Impact of Rehabilitation on Physical and Neuropsychological Health of Patients Who Acquired COVID-19 in the Workplace
Abstract
1. Introduction
1.1. Physical Health in Long-/Post-COVID
1.2. Neuropsychological Health in Long-/Post-COVID
2. Materials and Methods
2.1. Study Design and Participants
2.2. Measurements of Sociodemographic Variables, Anamnesis, and Post-COVID Symptoms
2.3. Physical Performance Measurements
2.4. Neuropsychological Health Measurements
2.5. Employment and Work Ability Measurements
2.6. Statistical Analyses
3. Results
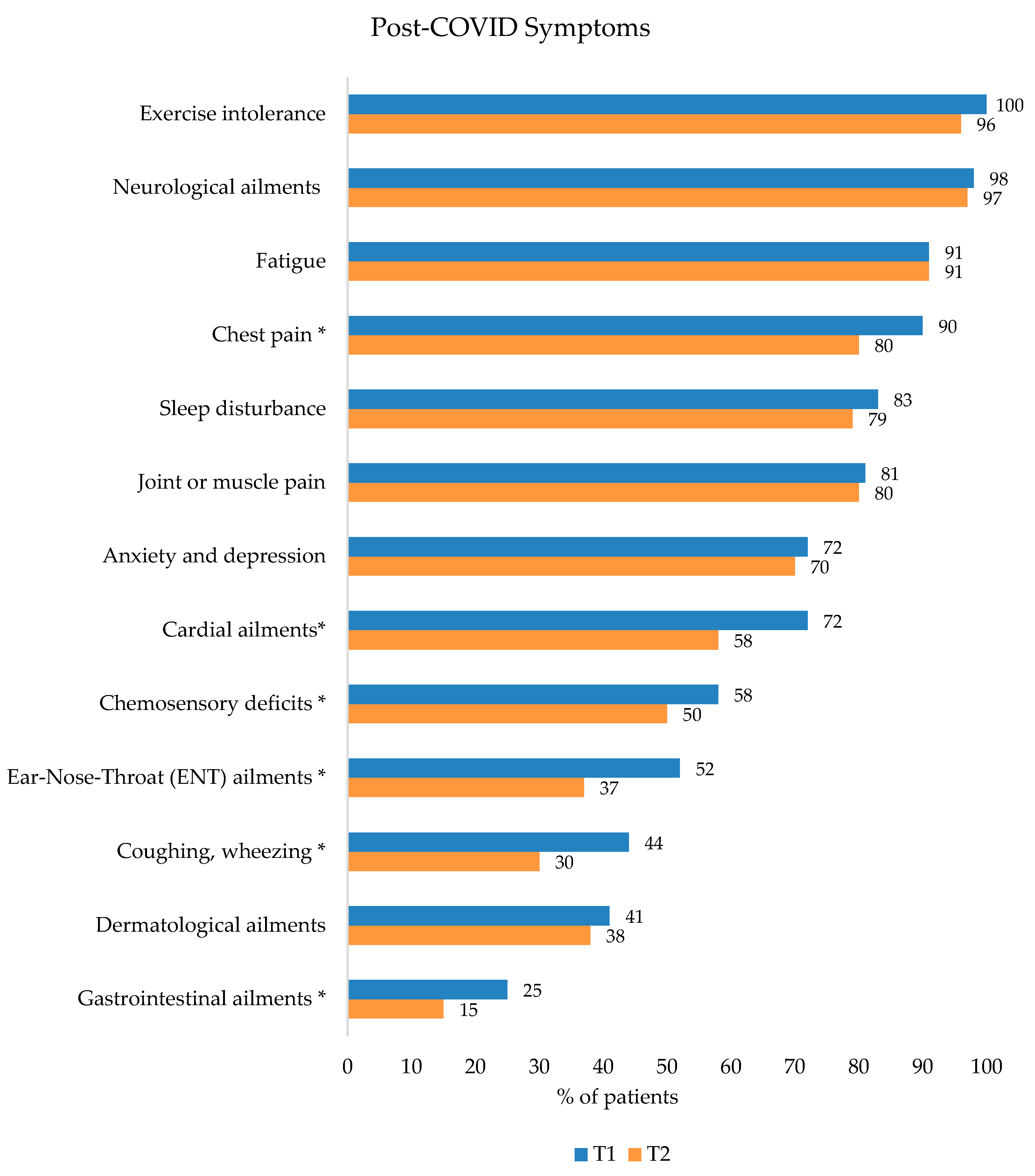
3.1. COVID-19 Infection, Risk Factors, and Post-COVID Symptoms
3.2. Group Differences at the Beginning of Rehabilitation in Comparison to Sex, COVID-Status, and Employment
3.2.1. Physical Health
3.2.2. Neuropsychological Health
3.2.3. Work Ability
3.3. Rehabilitation Outcomes
3.3.1. Physical Health
3.3.2. Neuropsychological Health
3.3.3. Work Ability
4. Discussion
4.1. Post-COVID Symptoms
4.2. Physical Health
4.3. Neuropsychological Health
4.4. Work Ability
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kluge, S.; Rabe, K.F. S3-Leitlinie Empfehlungen zur Stationären Therapie von Patienten mit COVID-19—Living Guideline. (S3-Guidline Recommendations for Inpatient Therapy of COVID-19 Patients—Living Guideline). Available online: https://register.awmf.org/assets/guidelines/113-001LGl_S3_Empfehlungen-zur-stationaeren-Therapie-von-Patienten-mit-COVID-19_2022-09_1.pdf (accessed on 30 November 2022).
