, contaminant treatment, and potential negative impacts. Chem. Eng. J. 2019, 373, 902–922. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, Y.H.; Wan, D.J.; Zhao, J.H.; He, Q.C.; Liu, Y.D. Synergistic adsorption and advanced oxidation activated by persulfate for degradation of tetracycline hydrochloride using iron-modified spent bleaching earth carbon. Environ. Sci. Pollut. Res. 2022, 29, 24704–24715. [Google Scholar] [CrossRef]
- Merikhy, A.; Heydari, A.; Eskandari, H.; Ghahraman-Rozegar, F. Carbonized spent bleaching earth as a low-cost adsorbent: A facile revalorization strategy via response surface methodology. Chem. Eng. Process. 2021, 158, 108167. [Google Scholar] [CrossRef]
- Zhu, X.L.; Zhang, T.; Wang, J.Q.; Zhao, H.H.; Shang, X.Q.; Shen, B.S.; Chen, C.; Wang, J. Adsorption effects of enrofloxacin by modified spent bleaching earth carbon composites. J. Agro-Environ. Sci. 2022, 41, 346–356. (In Chinese) [Google Scholar]
- Huang, F.; Gao, L.Y.; Wu, R.R.; Wang, H.; Xiao, R.B. Qualitative and quantitative characterization of adsorption mechanisms for Cd2+ by silicon-rich biochar. Sci. Total Environ. 2020, 731, 139163. [Google Scholar] [CrossRef]
- Wiriyathamcharoen, S.; Sarkar, S.; Jiemvarangkul, P.; Nguyen, T.T.; Klysubun, W.; Padungthon, S. Synthesis optimization of hybrid anion exchanger containing triethylamine functional groups and hydrated Fe(III) oxide nanoparticles for simultaneous nitrate and phosphate removal. Chem. Eng. J. 2020, 381, 122671. [Google Scholar] [CrossRef]
- Tang, J.; Zong, L.; Mu, B.; Kang, Y.R.; Wang, A.Q. Attapulgite/carbon composites as a recyclable adsorbent for antibiotics removal. Korean J. Chem. Eng. 2018, 35, 1650–1661. [Google Scholar] [CrossRef]
- Sui, L.; Tang, C.Y.; Du, Q.; Zhao, Y.; Cheng, K.; Yang, F. Preparation and characterization of boron-doped corn straw biochar: Fe (II) removal equilibrium and kinetics. J. Environ. Sci. 2021, 106, 116–123. [Google Scholar] [CrossRef]
- Ke, Y.X.; Cui, S.; Fu, Q.; Hough, R.; Zhang, Z.L.; Li, Y.F. Effects of pyrolysis temperature and aging treatment on the adsorption of Cd2+ and Zn2+ by coffee grounds biochar. Chemosphere 2022, 296, 134051. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Mu, B.; Zong, L.; Zheng, M.S.; Wang, A.Q. Fabrication of attapulgite/carbon composites from spent bleaching earth for the efficient adsorption of methylene blue. RSC Adv. 2015, 5, 38443–38451. [Google Scholar] [CrossRef]
- Tang, J.; Mu, B.; Zong, L.; Zheng, M.S.; Wang, A.Q. Facile and green fabrication of magnetically recyclable carboxyl-functionalized attapulgite/carbon nanocomposites derived from spent bleaching earth for wastewater treatment. Chem. Eng. J. 2017, 322, 102–114. [Google Scholar] [CrossRef]
- Tang, J.; Mu, B.; Zheng, M.S.; Wang, A.Q. One-step calcination of the spent bleaching earth for the efficient removal of heavy metal ions. ACS Sustain. Chem. Eng. 2015, 3, 1125–1135. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, W.B.; Zhang, J.P.; Liu, P.; Wang, A.Q. A comparative study about adsorption of natural palygorskite for methylene blue. Chem. Eng. J. 2015, 262, 390–398. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Lu, H.H.; Liu, Y.X.; Yang, S.M. Removal of phosphate from aqueous solution by SiO2-biochar nanocomposites prepared by pyrolysis of vermiculite treated algal biomass. RSC Adv. 2016, 6, 83534–83546. [Google Scholar] [CrossRef]
