Three-Dimensional Assessment of Temporomandibular Joint Morphology and Facial Asymmetry in Individuals with Different Vertical Skeletal Growth Patterns
Abstract
:1. Introduction
Aim
2. Materials and Methods
2.1. Sample
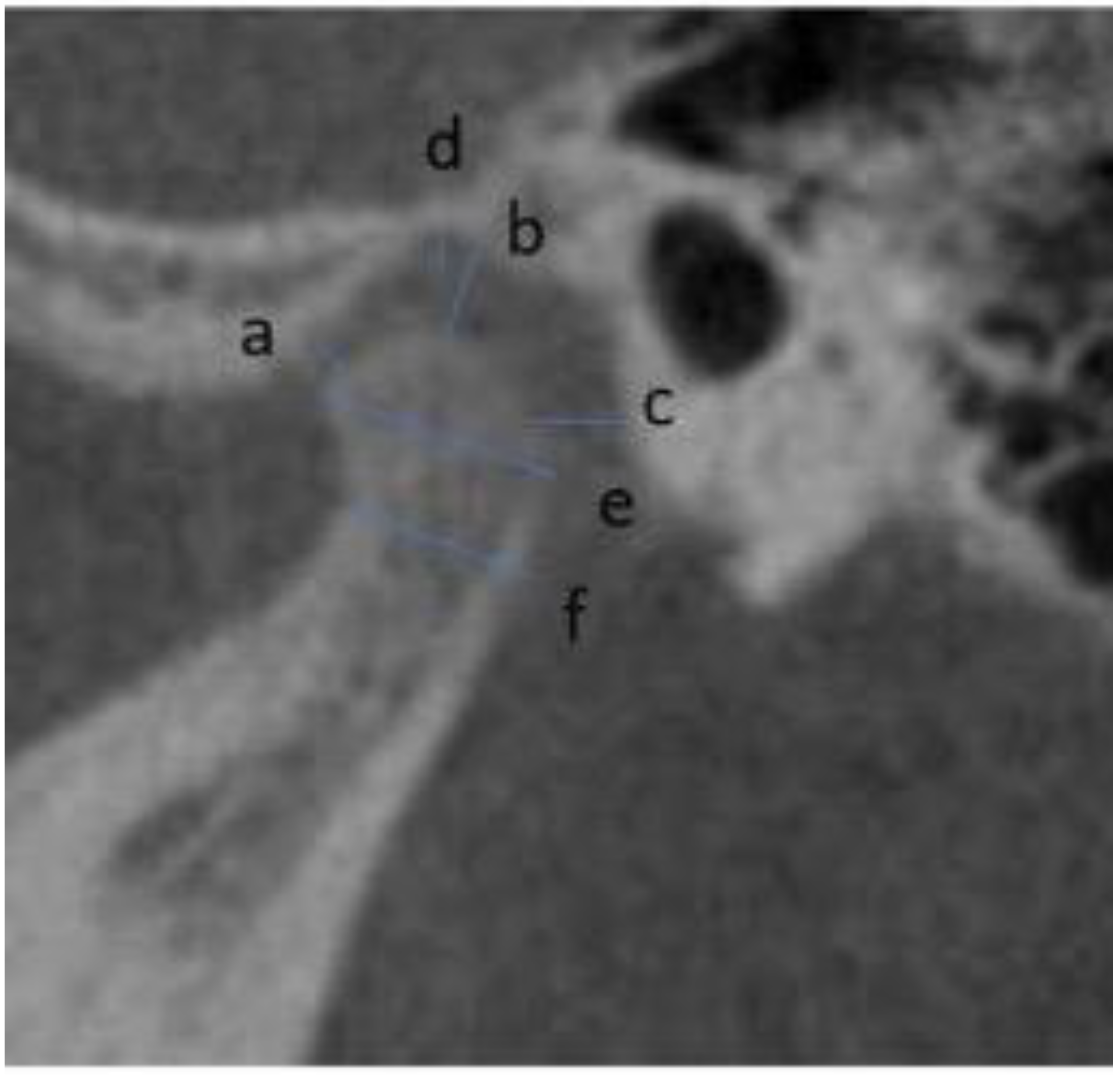
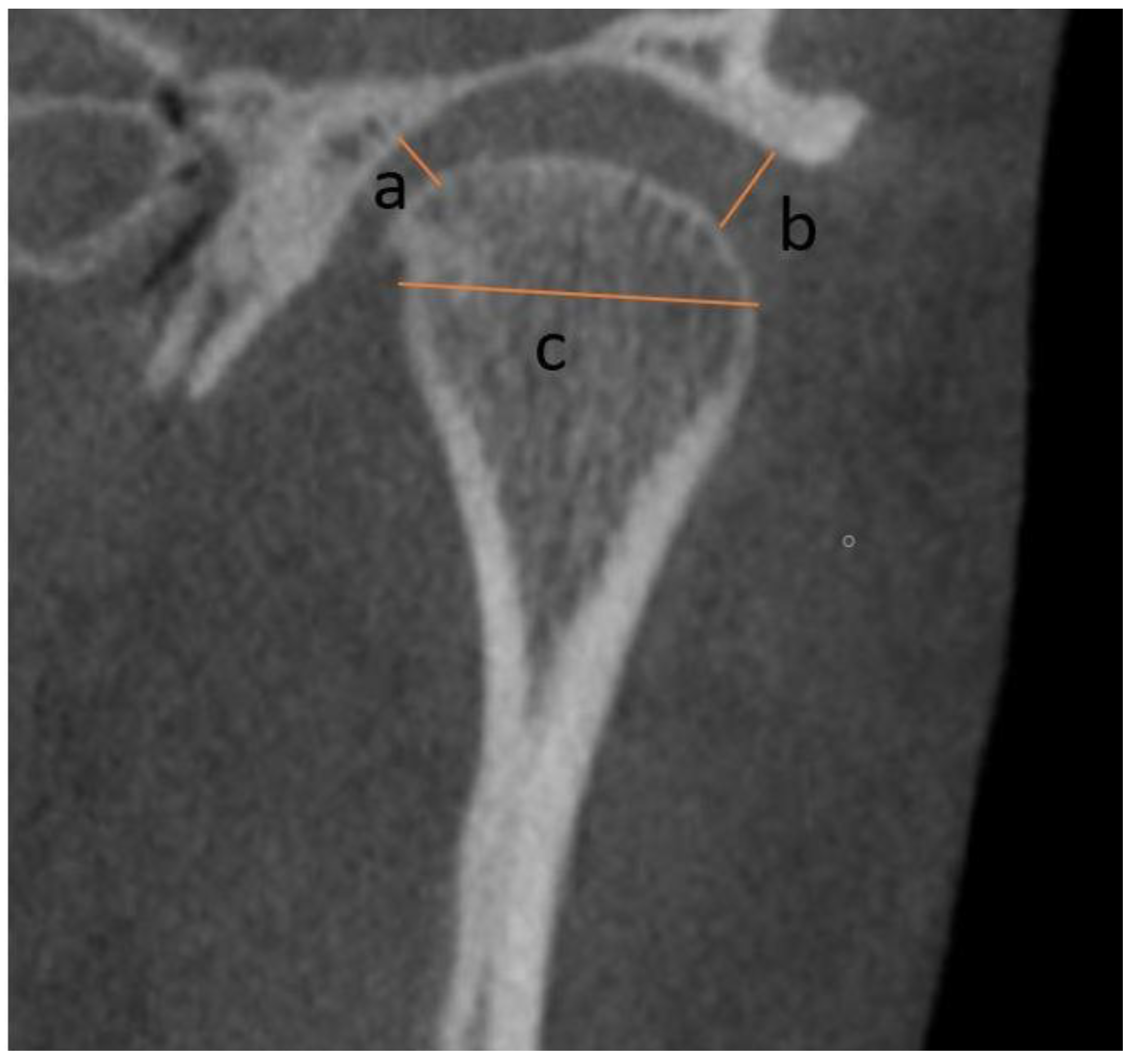
2.2. Measurements
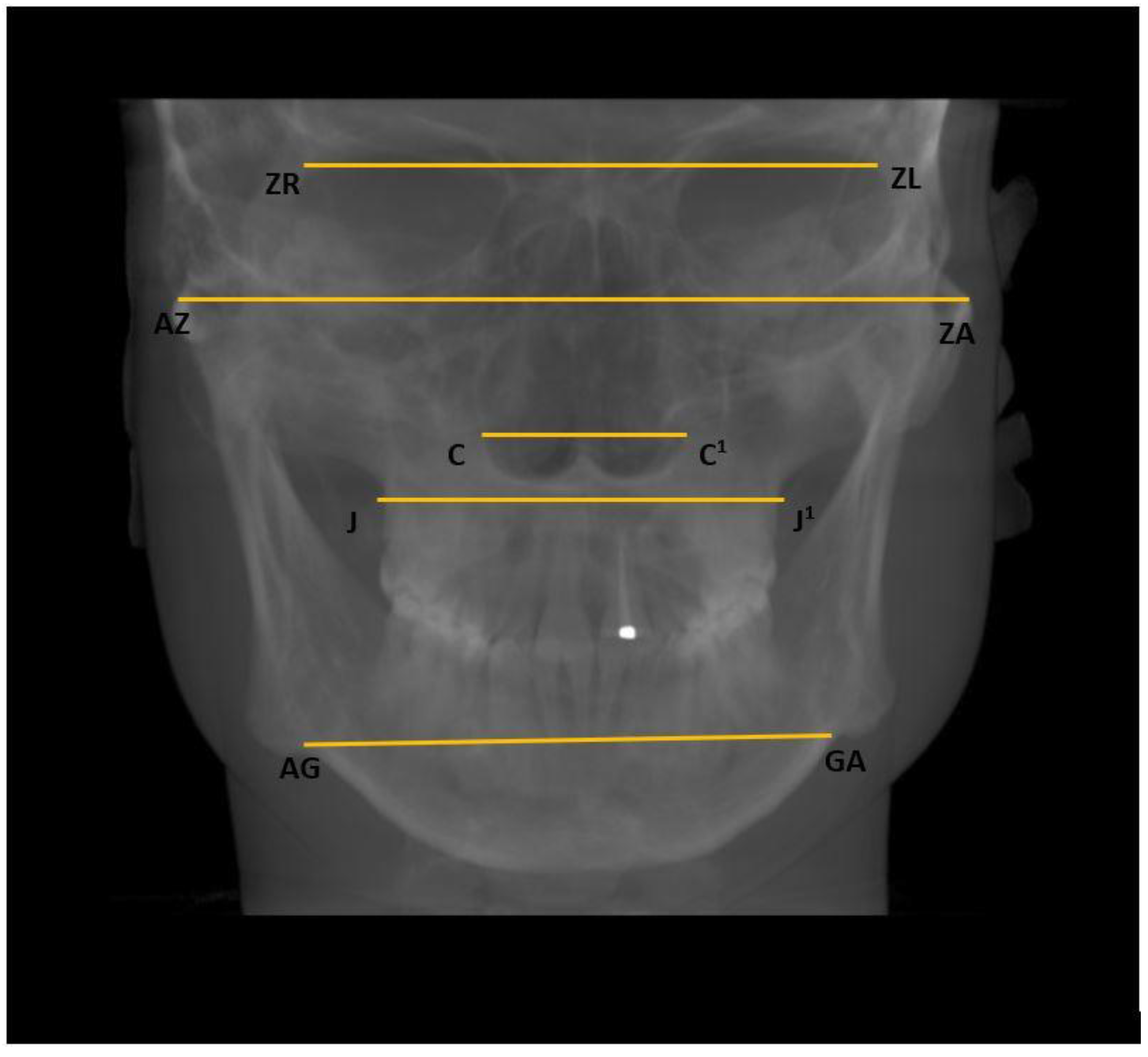
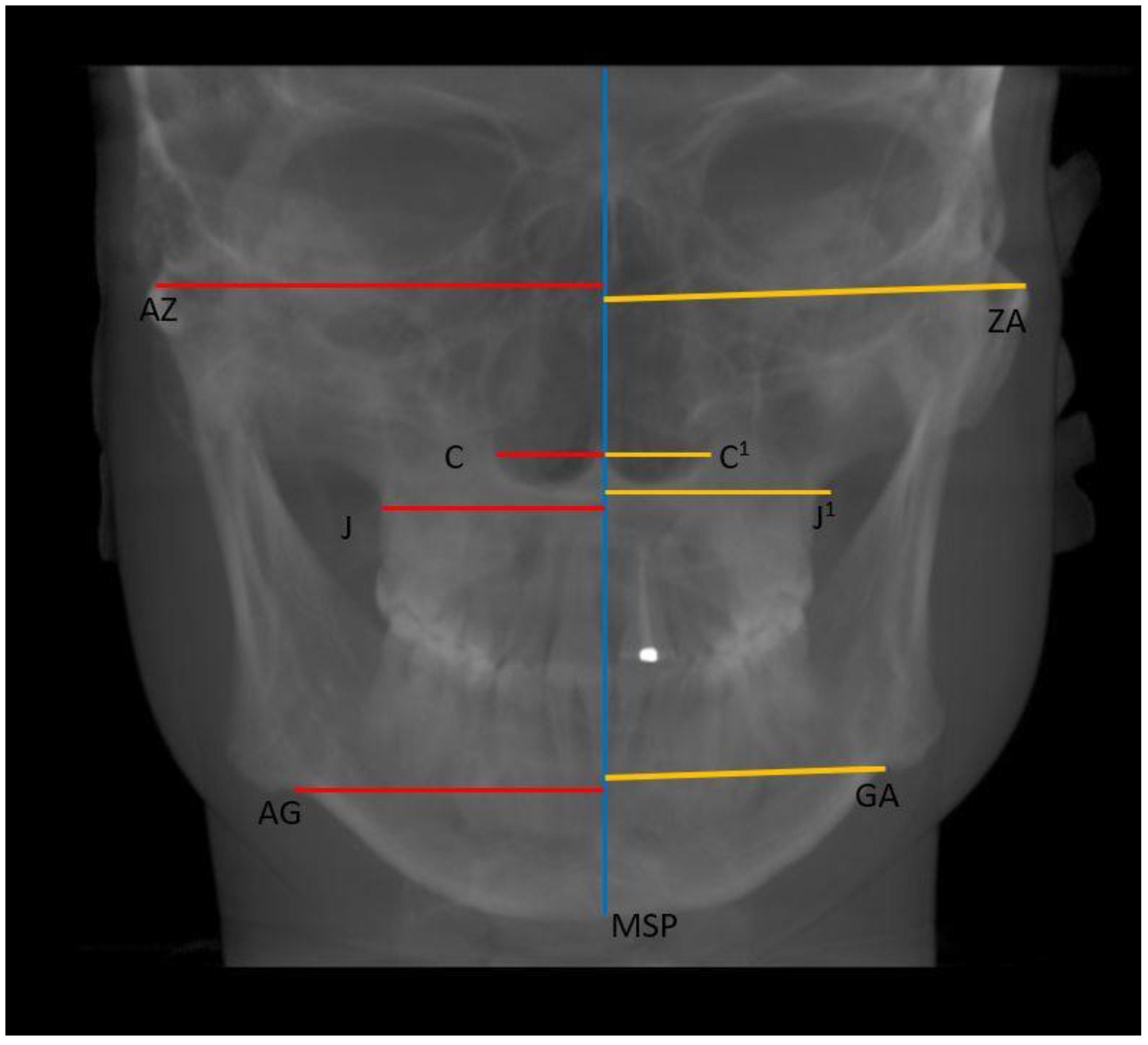
- Horizontal: Nasal cavity width (C–C1), distance between zygomaticofrontal sutures (ZR–ZL), distance between the centres of the roof of the zygomatic arch (AZ–ZA), distance between the jugal processes (J–J1), and distance between the antegonial points (AG–GA) (Figure 4);
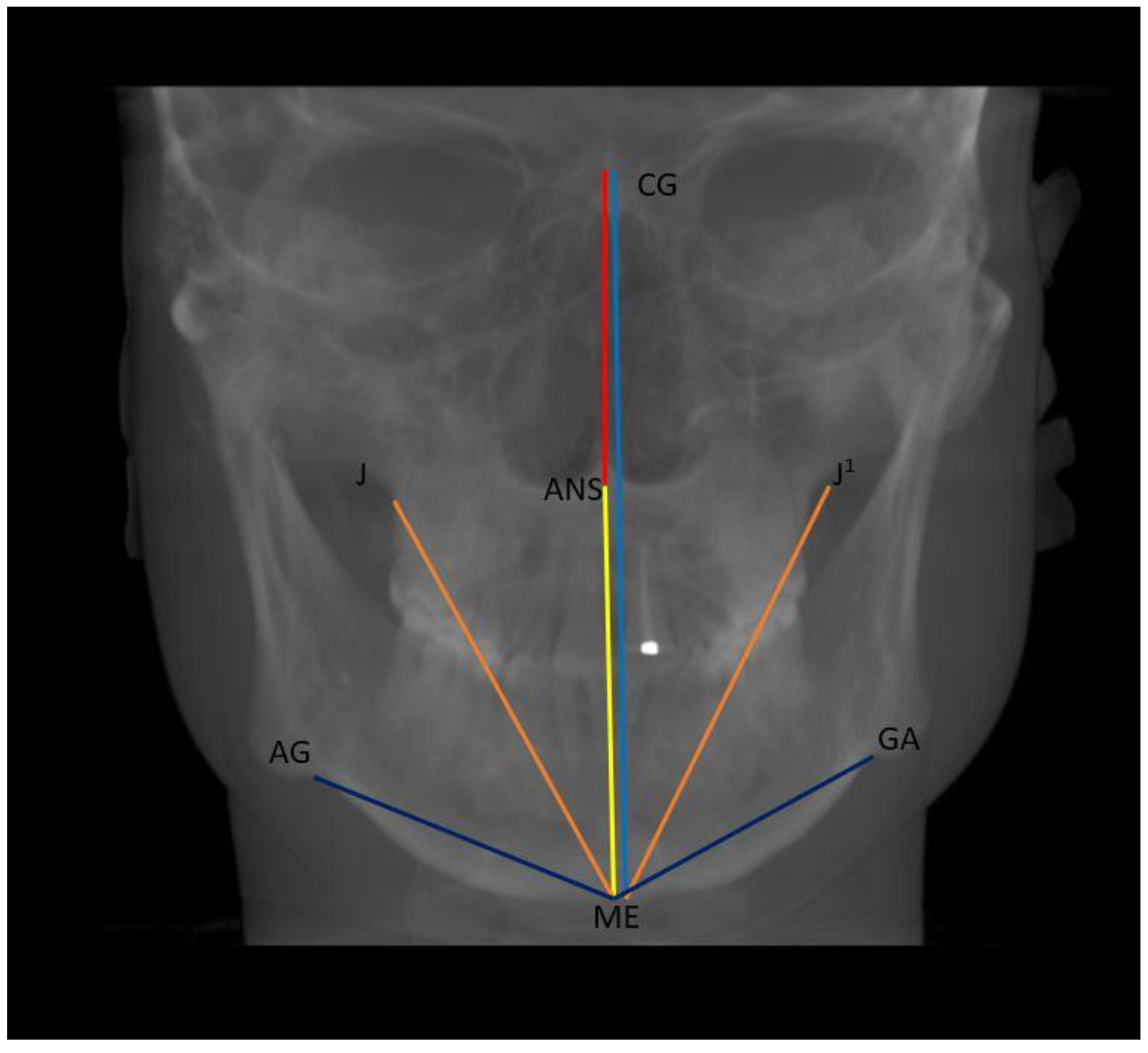
- Vertical: distance between Crista Galli to Menton (Cg–Me), distance between Anterior Nasal Spine and Menton (ANS–Me), distance between Crista Galli and Anterior Nasal Spine (Cg–ANS), distance between Jugal Process and Menton (J–Me left and right), distance between Antegonial notch and Menton (Ag–Me left and right) (Figure 5);
2.3. Statistical Analysis
3. Results
Fi-Index Tool
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosen, H.M. Evolution of a Surgical Philosophy in Orthognathic Surgery. Plast. Reconstr. Surg. 2017, 139, 978–990. [Google Scholar] [CrossRef]
- Thiesen, G.; Gribel, B.F.; Freitas, M.P.M. Facial Asymmetry: A Current Review. Dent. Press J. Orthod. 2015, 20, 110–125. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Miyamoto, J.J.; Takada, J.-I.; Moriyama, K. Association between 3-Dimensional Mandibular Morphology and Condylar Movement in Subjects with Mandibular Asymmetry. Am. J. Orthod. Dentofac. Orthop. 2017, 151, 324–334. [Google Scholar] [CrossRef] [PubMed]