- Khalatbari-Soltani, S.; Cumming, R.G.; Delpierre, C.; Kelly-Irving, M. Importance of collecting data on socioeconomic determinants from the early stage of the COVID-19 outbreak onwards. J. Epidemiol. Community Health 2020, 74, 620–623. [Google Scholar] [CrossRef]
- Burdorf, A.; Porru, F.; Rugulies, R. The COVID-19 pandemic: One year later—An occupational perspective. Scand. J. Work Environ. Health 2021, 47, 245–247. [Google Scholar] [CrossRef]
- Reuter, M.; Rigo, M.; Formazin, M.; Liebers, F.; Latza, U.; Castell, S.; Jockel, K.H.; Greiser, K.H.; Michels, K.B.; Krause, G.; et al. Occupation and SARS-CoV-2 infection risk among 108 960 workers during the first pandemic wave in Germany. Scand. J. Work Environ. Health 2022, 48, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Alshamrani, M.M.; El-Saed, A.; Al Zunitan, M.; Almulhem, R.; Almohrij, S. Risk of COVID-19 morbidity and mortality among healthcare workers working in a Large Tertiary Care Hospital. Int. J. Infect. Dis. 2021, 109, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Mutambudzi, M.; Niedzwiedz, C.; Macdonald, E.B.; Leyland, A.; Mair, F.; Anderson, J.; Celis-Morales, C.; Cleland, J.; Forbes, J.; Gill, J.; et al. Occupation and risk of severe COVID-19: Prospective cohort study of 120 075 UK Biobank participants. Occup. Environ. Med. 2021, 78, 307–314. [Google Scholar] [CrossRef]
- Gómez-Ochoa, S.A.; Franco, O.H.; Rojas, L.Z.; Raguindin, P.F.; Roa-Díaz, Z.M.; Wyssmann, B.M.; Guevara, S.L.R.; Echeverría, L.E.; Glisic, M.; Muka, T. COVID-19 in health-care workers: A living systematic review and meta-analysis of prevalence, risk factors, clinical characteristics, and outcomes. Am. J. Epidemiol. 2020, 190, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Deutsche Gesetzliche Unfallversicherung (German Social Accident Insurance). Berufskrankheiten und Arbeitsunfälle im Zusammenhang mit COVID-19. (Occupational Disease and Occupational Accidents According to COVID-19). Available online: https://www.dguv.de/medien/inhalt/mediencenter/hintergrund/covid/dguv_zahlen_covid.pdf (accessed on 30 November 2022).
- Gualano, M.R.; Rossi, M.F.; Borrelli, I.; Santoro, P.E.; Amantea, C.; Daniele, A.; Tumminello, A.; Moscato, U. Returning to work and the impact of post COVID-19 condition: A systematic review. Work 2022, 73, 405–413. [Google Scholar] [CrossRef] [PubMed]
- D’Ettorre, G.; Gentilini Cacciola, E.; Santinelli, L.; De Girolamo, G.; Spagnolello, O.; Russo, A.; Tarsitani, L.; Ciccozzi, M.; Mastroianni, C.M.; D’Ettorre, G.; et al. COVID-19 sequelae in working age patients: A systematic review. J. Med. Virol. 2022, 94, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Wachtler, B.; Neuhauser, H.; Haller, S.; Grabka, M.M.; Zinn, S.; Schaade, L.; Hövener, C.; Hoebel, J. The risk of infection with SARS-CoV-2 among healthcare workers during the pandemic. Dtsch Arztebl. Int. 2021, 118, 842–843. [Google Scholar] [CrossRef] [PubMed]
- Ferland, L.; Carvalho, C.; Gomes Dias, J.; Lamb, F.; Adlhoch, C.; Suetens, C.; Beauté, J.; Kinross, P.; Plachouras, D.; Hannila-Handelberg, T.; et al. Risk of hospitalization and death for healthcare workers with COVID-19 in nine European countries, January 2020–January 2021. J. Hosp. Infect. 2022, 119, 170–174. [Google Scholar] [CrossRef]
- Tabacof, L.; Tosto-Mancuso, J.; Wood, J.; Cortes, M.; Kontorovich, A.; McCarthy, D.; Rizk, D.; Rozanski, G.; Breyman, E.; Nasr, L.; et al. Post-acute COVID-19 syndrome negatively impacts physical function, cognitive function, health-related quality of life, and participation. Am. J. Phys. Med. Rehabil. 2022, 101, 48–52. [Google Scholar] [CrossRef]
- Delgado-Alonso, C.; Cuevas, C.; Oliver-Mas, S.; Díez-Cirarda, M.; Delgado-Álvarez, A.; Gil-Moreno, M.J.; Matías-Guiu, J.; Matias-Guiu, J.A. Fatigue and cognitive dysfunction are associated with occupational status in post-COVID syndrome. Int. J. Environ. Res. Public Health 2022, 19, 13368. [Google Scholar] [CrossRef]
- Mendola, M.; Leoni, M.; Cozzi, Y.; Manzari, A.; Tonelli, F.; Metruccio, F.; Tosti, L.; Battini, V.; Cucchi, I.; Costa, M.C.; et al. Long-term COVID symptoms, work ability and fitness to work in healthcare workers hospitalized for Sars-CoV-2 infection. Med. Lav. 2022, 113, e2022040. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.; Dulon, M.; Westermann, C.; Kozak, A.; Nienhaus, A. Long-Term Effects of COVID-19 on Workers in Health and Social Services in Germany. Int. J. Environ. Res. Public Health 2022, 19, 6983. [Google Scholar] [CrossRef] [PubMed]
- Almas, T.; Malik, J.; Alsubai, A.K.; Jawad Zaidi, S.M.; Iqbal, R.; Khan, K.; Ali, M.; Ishaq, U.; Alsufyani, M.; Hadeed, S.; et al. Post-acute COVID-19 syndrome and its prolonged effects: An updated systematic review. Ann. Med. Surg. 2022, 80, 103995. [Google Scholar] [CrossRef] [PubMed]
- Bastola, A.; Nepal, R.; Shrestha, B.; Maharjan, K.; Shrestha, S.; Chalise, B.S.; Neupane, J. Persistent symptoms in post-COVID-19 patients attending follow-up OPD at Sukraraj Tropical and Infectious Disease Hospital (STIDH), Kathmandu, Nepal. Trop. Med. Infect. Dis. 2021, 6, 113. [Google Scholar] [CrossRef]
- Carfì, A.; Bernabei, R.; Landi, F. Persistent symptoms in patients after acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef]
- Sivan, M.; Taylor, S. NICE guideline on long covid. BMJ 2020, 371, m4938. [Google Scholar] [CrossRef]
- Chen, C.; Haupert, S.R.; Zimmermann, L.; Shi, X.; Fritsche, L.G.; Mukherjee, B. Global prevalence of post-coronavirus disease 2019 (COVID-19) condition or long COVID: A meta-analysis and systematic review. J. Infect. Dis. 2022, 226, 1593–1607. [Google Scholar] [CrossRef]