- Huang, S.; Dong, C.Q.; Huang, J.S.; Deng, Y.Y.; Jiang, J.L. Effects of temperature and sieving treatments on physicochemical characteristics and cadmium adsorption capacity for biochars derived from pig manure and rice straw. Trans. Chin. Soc. Agric. Eng. 2018, 34, 235–243. (In Chinese) [Google Scholar]
- Aberkane, D.; Meziti, C.; Ihaddaden, S.; Boukerroui, A.; Cagnon, B. Calcium alginate-regenerated spent bleaching earth composite beads for efficient removal of methylene blue. Can. J. Chem. Eng. 2021, 100, 1001–1012. [Google Scholar] [CrossRef]
- Yulikasari, A.; Nurhayati, E.; Utama, W.; Warmadewanthi, I. Characterization of spent bleaching earth as an adsorbent material for dye removal. J. Ecol. Eng. 2022, 23, 96–104. [Google Scholar] [CrossRef]
- Ying, M.W.; Azmi, M.; Ganesan, S. Synthesis and characterization of spent bleaching clay-based geopolymer: An effort towards palm oil industry waste reutilization. Chem.-Asian J. 2022, 17, e202200286. [Google Scholar] [CrossRef]
- Wan, D.J.; Chen, Y.; Shi, Y.H.; Liu, Y.D.; Xiao, S.H. Effective adsorption of bisphenol A from aqueous solution over a novel mesoporous carbonized material based on spent bleaching earth. Environ. Sci. Pollut. Res. 2021, 28, 40035–40048. [Google Scholar] [CrossRef]
- Zhang, C.F.; Yu, M.Y.; Li, X.T.; Li, X.G.; Liu, Y.; Jin, Y.J.; Dai, J.J.; Wang, L.; Zhou, C.B.; Zhang, Y.W.; et al. Disposal, regeneration and pyrolysis products characterization of spent bleaching clay from vegetable oil refinery in a fluidized bed pyrolyser. J. Clean. Prod. 2022, 346, 131157. [Google Scholar] [CrossRef]
- Awang, N.A.; Salleh, W.N.W.; Ismail, A.F.; Yusof, N.; Aziz, F.; Jaafar, J. Adsorption behavior of chromium(Ⅵ) onto regenerated cellulose membrane. Ind. Eng. Chem. Res. 2019, 58, 720–728. [Google Scholar] [CrossRef]
- Cantrell, K.B.; Hunt, P.G.; Uchimiya, M.; Novak, J.M.; Ro, K.S. Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour. Technol. 2012, 107, 419–428. [Google Scholar] [CrossRef]
- Trevino-Cordero, H.; Juarez-Aguilar, L.G.; Mendoza-Castillo, D.I.; Hernandez-Montoya, V.; Bonilla-Petriciolet, A.; Montes-Moran, M.A. Synthesis and adsorption properties of activated carbons from biomass of Prunus domestica and Jacaranda mimosifolia for the removal of heavy metals and dyes from water. Ind. Crop. Prod. 2013, 42, 315–323. [Google Scholar] [CrossRef]
- Feng, Y.P.; Chen, G.; Zhang, Y.J.; Li, D.G.; Ling, C.; Wang, Q.Y.; Liu, G.G. Superhigh co-adsorption of tetracycline and copper by the ultrathin g-C3N4 modified graphene oxide hydrogels. J. Hazard. Mater. 2022, 424, 127362. [Google Scholar] [CrossRef]
- Dai, Y.J.; Zhang, K.X.; Meng, X.B.; Li, J.J.; Guan, X.T.; Sun, Q.Y.; Sun, Y.; Wang, W.S.; Lin, M.; Liu, M.; et al. New use for spent coffee ground as an adsorbent for tetracycline removal in water. Chemosphere 2019, 215, 163–172. [Google Scholar] [CrossRef]