- You, K.-H.; Kim, K.-H.; Lee, K.-J.; Baik, H.-S. Three-Dimensional Computed Tomography Analysis of Mandibular Morphology in Patients with Facial Asymmetry and Mandibular Retrognathism. Am. J. Orthod. Dentofac. Orthop. 2018, 153, 685–691. [Google Scholar] [CrossRef]
- Ma, Q.; Bimal, P.; Mei, L.; Olliver, S.; Farella, M.; Li, H. Temporomandibular Condylar Morphology in Diverse Maxillary-Mandibular Skeletal Patterns: A 3-Dimensional Cone-Beam Computed Tomography Study. J. Am. Dent. Assoc. 2018, 149, 589–598. [Google Scholar] [CrossRef]
- Lin, M.; Xu, Y.; Wu, H.; Zhang, H.; Wang, S.; Qi, K. Comparative Cone-Beam Computed Tomography Evaluation of Temporomandibular Joint Position and Morphology in Female Patients with Skeletal Class II Malocclusion. J. Int. Med. Res. 2020, 48, 0300060519892388. [Google Scholar] [CrossRef] [PubMed]
- Santander, P.; Quast, A.; Olbrisch, C.; Rose, M.; Moser, N.; Schliephake, H.; Meyer-Marcotty, P. Comprehensive 3D Analysis of Condylar Morphology in Adults with Different Skeletal Patterns—A Cross-Sectional Study. Head Face Med. 2020, 16, 33. [Google Scholar] [CrossRef] [PubMed]
- Greene, C.S. “The Ball on the Hill”: A New Perspective on TMJ Functional Anatomy. Orthod. Craniofac. Res. 2018, 21, 170–174. [Google Scholar] [CrossRef]
- Liu, Z.J.; King, G.J.; Herring, S.W. Alterations of Morphology and Microdensity in the Condyle after Mandibular Osteodistraction in the Rat. J. Oral Maxillofac. Surg. 2003, 61, 918–927. [Google Scholar] [CrossRef]
- Hilgers, M.L.; Scarfe, W.C.; Scheetz, J.P.; Farman, A.G. Accuracy of Linear Temporomandibular Joint Measurements with Cone Beam Computed Tomography and Digital Cephalometric Radiography. Am. J. Orthod. Dentofac. Orthop. 2005, 128, 803–811. [Google Scholar] [CrossRef]