- Rooney, S.; Webster, A.; Paul, L. Systematic review of changes and recovery in physical function and fitness after severe acute respiratory syndrome-related coronavirus infection: Implications for COVID-19 rehabilitation. Phys. Ther. 2020, 100, 1717–1729. [Google Scholar] [CrossRef]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Sanchez-Ramirez, D.C.; Normand, K.; Zhaoyun, Y.; Torres-Castro, R. Long-term impact of COVID-19: A systematic review of the literature and meta-analysis. Biomedicines 2021, 9, 900. [Google Scholar] [CrossRef]
- Baricich, A.; Borg, M.B.; Cuneo, D.; Cadario, E.; Azzolina, D.; Balbo, P.E.; Bellan, M.; Zeppegno, P.; Pirisi, M.; Cisari, C. Midterm functional sequelae and implications in rehabilitation after COVID-19: A cross-sectional study. Eur. J. Phys. Rehabil. Med. 2021, 57, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Taboada, M.; Cariñena, A.; Moreno, E.; Rodríguez, N.; Domínguez, M.J.; Casal, A.; Riveiro, V.; Diaz-Vieito, M.; Valdés, L.; Alvarez, J.; et al. Post-COVID-19 functional status six-months after hospitalization. J. Infect. 2021, 82, e31–e33. [Google Scholar] [CrossRef]
- Halpin, S.J.; McIvor, C.; Whyatt, G.; Adams, A.; Harvey, O.; McLean, L.; Walshaw, C.; Kemp, S.; Corrado, J.; Singh, R.; et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: A cross-sectional evaluation. J. Med. Virol. 2021, 93, 1013–1022. [Google Scholar] [CrossRef]
- Paneroni, M.; Simonelli, C.; Saleri, M.; Bertacchini, L.; Venturelli, M.; Troosters, T.; Ambrosino, N.; Vitacca, M. Muscle strength and physical performance in patients without previous disabilities recovering from COVID-19 pneumonia. Am. J. Phys. Med. Rehabil. 2021, 100, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Kedor, C.; Freitag, H.; Meyer-Arndt, L.; Wittke, K.; Hanitsch, L.G.; Zoller, T.; Steinbeis, F.; Haffke, M.; Rudolf, G.; Heidecker, B.; et al. A prospective observational study of post-COVID-19 chronic fatigue syndrome following the first pandemic wave in Germany and biomarkers associated with symptom severity. Nat. Commun. 2022, 13, 5104. [Google Scholar] [CrossRef] [PubMed]
- Rinaldo, R.F.; Mondoni, M.; Parazzini, E.M.; Pitari, F.; Brambilla, E.; Luraschi, S.; Balbi, M.; Sferrazza Papa, G.F.; Sotgiu, G.; Guazzi, M.; et al. Deconditioning as main mechanism of impaired exercise response in COVID-19 survivors. Eur. Respir. J. 2021, 58, 2100870. [Google Scholar] [CrossRef]
- Skjørten, I.; Ankerstjerne, O.A.W.; Trebinjac, D.; Brønstad, E.; Rasch-Halvorsen, Ø.; Einvik, G.; Lerum, T.V.; Stavem, K.; Edvardsen, A.; Ingul, C.B. Cardiopulmonary exercise capacity and limitations 3 months after COVID-19 hospitalisation. Eur. Respir. J. 2021, 58, 2100996. [Google Scholar] [CrossRef] [PubMed]
- Liao, T.; Meng, D.; Xiong, L.; Wu, S.; Yang, L.; Wang, S.; Zhou, M.; He, X.; Cao, X.; Xiong, H.; et al. Long-term effects of COVID-19 on health care workers 1-year post-discharge in Wuhan. Infect. Dis. Ther. 2022, 11, 145–163. [Google Scholar] [CrossRef]
- Hasenoehrl, T.; Palma, S.; Huber, D.F.X.; Kastl, S.; Steiner, M.; Jordakieva, G.; Crevenna, R. Post-COVID: Effects of physical exercise on functional status and work ability in health care personnel. Disabil. Rehabil. 2022, 13, 5104. [Google Scholar] [CrossRef]
- Ahmed, I.; Mustafaoglu, R.; Yeldan, I.; Yasaci, Z.; Erhan, B. Effect of pulmonary rehabilitation approaches on dyspnea, exercise capacity, fatigue, lung functions, and quality of life in patients with COVID-19: A systematic review and meta-analysis. Arch. Phys. Med. Rehabil. 2022, 103, 2051–2062. [Google Scholar] [CrossRef]
- Bailly, M.; Pelissier, L.; Coudeyre, E.; Evrard, B.; Bingula, R.; Rochette, C.; Meriade, L.; Blavignac, C.; Fournier, A.C.; Bignon, Y.J.; et al. Systematic review of COVID-19-related physical activity-based rehabilitations: Benefits to be confirmed by more robust methodological approaches. Int. J. Environ. Res. Public Health 2022, 19, 9025. [Google Scholar] [CrossRef]
- Glöckl, R.; Leitl, D.; Jarosch, I.; Schneeberger, T.; Nell, C.; Stenzel, N.; Vogelmeier, C.F.; Kenn, K.; Koczulla, A.R. Benefits of pulmonary rehabilitation in COVID-19: A prospective observational cohort study. ERJ Open Res. 2021, 7, 00108–2021. [Google Scholar] [CrossRef]
- Nopp, S.; Moik, F.; Klok, F.A.; Gattinger, D.; Petrovic, M.; Vonbank, K.; Koczulla, A.R.; Ay, C.; Zwick, R.H. Outpatient pulmonary rehabilitation in patients with long COVID improves exercise capacity, functional status, dyspnea, fatigue, and quality of life. Respiration 2022, 101, 593–601. [Google Scholar] [CrossRef]
- Chen, H.; Shi, H.; Liu, X.; Sun, T.; Wu, J.; Liu, Z. Effect of pulmonary rehabilitation for patients with post-COVID-19: A systematic review and meta-analysis. Front. Med. 2022, 9, 837420. [Google Scholar] [CrossRef]
- Al-Mhanna, S.B.; Mohamed, M.; Noor, N.M.; Afolabi, H.A.; Irekeola, A.A.; Bello, K.E.; Aldhahi, M.I.; Wan Ghazali, W.S. Effectiveness of pulmonary rehabilitation among COVID-19 patients: A systematic review and meta-analysis. Healthcare 2022, 10, 2130. [Google Scholar] [CrossRef]
- Vahtra, M.; Fahey, K.; Malina, A.; Dreyer, S.; Roth, E.; Grafman, J.; Jayabalan, P.; Cohen-Zimerman, S. Cognitive status and rehabilitation outcomes of patients in acute rehabilitation post COVID-19. medRxiv 2022. [Google Scholar] [CrossRef]
- Crivelli, L.; Palmer, K.; Calandri, I.; Guekht, A.; Beghi, E.; Carroll, W.; Frontera, J.; García-Azorín, D.; Westenberg, E.; Winkler, A.S.; et al. Changes in cognitive functioning after COVID-19: A systematic review and meta-analysis. Alzheimer’s Dement. 2022, 18, 1047–1066. [Google Scholar] [CrossRef] [PubMed]