- Fan, Y.Y.; Lan, H.C.; Qi, Z.L.; Liu, R.P.; Hu, C.Z. Removal of nickel and copper ions in strongly acidic conditions by in-situ formed amyloid fibrils. Chemosphere 2022, 297, 134241. [Google Scholar] [CrossRef]
- Bogusz, A.; Oleszczuk, P.; Dobrowolski, R. Application of laboratory prepared and commercially available biochars to adsorption of cadmium, copper and zinc ions from water. Bioresour. Technol. 2015, 196, 540–549. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Liu, X.C.; Xiang, Y.J.; Wang, P.; Zhang, J.C.; Zhang, F.F.; Wei, J.H.; Luo, L.; Lei, M.; Tang, L. Modification of biochar derived from sawdust and its application in removal of tetracycline and copper from aqueous solution: Adsorption mechanism and modelling. Bioresour. Technol. 2017, 245, 266–273. [Google Scholar] [CrossRef]
- Al-Khateeb, L.A.; Almotiry, S.; Salam, M.A. Adsorption of pharmaceutical pollutants onto graphene nanoplatelets. Chem. Eng. J. 2014, 248, 191–199. [Google Scholar] [CrossRef]
- Li, X.; Huang, Y.F.; Liang, X.L.; Huang, L.X.; Wei, L.; Zheng, X.D.; Albert, H.A.; Huang, Q.; Liu, Z.Z.; Li, Z.J. Characterization of biochars from woody agricultural wastes and sorption behavior comparison of cadmium and atrazine. Biochar 2022, 4, 27. [Google Scholar] [CrossRef]
- Ahmad, S.; Gao, F.L.; Lyu, H.H.; Ma, J.K.; Zhao, B.B.; Xu, S.Y.; Ri, C.; Tang, J.C. Temperature-dependent carbothermally reduced iron and nitrogen doped biochar composites for removal of hexavalent chromium and nitrobenzene. Chem. Eng. J. 2022, 450, 138006. [Google Scholar] [CrossRef]
- Shao, P.L.; Yin, H.; Li, Y.C.; Cai, Y.H.; Yan, C.Y.; Yuan, Y.B.; Dang, Z. Remediation of Cu and As contaminated water and soil utilizing biochar supported layered double hydroxide: Mechanisms and soil environment altering. J. Environ. Sci. 2022, 126, 275–286. [Google Scholar] [CrossRef]
- Meng, J.; Feng, X.L.; Dai, Z.M.; Liu, X.M.; Wu, J.J.; Xu, J.M. Adsorption characteristics of Cu(II) from aqueous solution onto biochar derived from swine manure. Environ. Sci. Pollut. Res. 2014, 21, 7035–7046. [Google Scholar] [CrossRef]
- Paulino, A.G.; da Cunha, A.J.; Alfaya, R.V.D.; Alfaya, A.A.D. Chemically modified natural cotton fiber: A low-cost biosorbent for the removal of the Cu(II), Zn(II), Cd(II), and Pb(II) from natural water. Desalin. Water Treat. 2014, 52, 4223–4233. [Google Scholar] [CrossRef]
- Li, L.; Cao, J. Study on the adsorption properties of copper from aqueous solution by blast furnace slag. Appl. Chem. Ind. 2016, 45, 1299–1303. (In Chinese) [Google Scholar]
- Liu, Y.; Chen, Q.; Singh, R.P. Low-cost RSAC and adsorption characteristics in the removal of copper ions from wastewater. Appl. Sci. 2022, 12, 5612. [Google Scholar] [CrossRef]
- Ding, R.; Zhang, P.F.; Seredych, M.; Bandosz, T.J. Removal of antibiotics from water using sewage sludge- and waste oil sludge-derived adsorbents. Water Res. 2012, 46, 4081–4090. [Google Scholar] [CrossRef]
- Lin, Y.X.; Xu, S.; Jia, L. Fast and highly efficient tetracyclines removal from environmental waters by graphene oxide functionalized magnetic particles. Chem. Eng. J. 2013, 225, 679–685. [Google Scholar] [CrossRef]