- Al-koshab, M.; Nambiar, P.; John, J. Assessment of Condyle and Glenoid Fossa Morphology Using CBCT in South-East Asians. PLoS ONE 2015, 10, e0121682. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.F.; Fraga, M.R.; Vitral, R.W.F. Computed Tomography Evaluation of the Temporomandibular Joint in Class II Division 1 and Class III Malocclusion Patients: Condylar Symmetry and Condyle-Fossa Relationship. Am. J. Orthod. Dentofac. Orthop. 2009, 136, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Chae, J.-M.; Park, J.H.; Tai, K.; Mizutani, K.; Uzuka, S.; Miyashita, W.; Seo, H.Y. Evaluation of Condyle-Fossa Relationships in Adolescents with Various Skeletal Patterns Using Cone-Beam Computed Tomography. Angle Orthod. 2020, 90, 224–232. [Google Scholar] [CrossRef] [Green Version]
- Burke, G.; Major, P.; Glover, K.; Prasad, N. Correlations between Condylar Characteristics and Facial Morphology in Class II Preadolescent Patients. Am. J. Orthod. Dentofac. Orthop. 1998, 114, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, K.D.; Schmidt, B.; Stoustrup, P.; Pedersen, T.K. Idiopathic Condylar Resorptions: 3-Dimensional Condylar Bony Deformation, Signs and Symptoms. Am. J. Orthod. Dentofac. Orthop. 2017, 152, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Fiorillo, L. Fi-Index: A New Method to Evaluate Authors Hirsch-Index Reliability. Publ. Res. Q. 2022, 38, 466–474. [Google Scholar] [CrossRef]
- Fiorillo, L.; Cicciù, M. The Use of Fi-Index Tool to Assess Per-manuscript Self-citations. Publ. Res. Q. 2022, 38, 684–692. [Google Scholar] [CrossRef]