- Ceban, F.; Ling, S.; Lui, L.M.W.; Lee, Y.; Gill, H.; Teopiz, K.M.; Rodrigues, N.B.; Subramaniapillai, M.; Di Vincenzo, J.D.; Cao, B.; et al. Fatigue and cognitive impairment in post-COVID-19 syndrome: A systematic review and meta-analysis. Brain. Behav. Immun. 2022, 101, 93–135. [Google Scholar] [CrossRef] [PubMed]
- Omar, A.K.A.-E.; Dahesh, S.M.A.; Ellakwa, D.E.-S.; Gomaa, M.K.; Abdulsamad, B.; Hanafy, R.; Al Metwally, H.G.; Mohammad, R.N.E.M.; Badawy, S.S.; El Saleh, R.M.; et al. Cognitive impairment in health care workers recovering from COVID-19 infection: A cross-sectional comparative study. Middle East Curr. Psychiatry 2022, 29, 79. [Google Scholar] [CrossRef]
- Dressing, A.; Bormann, T.; Blazhenets, G.; Schroeter, N.; Walter, L.I.; Thurow, J.; August, D.; Hilger, H.; Stete, K.; Gerstacker, K.; et al. Neuropsychologic profiles and cerebral glucose metabolism in neurocognitive long COVID syndrome. J. Nucl. Med. 2022, 63, 1058–1063. [Google Scholar] [CrossRef] [PubMed]
- Hartung, T.J.; Neumann, C.; Bahmer, T.; Chaplinskaya-Sobol, I.; Endres, M.; Geritz, J.; Haeusler, K.G.; Heuschmann, P.U.; Hildesheim, H.; Hinz, A.; et al. Fatigue and cognitive impairment after COVID-19: A prospective multicentre study. eClinicalMedicine 2022, 53, 101651. [Google Scholar] [CrossRef]
- Becker, J.H.; Lin, J.J.; Doernberg, M.; Stone, K.; Navis, A.; Festa, J.R.; Wisnivesky, J.P. Assessment of cognitive function in patients after COVID-19 infection. JAMA 2021, 4, e2130645. [Google Scholar] [CrossRef]
- Carazo, S.; Skowronski, D.M.; Laforce, R., Jr.; Talbot, D.; Falcone, E.L.; Laliberté, D.; Denis, G.; Deshaies, P.; Hegg-Deloye, S.; De Serres, G. Physical, psychological, and cognitive profile of post-COVID conditions in healthcare workers, Quebec, Canada. Open Forum Infect. Dis. 2022, 9, ofac386. [Google Scholar] [CrossRef]
- Daynes, E.; Gerlis, C.; Chaplin, E.; Gardiner, N.; Singh, S.J. Early experiences of rehabilitation for individuals post-COVID to improve fatigue, breathlessness exercise capacity and cognition—A cohort study. Chron. Respir. Dis. 2021, 18, 147997312110156. [Google Scholar] [CrossRef] [PubMed]
- Shanbehzadeh, S.; Tavahomi, M.; Zanjari, N.; Ebrahimi-Takamjani, I.; Amiri-Arimi, S. Physical and mental health complications post-COVID-19: Scoping review. J. Psychosom. Res. 2021, 147, 110525. [Google Scholar] [CrossRef]
- Premraj, L.; Kannapadi, N.V.; Briggs, J.; Seal, S.M.; Battaglini, D.; Fanning, J.; Suen, J.; Robba, C.; Fraser, J.; Cho, S.M. Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: A meta-analysis. J. Neurol. Sci. 2022, 434, 120162. [Google Scholar] [CrossRef]
- Deng, J.; Zhou, F.; Hou, W.; Silver, Z.; Wong, C.Y.; Chang, O.; Huang, E.; Zuo, Q.K. The prevalence of depression, anxiety, and sleep disturbances in COVID-19 patients: A meta-analysis. Ann. N. Y. Acad. Sci. 2021, 1486, 90–111. [Google Scholar] [CrossRef]
- D’Ávila, K.G.; Monaiar, L.R.; Dantas, L.D.P.; Freitas, A.A.; Rodrigues, M.M.; Bonamigo, R.R.; Dantas Filho, F.F.; Silva, D.R. Decrease in health-related quality of life and post-COVID-19 syndrome in healthcare workers after SARS-CoV-2 infection: A cohort study. J. Occup. Environ. Med. 2022, 65, e1–e3. [Google Scholar] [CrossRef] [PubMed]
- Fouad, M.M.; Zawilla, N.H.; Maged, L.A. Work performance among healthcare workers with post COVID-19 syndrome and its relation to antibody response. Infection 2022. [Google Scholar] [CrossRef] [PubMed]
- Houben-Wilke, S.; Goërtz, Y.M.; Delbressine, J.M.; Vaes, A.W.; Meys, R.; Machado, F.V.; Van Herck, M.; Burtin, C.; Posthuma, R.; Franssen, F.M.; et al. The impact of long COVID-19 on mental health: Observational 6-month follow-up study. JMIR Ment. Health 2022, 9, e33704. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhang, W.; Yang, Y.; Zhang, J.; Li, Y.; Chen, Y. Respiratory rehabilitation in elderly patients with COVID-19: A randomized controlled study. Complement. Ther. Clin. Pract. 2020, 39, 101166. [Google Scholar] [CrossRef]
- Hayden, M.C.; Limbach, M.; Schuler, M.; Merkl, S.; Schwarzl, G.; Jakab, K.; Nowak, D.; Schultz, K. Effectiveness of a three-week inpatient pulmonary rehabilitation program for patients after COVID-19: A prospective observational study. Int. J. Environ. Res. Public Health 2021, 18, 9001. [Google Scholar] [CrossRef]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.; Cuapio, A.; Villapol, S. More than 50 long-term effects of COVID-19: A systematic review and meta-analysis. Res. Sq. 2021. preprint. [Google Scholar] [CrossRef]
- Garrigues, E.; Janvier, P.; Kherabi, Y.; Le Bot, A.; Hamon, A.; Gouze, H.; Doucet, L.; Berkani, S.; Oliosi, E.; Mallart, E.; et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J. Infect. 2020, 81, e4–e6. [Google Scholar] [CrossRef]
- Haller, J.; Kocalevent, R.-D.; Nienhaus, A.; Peters, C.; Bergelt, C.; Koch-Gromus, U. Anhaltende Fatigue als Folge einer COVID-19-Infektion bei Beschäftigten im Gesundheitswesen: Risikofaktoren und Auswirkungen auf die Lebensqualität. (Persistent fatigue as a consequence of COVID-19 infection in healthcare workers: Risk factors and impact on quality of life). Bundesgesundheitsblatt 2022, 65, 471–480. [Google Scholar] [CrossRef]
- De Sire, A.; Moggio, L.; Marotta, N.; Agostini, F.; Tasselli, A.; Drago Ferrante, V.; Curci, C.; Calafiore, D.; Ferraro, F.; Bernetti, A.; et al. Impact of rehabilitation on fatigue in post-COVID-19 patients: A systematic review and meta-analysis. Appl. Sci. 2022, 12, 8593. [Google Scholar] [CrossRef]