- Anton-Herrero, R.; Garcia-Delgado, C.; Alonso-Izquierdo, M.; Garcia-Rodriguez, G.; Cuevas, J.; Eymar, E. Comparative adsorption of tetracyclines on biochars and stevensite: Looking for for the most effective adsorbent. Appl. Clay Sci. 2018, 160, 163–172. [Google Scholar] [CrossRef]
- Caroni, A.L.P.F.; de Lima, C.R.M.; Pereira, M.R.; Fonseca, J.L.C. Tetracycline adsorption on chitosan: A mechanistic description based on mass uptake and zeta potential measurements. Colloid. Surf. B-Biointerfaces 2012, 100, 222–228. [Google Scholar] [CrossRef]
- Mihaly-Cozmuta, L.; Mihaly-Cozmuta, A.; Peter, A.; Nicula, C.; Tutu, H.; Silipas, D.; Indrea, E. Adsorption of heavy metal cations by Na-clinoptilolite: Equilibrium and selectivity studies. J. Environ. Manag. 2014, 137, 69–80. [Google Scholar] [CrossRef]
- Li, K.; Wang, Y.W.; Huang, M.; Yan, H.; Yang, H.; Xiao, S.J.; Li, A.M. Preparation of chitosan-graft-polyacrylamide magnetic composite microspheres for enhanced selective removal of mercury ions from water. J. Colloid Interface Sci. 2015, 455, 261–270. [Google Scholar] [CrossRef]
- Batool, S.; Idrees, M.; Hussain, Q.; Kong, J. Adsorption of copper (II) by using derived-farmyard and poultry manure biochars: Efficiency and mechanism. Chem. Phys. Lett. 2017, 689, 190–198. [Google Scholar] [CrossRef]
- Cui, S.; Ke, Y.X.; Fu, Q.; Hough, R.; Zhang, Z.L.; Shen, Z.X.; An, L.H.; Li, Y.F. Optimization preparation of biochar from garden waste and quantitative analysis for Cd2+ adsorption mechanism in aqueous solution. Biomass Convers. Biorefinery 2022. [Google Scholar] [CrossRef]
- Deng, J.Q.; Liu, Y.Q.; Liu, S.B.; Zeng, G.M.; Tan, X.F.; Huang, B.Y.; Tang, X.J.; Wang, S.F.; Hua, Q.; Yan, Z.L. Competitive adsorption of Pb(II), Cd(II) and Cu(II) onto chitosan-pyromellitic dianhydride modified biochar. J. Colloid Interface Sci. 2017, 506, 355–364. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Li, Y.; Ding, L.; Yu, J.; Zhou, Q.; Kong, Y.L.; Ma, J.Y. Novel sodium bicarbonate activation of cassava ethanol sludge derived biochar for removing tetracycline from aqueous solution: Performance assessment and mechanism insight. Bioresour. Technol. 2021, 330, 124949. [Google Scholar] [CrossRef]
- Hu, Z.T.; Ding, Y.; Shao, Y.; Cai, L.; Jin, Z.Y.; Liu, Z.; Zhao, J.; Li, F.; Pan, Z.; Li, X.; et al. Banana peel biochar with nanoflake-assembled structure for cross contamination treatment in water: Interaction behaviors between lead and tetracycline. Chem. Eng. J. 2021, 420, 129807. [Google Scholar] [CrossRef]
- Ngambia, A.; Ifthikar, J.; Shahib, I.I.; Jawad, A.; Shahzad, A.; Zhao, M.M.; Wang, J.; Chen, Z.L.; Chen, Z.Q. Adsorptive purification of heavy metal contaminated wastewater with sewage sludge derived carbon-supported Mg(II) composite. Sci. Total Environ. 2020, 691, 306–321. [Google Scholar] [CrossRef]
- Chen, T.W.; Luo, L.; Deng, S.H.; Shi, G.Z.; Zhang, S.R.; Zhang, Y.Z.; Deng, O.P.; Wang, L.L.; Zhang, J.; Wei, L.Y. Sorption of tetracycline on H3PO4 modified biochar derived from rice straw and swine manure. Bioresour. Technol. 2018, 267, 431–437. [Google Scholar] [CrossRef]