- Park, I.-Y.; Kim, J.-H.; Park, Y.-H. Three-Dimensional Cone-Beam Computed Tomography Based Comparison of Condylar Position and Morphology According to the Vertical Skeletal Pattern. Korean J. Orthod. 2015, 45, 66–73. [Google Scholar] [CrossRef] [Green Version]
- Paknahad, M.; Shahidi, S. Association between Condylar Position and Vertical Skeletal Craniofacial Morphology: A Cone Beam Computed Tomography Study. Int. Orthod. 2017, 15, 740–751. [Google Scholar] [CrossRef]
- Song, J.; Cheng, M.; Qian, Y.; Chu, F. Cone-Beam CT Evaluation of Temporomandibular Joint in Permanent Dentition According to Angle’s Classification. Oral Radiol. 2020, 36, 261–266. [Google Scholar] [CrossRef]
- Fan, X.C.; Ma, L.S.; Chen, L.; Singh, D.M.; Rausch-Fan, X.; Huang, X.F. Temporomandibular Joint Osseous Morphology of Class I and Class II Malocclusions in the Normal Skeletal Pattern: A Cone-Beam Computed Tomography Study. Diagnostics 2021, 11, 541. [Google Scholar] [CrossRef] [PubMed]
- John, Z.A.S.; Shrivastav, S.S.; Kamble, R.; Jaiswal, E.; Dhande, R. Three-Dimensional Comparative Evaluation of Articular Disc Position and Other Temporomandibular Joint Morphology in Class II Horizontal and Vertical Cases with Class I Malocclusion. Angle Orthod. 2020, 90, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, D.; Segù, M.; Arveda, N.; Lombardo, L.; Siciliani, G.; Rossi, A.; Guarda-Nardini, L. Temporomandibular Joint Disorders in Patients with Different Facial Morphology. A Systematic Review of the Literature. J. Oral Maxillofac. Surg. 2016, 74, 29–46. [Google Scholar] [CrossRef]