- Müller, K.; Zwingmann, K.; Auerswald, T.; Berger, I.; Thomas, A.; Schultz, A.L.; Wilhelm, E.; Weber, R.C.; Kolb, F.; Wastlhuber, A.; et al. Rehabilitation and return-to-work of patients acquiring COVID-19 in the workplace: A study protocol for an observational cohort study. Front. Rehabil. Sci. 2022, 2, 754468. [Google Scholar] [CrossRef] [PubMed]
- Lampert, T.; Kroll, L.; Müters, S.; Stolzenberg, H. Messung des sozioökonomischen Status in der Studie zur Gesundheit Erwachsener in Deutschland (DEGS1). (Measurement of socioeconomic status in the German health interview and examination survey for adults (DEGS1)). Bundesgesundheitsblatt 2013, 56, 631–636. [Google Scholar] [CrossRef]
- Scheidt-Nave, C.; Kamtsiuris, P.; Gößwald, A.; Hölling, H.; Lange, M.; Busch, M.A.; Dahm, S.; Dölle, R.; Ellert, U.; Fuchs, J. German health interview and examination survey for adults (DEGS)-design, objectives and implementation of the first data collection wave. BMC Public Health 2012, 12, 730. [Google Scholar] [CrossRef] [PubMed]
- Koczulla, A.R.; Ankermann, T.; Behrends, U.; Berlit, P.; Böing, S.; Brinkmann, F.; Franke, C.; Glöckl, R.; Gogoll, C.; Hummel, T.; et al. S1-Leitlinie “Post-COVID/Long-COVID“. (S1-Guideline “Post-COVID/Long-COVID)”. Available online: https://register.awmf.org/assets/guidelines/020-027l_S1_Post_COVID_Long_COVID_2022-08.pdf (accessed on 1 December 2022).
- Hasselhorn, H.-M.; Freude, G. Der Work-Ability-Index: Ein Leitfaden (The Work-Ability-Index Guideline); Wirtschaftsverlag NW: Bremerhaven, Germany, 2007; p. 54. [Google Scholar]
- Klok, F.A.; Boon, G.J.; Barco, S.; Endres, M.; Geelhoed, J.M.; Knauss, S.; Rezek, S.A.; Spruit, M.A.; Vehreschild, J.; Siegerink, B. The post-COVID-19 functional Status scale: A tool to measure functional status over time after COVID-19. Eur. Respir. J. 2020, 56, 2001494. [Google Scholar] [CrossRef] [PubMed]
- Holland, A.E.; Spruit, M.A.; Troosters, T.; Puhan, M.A.; Pepin, V.; Saey, D.; McCormack, M.C.; Carlin, B.W.; Sciurba, F.C.; Pitta, F. An official European Respiratory Society/American Thoracic Society technical standard: Field walking tests in chronic respiratory disease. Eur. Respir. J. 2014, 44, 1428–1446. [Google Scholar] [CrossRef] [PubMed]
- Schneeberger, T.; Gloeckl, R.; Jarosch, I.; Drechsel, F.; Koczulla, A.R.; Kenn, K. The minimal important difference for the 1-minute sit-to-stand test following pulmonary rehabilitation in patients with COPD—A prospective observational trial. Eur. Respir. J. 2018, 52, PA1431. [Google Scholar] [CrossRef]
- Bohannon, R.W.; Crouch, R. 1-Minute sit-to-stand test: Systematic review of procedures, performance, and clinimetric properties. J. Cardiopulm. Rehabil. Prev. 2019, 39, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Wong, G.K.C.; Mak, J.S.Y.; Wong, A.; Zheng, V.Z.Y.; Poon, W.S.; Abrigo, J.; Mok, V.C.T. Minimum clinically important difference of Montreal Cognitive Assessment in aneurysmal subarachnoid hemorrhage patients. J. Clin. Neurosci. 2017, 46, 41–44. [Google Scholar] [CrossRef]
- Jaeger, J. Digit symbol substitution test: The case for sensitivity over specificity in neuropsychological testing. J. Clin. Psychopharmacol. 2018, 38, 513–519. [Google Scholar] [CrossRef]
- Hermann-Lingen, C.; Buss, U.; Snaith, R. HADS-D Hospital Anxiety and Depression Scale-German Version, 4th ed.; Hogrefe: Bern, Switzerland, 2018; p. 72. [Google Scholar]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Lemay, K.R.; Tulloch, H.E.; Pipe, A.L.; Reed, J.L. Establishing the minimal clinically important difference for the Hospital Anxiety and Depression Scale in patients with cardiovascular disease. J. Cardiopulm. Rehabil. Prev. 2019, 39, E6–E11. [Google Scholar] [CrossRef]
- Radbruch, L.; Sabatowski, R.; Elsner, F.; Everts, J.; Mendoza, T.; Cleeland, C. Validation of the german version of the Brief Fatigue Inventory. J. Pain Symptom Manag. 2003, 25, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, T.R.; Wang, X.S.; Cleeland, C.S.; Morrissey, M.; Johnson, B.A.; Wendt, J.K.; Huber, S.L. The rapid assessment of fatigue severity in cancer patients. Cancer 1999, 85, 1186–1196. [Google Scholar] [CrossRef]
- Fisk, J.D.; Ritvo, P.G.; Ross, L.; Haase, D.A.; Marrie, T.J.; Schlech, W.F. Measuring the functional impact of fatigue: Initial validation of the fatigue impact scale. Clin. Infect. Dis. 1994, 18, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Martus, P.; Jakob, O.; Rose, U.; Seibt, R.; Freude, G. A comparative analysis of the Work Ability Index. Occup. Med. 2010, 60, 517–524. [Google Scholar] [CrossRef]
- Fritz, C.O.; Morris, P.E.; Richler, J.J. Effect size estimates: Current use, calculations, and interpretation. J. Exp. Psychol. Gen. 2012, 141, 2–18. [Google Scholar] [CrossRef]
- Taquet, M.; Sillett, R.; Zhu, L.; Mendel, J.; Camplisson, I.; Dercon, Q.; Harrison, P.J. Neurological and psychiatric risk trajectories after SARS-CoV-2 infection: An analysis of 2-year retrospective cohort studies including 1 284 437 patients. Lancet Psychiatry 2022, 9, 815–827. [Google Scholar] [CrossRef]
- Bahmer, T.; Borzikowsky, C.; Lieb, W.; Horn, A.; Krist, L.; Fricke, J.; Scheibenbogen, C.; Rabe, K.F.; Maetzler, W.; Maetzler, C.; et al. Severity, predictors and clinical correlates of Post-COVID syndrome (PCS) in Germany: A prospective, multi-centre, population-based cohort study. eClinicalMedicine 2022, 51, 101549. [Google Scholar] [CrossRef]