- Chen, Y.N.; Li, M.L.; Li, Y.P.; Liu, Y.H.; Chen, Y.R.; Li, H.; Li, L.S.Z.; Xu, F.T.; Jiang, H.J.; Chen, L. Hydroxyapatite modified sludge-based biochar for the adsorption of Cu2+ and Cd2+: Adsorption behavior and mechanisms. Bioresour. Technol. 2021, 321, 124413. [Google Scholar] [CrossRef]
- Zhu, X.D.; Liu, Y.C.; Qian, F.; Zhou, C.; Zhang, S.C.; Chen, J.M. Preparation of magnetic porous carbon from waste hydrochar by simultaneous activation and magnetization for tetracycline removal. Bioresour. Technol. 2014, 154, 209–214. [Google Scholar] [CrossRef]
- Wang, R.Z.; Huang, D.L.; Liu, Y.G.; Zhang, C.; Lai, C.; Wang, X.; Zeng, G.M.; Zhang, Q.; Gong, X.M.; Xu, P. Synergistic removal of copper and tetracycline from aqueous solution by steam-activated bamboo-derived biochar. J. Hazard. Mater. 2020, 384, 121470. [Google Scholar] [CrossRef]
- Liu, S.; Xu, W.H.; Liu, Y.G.; Tan, X.F.; Zeng, G.M.; Li, X.; Liang, J.; Zhou, Z.; Yan, Z.L.; Cai, X.X. Facile synthesis of Cu(II) impregnated biochar with enhanced adsorption activity for the removal of doxycycline hydrochloride from water. Sci. Total Environ. 2017, 592, 546–553. [Google Scholar] [CrossRef]



| Adsorbent | Cu(II) (mg/g) | TC (mg/g) | References |
|---|---|---|---|
| A&C500 | 14.70 | 44.90 | This study |
| Swine manure biochar | 8.98 | [51] | |
| Cotton fiber | 6.12 | [52] | |
| Blast furnace slag | 8.49 | [53] | |
| Residue & soil adsorption composite | 2.63 | [54] | |
| Sludge-derived adsorbent | 30.00 | [55] | |
| Graphene oxide functionalized magnetic particles | 39.10 | [56] | |
| Oak biochar | 11.85 | [57] | |
| Chitosan | 31.00 | [58] |
| C0 | Kd (TC) | Kd (Cu) | |||
|---|---|---|---|---|---|
| A&C500 | 10 | 249.376 | 7.418 | 33.620 | 0.030 |
| 50 | 16.585 | 0.929 | 17.845 | 0.056 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ke, Y.; Zhu, X.; Si, S.; Zhang, T.; Wang, J.; Zhang, Z. A Novel Adsorbent of Attapulgite & Carbon Composites Derived from Spent Bleaching Earth for Synergistic Removal of Copper and Tetracycline in Water. Int. J. Environ. Res. Public Health 2023, 20, 1573. https://doi.org/10.3390/ijerph20021573
Ke Y, Zhu X, Si S, Zhang T, Wang J, Zhang Z. A Novel Adsorbent of Attapulgite & Carbon Composites Derived from Spent Bleaching Earth for Synergistic Removal of Copper and Tetracycline in Water. International Journal of Environmental Research and Public Health. 2023; 20(2):1573. https://doi.org/10.3390/ijerph20021573
Chicago/Turabian StyleKe, Yuxin, Xiaoli Zhu, Shaocheng Si, Ting Zhang, Junqiang Wang, and Ziye Zhang. 2023. "A Novel Adsorbent of Attapulgite & Carbon Composites Derived from Spent Bleaching Earth for Synergistic Removal of Copper and Tetracycline in Water" International Journal of Environmental Research and Public Health 20, no. 2: 1573. https://doi.org/10.3390/ijerph20021573
APA StyleKe, Y., Zhu, X., Si, S., Zhang, T., Wang, J., & Zhang, Z. (2023). A Novel Adsorbent of Attapulgite & Carbon Composites Derived from Spent Bleaching Earth for Synergistic Removal of Copper and Tetracycline in Water. International Journal of Environmental Research and Public Health, 20(2), 1573. https://doi.org/10.3390/ijerph20021573