- Aboalnaga, A.A.; Amer, N.M.; Elnahas, M.O.; Fayed, M.M.S.; Soliman, S.A.; ElDakroury, A.E.; Labib, A.H.; Fahim, F.H. Malocclusion and Temporomandibular Disorders: Verification of the Controversy. J. Oral Facial Pain Headache 2019, 33, 440–450. [Google Scholar] [CrossRef]
- Nickel, J.C.; Weber, A.L.; Riddle, P.C.; Liu, Y.; Liu, H.; Iwasaki, L.R. Mechanobehaviour in Dolichofacial and Brachyfacial Adolescents. Orthod. Craniofac. Res. 2017, 20 (Suppl. 1), 139–144. [Google Scholar] [CrossRef] [PubMed]
- Nickel, J.C.; Iwasaki, L.R.; Gonzalez, Y.M.; Gallo, L.M.; Yao, H. Mechanobehavior and Ontogenesis of the Temporomandibular Joint. J. Dent. Res. 2018, 97, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Colonna, A.; Manfredini, D.; Lombardo, L.; Muscatello, L.; Marchese-Ragona, R.; Arveda, N.; Siciliani, G. Comparative Analysis of Jaw Morphology and Temporomandibular Disorders: A Three-Dimension Imaging Study. Cranio 2020, 38, 158–167. [Google Scholar] [CrossRef]
- Michelotti, A.; Rongo, R.; D’Antò, V.; Bucci, R. Occlusion, Orthodontics, and Temporomandibular Disorders: Cutting Edge of the Current Evidence. J. World Fed. Orthod. 2020, 9, S15–S18. [Google Scholar] [CrossRef]
- Good, S.; Edler, R.; Wertheim, D.; Greenhill, D. A Computerized Photographic Assessment of the Relationship between Skeletal Discrepancy and Mandibular Outline Asymmetry. Eur. J. Orthod. 2006, 28, 97–102. [Google Scholar] [CrossRef] [Green Version]
- Mendoza, L.V.; Bellot-Arcís, C.; Montiel-Company, J.M.; García-Sanz, V.; Almerich-Silla, J.M.; Paredes-Gallardo, V. Linear and Volumetric Mandibular Asymmetries in Adult Patients With Different Skeletal Classes and Vertical Patterns: A Cone-Beam Computed Tomography Study. Sci. Rep. 2018, 8, 12319. [Google Scholar] [CrossRef]