- Forster, C.; Colombo, M.G.; Wetzel, A.J.; Martus, P.; Joos, S. Persisting symptoms after COVID-19. Dtsch Arztebl. Int. 2022, 119, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Zheng, B.; Daines, L.; Sheikh, A. Long-Term Sequelae of COVID-19: A systematic review and meta-analysis of one-year follow-up studies on post-COVID symptoms. Pathogens 2022, 11, 269. [Google Scholar] [CrossRef]
- Mazza, M.G.; Palladini, M.; De Lorenzo, R.; Bravi, B.; Poletti, S.; Furlan, R.; Ciceri, F.; Vai, B.; Bollettini, I.; Melloni, E.M.T.; et al. One-year mental health outcomes in a cohort of COVID-19 survivors. J. Psychiatr. Res. 2022, 145, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Gaber, T.A.-Z.K.; Ashish, A.; Unsworth, A. Persistent post-covid symptoms in healthcare workers. Occup. Med. 2021, 71, 144–146. [Google Scholar] [CrossRef] [PubMed]
- Soril, L.J.J.; Damant, R.W.; Lam, G.Y.; Smith, M.P.; Weatherald, J.; Bourbeau, J.; Hernandez, P.; Stickland, M.K. The effectiveness of pulmonary rehabilitation for Post-COVID symptoms: A rapid review of the literature. Respir. Med. 2022, 195, 106782. [Google Scholar] [CrossRef] [PubMed]
- Brehon, K.; Niemeläinen, R.; Hall, M.; Bostick, G.P.; Brown, C.A.; Wieler, M.; Gross, D.P. Return-to-work following occupational rehabilitation for long COVID: Descriptive cohort study. JMIR Rehabil. Assist. Technol. 2022, 9, e39883. [Google Scholar] [CrossRef] [PubMed]
- Bishop, P.; Cureton, K.; Collins, M. Sex difference in muscular strength in equally-trained men and women. Ergonomics 1987, 30, 675–687. [Google Scholar] [CrossRef]
- Hermann, M.; Pekacka-Egli, A.M.; Witassek, F.; Baumgaertner, R.; Schoendorf, S.; Spielmanns, M. Feasibility and efficacy of cardiopulmonary rehabilitation after COVID-19. Am. J. Phys. Med. Rehabil. 2020, 99, 865–869. [Google Scholar] [CrossRef]
- Al Chikhanie, Y.; Veale, D.; Schoeffler, M.; Pépin, J.L.; Verges, S.; Hérengt, F. Effectiveness of pulmonary rehabilitation in COVID-19 respiratory failure patients post-ICU. Respir. Physiol. Neurobiol. 2021, 287, 103639. [Google Scholar] [CrossRef]
- Cevei, M.; Onofrei, R.R.; Gherle, A.; Gug, C.; Stoicanescu, D. Rehabilitation of post-COVID-19 musculoskeletal sequelae in geriatric patients: A case series study. Int. J. Environ. Res. Public Health 2022, 19, 15350. [Google Scholar] [CrossRef]
- Rodriguez-Blanco, C.; Gonzalez-Gerez, J.J.; Bernal-Utrera, C.; Anarte-Lazo, E.; Perez-Ale, M.; Saavedra-Hernandez, M. Short-term effects of a conditioning telerehabilitation program in confined patients affected by COVID-19 in the acute phase. A pilot randomized controlled trial. Medicina 2021, 57, 684. [Google Scholar] [CrossRef]
- Jimeno-Almazán, A.; Pallarés, J.G.; Buendía-Romero, Á.; Martínez-Cava, A.; Franco-López, F.; Sánchez-Alcaraz Martínez, B.J.; Bernal-Morel, E.; Courel-Ibáñez, J. Post-COVID-19 syndrome and the potential benefits of exercise. Int. J. Environ. Res. Public Health 2021, 18, 5329. [Google Scholar] [CrossRef]
- Schumann, M.; Marschall, J.; Hildebrandt, S.; Nolting, H. Gesundheitsreport 2022 (Health Report); Medhochzwei: Heidelberg, Germany, 2022; 186p. [Google Scholar]
- Calabria, M.; García-Sánchez, C.; Grunden, N.; Pons, C.; Arroyo, J.A.; Gómez-Anson, B.; Estévez García, M.D.C.; Belvís, R.; Morollón, N.; Vera Igual, J.; et al. Post-COVID-19 fatigue: The contribution of cognitive and neuropsychiatric symptoms. J. Neurol. 2022, 269, 3990–3999. [Google Scholar] [CrossRef]
- Mirfazeli, F.S.; Sarabi-Jamab, A.; Pereira-Sanchez, V.; Kordi, A.; Shariati, B.; Shariat, S.V.; Bahrami, S.; Nohesara, S.; Almasi-Dooghaee, M.; Faiz, S.H.R. Chronic fatigue syndrome and cognitive deficit are associated with acute-phase neuropsychiatric manifestations of COVID-19: A 9-month follow-up study. Neurol. Sci. 2022, 43, 2231–2239. [Google Scholar] [CrossRef]
- Guo, P.; Benito Ballesteros, A.; Yeung, S.P.; Liu, R.; Saha, A.; Curtis, L.; Kaser, M.; Haggard, M.P.; Cheke, L.G. COVCOG 2: Cognitive and memory deficits in long COVID: A second publication from the COVID and cognition study. Front. Aging Neurosci. 2022, 14, 804937. [Google Scholar] [CrossRef]
- Douaud, G.; Lee, S.; Alfaro-Almagro, F.; Arthofer, C.; Wang, C.; Mccarthy, P.; Lange, F.; Andersson, J.L.R.; Griffanti, L.; Duff, E.; et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature 2022, 604, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Alpert, O.; Begun, L.; Garren, P.; Solhkhah, R. Cytokine storm induced new onset depression in patients with COVID-19. A new look into the association between depression and cytokines -two case reports. Brain Behav. Immun. Health 2020, 9, 100173. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.C.; Kern, F.; Losada, P.M.; Agam, M.R.; Maat, C.A.; Schmartz, G.P.; Fehlmann, T.; Stein, J.A.; Schaum, N.; Lee, D.P.; et al. Dysregulation of brain and choroid plexus cell types in severe COVID-19. Nature 2021, 595, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, M.E.; Madore, C.; Bordeleau, M.; Tian, L.; Verkhratsky, A. Neuropathobiology of COVID-19: The role for glia. Front. Cell. Neurosci. 2020, 14, 592214. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’Em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. eClinicalMedicine 2021, 38, 101019. [Google Scholar] [CrossRef]
- Havervall, S.; Rosell, A.; Phillipson, M.; Mangsbo, S.M.; Nilsson, P.; Hober, S.; Thålin, C. Symptoms and functional impairment assessed 8 months after mild COVID-19 among health care workers. JAMA 2021, 325, 2015. [Google Scholar] [CrossRef]
- Pauwels, S.; Boets, I.; Polli, A.; Mylle, G.; De Raeve, H.; Godderis, L. Return to Work after Long COVID: Evidence at 8th March 2021; Health and Safety Executive: London, UK, 2021.