- Lemes, C.R.; Tozzi, C.F.; Gribel, S.; Gribel, B.F.; Venezian, G.C.; do Carmo Menezes, C.; Custodio, W. Mandibular Ramus Height and Condyle Distance Asymmetries in Individuals with Different Facial Growth Patterns: A Cone-Beam Computed Tomography Study. Surg. Radiol. Anat. 2021, 43, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Dygas, S.; Szarmach, I.; Radej, I. Assessment of the Morphology and Degenerative Changes in the Temporomandibular Joint Using CBCT According to the Orthodontic Approach: A Scoping Review. Biomed Res. Int. 2022, 2022, 6863014. [Google Scholar] [CrossRef] [PubMed]

| Group | AS | PS | SS | Condylar Length | Condylar Neck Width | Depth of Glenoid Fossa | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R | L | p-Value R vs. L | R | L | p-Value R vs. L | R | L | p-Value R vs. L | R | L | p-Value R vs. L | R | L | p-Value R vs. L | R | L | p-Value R vs. L | ||
| Normo | Mean | 1.99 a | 1.98 | 0.065 | 2.75 | 2.65 | 0.448 | 3.07 | 6.89 | 0.790 | 6.74 | 3.10 | 0.010 | 7.07 | 6.96 | 0.594 | 1.17 | 1.53 | 0.801 |
| SD | 0.59 | 0.79 | 0.89 | 0.81 | 0.80 | 1.34 | 1.51 | 0.76 | 1.37 | 1.36 | 0.35 | 0.23 | |||||||
| Hypo | Mean | 1.69 a | 1.78 | 0.841 | 2.88 | 2.98 | 0.203 | 3.33 | 7.37 | 0.850 | 7.21 | 3.38 | 0.055 | 7.40 | 7.57 | 0.047 | 1.25 | 1.08 | 0.699 |
| SD | 0.51 | 0.69 | 0.99 | 1.39 | 1.37 | 1.44 | 1.43 | 1.31 | 1.18 | 1.44 | 0.38 | 0.23 | |||||||
| Hyper | Mean | 2.43 b | 1.98 | 0.577 | 2.79 | 2.55 | 0.731 | 3.24 | 6.11 | 0.643 | 6.46 | 3.11 | 0.202 | 7.18 | 7.31 | 0.195 | 1.29 | 1.23 | 0.493 |
| SD | 0.84 | 0.70 | 0.96 | 0.84 | 1.02 | 1.79 | 1.73 | 0.69 | 2.22 | 1.48 | 0.29 | 0.29 | |||||||
| p-value ANOVA | 0.005 | 0.610 | 0.900 | 0.440 | 0.740 | 0.060 | 0.330 | 0.610 | 0.590 | 0.390 | 0.580 | 0.240 | |||||||
| Group | AP Condyle Diameter | ML Condyle Diameter | Condylar Axis Angle | Condyle-MSP | AP Diff Condyle-MSP | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R | L | p-Value R vs. L | R | L | p-Value R vs. L | R | L | p-Value R vs. L | R | L | p-Value R vs. L | |||
| Normo | Mean | 5.91 | 6.04 | 0.008 | 18.69 a | 18.08 a | 0.509 | 68.76 | 73.25 | 0.304 | 47.53 | 41.51 | 0.001 | 8.00 |
| SD | 0.59 | 1.29 | 2.17 | 2.13 | 10.37 | 8.45 | 4.48 | 7.25 | 4.09 | |||||
| Hypo | Mean | 6.46 | 6.44 | 0.579 | 18.79 a | 18.26 a | 0.602 | 64.59 | 70.24 | 0.124 | 47.13 | 40.60 | 0.001 | 4.95 |
| SD | 1.56 | 1.28 | 2.44 | 2.79 | 8.80 | 9.31 | 6.09 | 5.32 | 4.18 | |||||
| Hyper | Mean | 5.57 | 5.72 | 0.024 | 15.75 b | 15.46 b | 0.752 | 63.54 | 73.47 | 0.098 | 47.83 | 40.17 | 0.012 | 6.67 |
| SD | 1.77 | 1.59 | 1.79 | 2.35 | 9.15 | 11.56 | 6.77 | 4.53 | 4.49 | |||||
| p-value ANOVA | 0.150 | 0.300 | 0.010 | 0.002 | 0.220 | 0.510 | 0.940 | 0.790 | 0.080 | |||||
| Group | LS | MS | ML Condyle Thickness | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R | L | p-Value R vs. L | R | L | p-Value R vs. L | R | L | p-Value R vs. L | ||
| Normo | Mean | 2.57 | 2.52 | 0.579 | 2.53 | 2.79 | 0.367 | 15.53 | 16.32 a | 0.013 |
| SD | 0.69 | 0.76 | 0.89 | 0.84 | 3.38 | 2.53 | ||||
| Hypo | Mean | 2.89 | 2.63 | 0.093 | 2.48 | 2.65 | 0.635 | 14.78 | 16.55 a | 0.683 |
| SD | 1.03 | 1.11 | 1.02 | 1.09 | 2.85 | 2.98 | ||||
| Hyper | Mean | 2.71 | 2.60 | 0.013 | 2.49 | 2.66 | 0.324 | 12.96 | 13.92 b | 0.355 |
| SD | 1.15 | 0.73 | 0.83 | 1.09 | 3.34 | 3.01 | ||||
| p-value ANOVA | 0.550 | 0.920 | 0.990 | 0.880 | 0.070 | 0.020 | ||||