- Andrade, M.A.; Castro, C.S.M.; Batistão, M.V.; Mininel, V.A.; Sato, T.O. Occupational profile, psychosocial aspects, and work ability of brazilian workers during COVID-19 pandemic: IMPPAC cohort. Saf. Health Work 2022, 13, 104–111. [Google Scholar] [CrossRef]
- Jacobsen, P.A.; Andersen, M.P.; Gislason, G.; Phelps, M.; Butt, J.H.; Køber, L.; Schou, M.; Fosbøl, E.; Christensen, H.C.; Torp-Pedersen, C.; et al. Return to work after COVID-19 infection—A Danish nationwide registry study. Public Health 2022, 203, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Böckerman, P.; Ilmakunnas, P. Unemployment and self-assessed health: Evidence from panel data. Health Econ. 2009, 18, 161–179. [Google Scholar] [CrossRef] [PubMed]
- Lunt, J.; Hemming, S.; Burton, K.; Elander, J.; Baraniak, A. What workers can tell us about post-COVID workability. Occup. Med. 2022, kqac086. [Google Scholar] [CrossRef]
- Vooijs, M.; Leensen, M.C.; Hoving, J.L.; Wind, H.; Frings-Dresen, M.H. Interventions to enhance work participation of workers with a chronic disease: A systematic review of reviews. Occup. Environ. Med. 2015, 72, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Wegrzynek, P.A.; Wainwright, E.; Ravalier, J. Return to work interventions for chronic pain: A systematic review. Occup. Med. 2020, 70, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Popa, A.E.; Bejenaru, A.; Mitrea, E.C.; Morândău, F.; Pogan, L. Return to work after chronic disease: A theoretical framework for understanding the worker-employer dynamic. Chronic Illn. 2022, 17423953221117852. [Google Scholar] [CrossRef]
- Munblit, D.; Nicholson, T.R.; Needham, D.M.; Seylanova, N.; Parr, C.; Chen, J.; Kokorina, A.; Sigfrid, L.; Buonsenso, D.; Bhatnagar, S.; et al. Studying the post-COVID-19 condition: Research challenges, strategies, and importance of core Outcome set development. BMC Med. 2022, 20, 50. [Google Scholar] [CrossRef]
- Li, J.A.; Xia, W.; Zhan, C.; Liu, S.; Yin, Z.; Wang, J.; Chong, Y.; Zheng, C.; Fang, X.; Cheng, W.; et al. A telerehabilitation programme in post-discharge COVID-19 patients (TERECO): A randomised controlled trial. Thorax 2022, 77, 697–706. [Google Scholar] [CrossRef] [PubMed]
- WHO. Delivered by Women, Led by Men: A Gender and Equity Analysis of the Global Health and Social Workforce; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Bond, A.E.; Wagler, K.; Anestis, M.D. Essential workers: Past month suicidal ideation and COVID-19 stress. J. Clin. Psychol. 2021, 77, 2849–2859. [Google Scholar] [CrossRef]
| N (%) | Mean | SD | Min | Max | |
|---|---|---|---|---|---|
| Sex female | |||||
| • male | 30 (23.6) | ||||
| • female | 97 (76.4) | ||||
| Age [years] | 50.62 | 10.74 | 21 | 69 | |
| BMI [kg/m2] | 31.47 | 6.61 | 19.1 | 54.5 | |
| • Normal | 19 (14.8) | ||||
| • Overweight | 41 (32.0) | ||||
| • Obesity class I | 36 (28.3) | ||||
| • Obesity class II | 20 (15.6) | ||||
| • Obesity class III | 11 (8.6) | ||||
| Smoking status | |||||
| • Currently | 11 (8.7) | ||||
| • Former | 49 (38.6) | ||||
| • Never | 67 (52.7) | ||||
| Healthcare workers | 89 (70.0) | ||||
| • Nursing staff | 71 (55.9) | ||||
| • Medical staff | 7 (5.5) | ||||
| • Paramedical staff | 6 (4.7) | ||||
| • Therapeutic staff | 5 (3.9) | ||||
| Non-Healthcare workers | 38 (30.0) | ||||
| • Administrative staff | 11 (8.7) | ||||
| • Industrial-/Building technicians | 9 (7.1) | ||||
| • Social educational staff and teachers | 7 (5.5) | ||||
| • Cosmeticians | 3 (2.4) | ||||
| • Retail salesman/-woman | 3 (2.4) | ||||
| • Personal service staff | 3 (2.4) | ||||
| • Other occupations | 2 (1.6) | ||||
| Occupational inability | |||||
| • Occupational inability prior to rehabilitation [days] | 135.27 | 153.85 | 6 | 544 | |
| • Pre rehabilitation o Healthcare workers (n = 89) o Non-healthcare workers (n = 38) | 86 (67.7) 60 (69.8) 26 (30.2) | ||||
| Post rehabilitation (N/A = 3) o Healthcare workers (n = 87) o Non-Healthcare workers (n = 37) | 90 (72.5) 66 (73.3) 24 (26.7) |
| N (%) | Mean | SD | Min | Max | |
|---|---|---|---|---|---|
| Hospitalization | 33 (25.9) | ||||
| • Duration of hospitalization [days] | 14.10 | 19.01 | 1 | 100 | |
| ICU stay | 10 (7.9) | ||||
| • Duration of ICU stay [days] | 10.83 | 5.46 | 5 | 21 | |
| Disease severity according to WHO | |||||
| • Mild/moderate | 91 (71.7) | ||||
| • Severe | 28 (22.0) | ||||
| • Critical | 8 (6.3) | ||||
| O2 therapy during hospitalization | 27 (21.2) | ||||
| Mechanical ventilation | 6 (4.7) | ||||
| Duration between first positive PCR test and rehabilitation admission [days] | 408.81 | 140.89 | 124 | 813 | |
| Duration of rehabilitation [days] | 28.77 | 5.44 | 9 | 42 | |
| Comorbidities prior to COVID-19 | |||||
| • Hypertension | 38 (29.9) | ||||
| • Coronary heart disease | 5 (3.9) | ||||
| • Chronic bronchitis | 5 (3.9) | ||||
| • Asthma | 21 (16.5) | ||||
| • Diabetes mellitus | 10 (7.8) | ||||
| • Neurological diseases | 9 (7.1) | ||||
| • Oncological diseases | 14 (11.0) |
| N | Pre Median (IQR) | Post Median (IQR) | Δ | z | p | r | |
|---|---|---|---|---|---|---|---|
| Physical Performance | |||||||
| 6MWD [m] | 119 | 520.00 (447.00–570.00) | 576.00 (522.00–636.00) | 64.00 (32.00–112.00) | −9.032 | <0.001 | −0.828 |
| 1MSTST | 118 | 20 (16–24) | 22 (17–26) | 2 (−1–5) | −4.960 | 0.001 | −0.456 |
| Handgrip strength [kg] | 121 | 27.63 (20.63–35.07) | 29.47 (21.02–36.17) | −0.17 (−2.95–4.02) | −0.960 | 0.337 | −0.087 |
| Quadriceps strength [kg] | 120 | 95.43 (69.36–131.83) | 110.13 (88.49–146.42) | 12.80 (0.51–29.19) | −6.973 | <0.001 | −0.637 |
| Subjective physical ability | 112 | 4.67 (3.44–6.11) | 5.78 (4.56–7.22) | 1.06 (0.22–2.22) | −6.148 | <0.001 | −0.581 |
| Neuropsychological Parameters | |||||||
| MoCA score | 122 | 27 (25–28) | 27 (26–29) | 0 (−1–2) | 2.434 | 0.015 | 0.220 |
| DSST | 122 | 46 (37–53) | 50 (40–57) | 2.5 (−1–6) | 4666 | <0.001 | 0.422 |
| HADSdepression | 122 | 7 (4–11) | 6 (3–10) | −1 (−3–0) | −4.477 | <0.001 | −0.405 |
| HADSanxiety | 122 | 7 (4–11) | 5 (3–10) | −1 (−3–0) | −4.444 | <0.001 | −0.402 |
| FIS | 120 | 97 (73–113) | 85.5 (65–111.75) | −5 (−16–4) | −3.262 | 0.001 | −0.297 |
| BFI | 122 | 5.6 (4.6–6.7) | 5.3 (3.9–6.6) | −0.21 (−0.99–0.43) | −2.848 | 0.004 | −0.257 |
| Subjective mental health | 122 | 5.3 (4.5–6.6) | 5.8 (4.5–7.3) | 0.31 (−0.39–1.26) | 3747 | <0.001 | 0.339 |
| Work ability | |||||||
| WAI score | 115 | 24.75 (21–28) | 24.75 (21–28) | 0 (−2–2) | −0.827 | 0.408 | −0.077 |
| • Work ability | 118 | 3 (0–6) | 3 (0.75–6) | 0 (−1–2) | −1.787 | 0.074 | −0.165 |
| • Work requirements | 117 | 7 (6–9) | 7 (6–8) | 0 (−1–1) | −2.584 | 0.010 | −0.239 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller, K.; Poppele, I.; Ottiger, M.; Zwingmann, K.; Berger, I.; Thomas, A.; Wastlhuber, A.; Ortwein, F.; Schultz, A.-L.; Weghofer, A.; et al. Impact of Rehabilitation on Physical and Neuropsychological Health of Patients Who Acquired COVID-19 in the Workplace. Int. J. Environ. Res. Public Health 2023, 20, 1468. https://doi.org/10.3390/ijerph20021468
Müller K, Poppele I, Ottiger M, Zwingmann K, Berger I, Thomas A, Wastlhuber A, Ortwein F, Schultz A-L, Weghofer A, et al. Impact of Rehabilitation on Physical and Neuropsychological Health of Patients Who Acquired COVID-19 in the Workplace. International Journal of Environmental Research and Public Health. 2023; 20(2):1468. https://doi.org/10.3390/ijerph20021468
Chicago/Turabian StyleMüller, Katrin, Iris Poppele, Marcel Ottiger, Katharina Zwingmann, Ivo Berger, Andreas Thomas, Alois Wastlhuber, Franziska Ortwein, Anna-Lena Schultz, Anna Weghofer, and et al. 2023. "Impact of Rehabilitation on Physical and Neuropsychological Health of Patients Who Acquired COVID-19 in the Workplace" International Journal of Environmental Research and Public Health 20, no. 2: 1468. https://doi.org/10.3390/ijerph20021468
APA StyleMüller, K., Poppele, I., Ottiger, M., Zwingmann, K., Berger, I., Thomas, A., Wastlhuber, A., Ortwein, F., Schultz, A.-L., Weghofer, A., Wilhelm, E., Weber, R.-C., Meder, S., Stegbauer, M., & Schlesinger, T. (2023). Impact of Rehabilitation on Physical and Neuropsychological Health of Patients Who Acquired COVID-19 in the Workplace. International Journal of Environmental Research and Public Health, 20(2), 1468. https://doi.org/10.3390/ijerph20021468