| Group | ZR-ZL | AZ-ZA | J-J1 | AG-GA | C-C1 | |
|---|---|---|---|---|---|---|
| Normo | Mean | 99.22 | 124.90 | 68.11 | 85.68 a | 26.76 |
| SD | 7.27 | 7.14 | 3.97 | 5.84 | 3.16 | |
| Hypo | Mean | 100.16 | 123.58 | 68.31 | 80.69 b | 25.89 |
| SD | 5.41 | 6.59 | 5.55 | 5.41 | 2.19 | |
| Hyper | Mean | 96.55 | 120.23 | 66.95 | 80.59 b | 26.68 |
| SD | 5.69 | 7.21 | 6.66 | 5.14 | 2.80 | |
| p-value ANOVA | 0.230 | 0.150 | 0.740 | 0.007 | 0.540 |
| Group | Cg-Me | ANS-Me | Cg-ANS | J-Me | Ag-Me | |||
|---|---|---|---|---|---|---|---|---|
| R | L | R | L | |||||
| Normo | Mean | 113.13 | 63.13 a | 50.54 | 70.41 | 68.32 | 49.57 a | 44.39 a |
| SD | 5.64 | 5.67 | 4.67 | 6.48 | 5.67 | 5.59 | 5.52 | |
| Hypo | Mean | 109.10 | 58.03 b | 51.19 | 67.46 | 64.52 | 44.03 b | 40.31 b |
| SD | 9.31 | 6.01 | 4.85 | 6.64 | 7.29 | 4.35 | 4.39 | |
| Hyper | Mean | 111.09 | 62.08 a | 49.86 | 70.29 | 68.01 | 48.41 a | 43.76 a |
| SD | 6.96 | 5.33 | 3.45 | 6.75 | 6.69 | 3.51 | 4.02 | |
| p-value ANOVA | 0.240 | 0.020 | 0.680 | 0.290 | 0.140 | 0.001 | 0.020 | |
| Group | Az-MSP | C-MSP | J-MSP | Ag-MSP | |||||
|---|---|---|---|---|---|---|---|---|---|
| R | L | R | L | R | L | R | L | ||
| Normo | Mean | 63.96 | 58.89 | 13.82 | 12.23 | 35.80 | 31.73 | 45.47 a | 39.48 |
| SD | 5.74 | 4.42 | 2.51 | 1.33 | 3.58 | 3.24 | 4.44 | 3.37 | |
| Hypo | Mean | 64.24 | 59.64 | 13.25 | 12.49 | 35.43 | 32.19 | 42.58 b | 37.99 |
| SD | 3.95 | 3.71 | 2.10 | 1.45 | 3.49 | 2.69 | 3.72 | 3.62 | |
| Hyper | Mean | 62.21 | 57.68 | 13.20 | 13.09 | 34.66 | 31.30 | 42.88 b | 37.72 |
| SD | 4.11 | 5.59 | 1.55 | 1.82 | 4.04 | 4.20 | 3.53 | 3.88 | |
| p-value ANOVA | 0.410 | 0.450 | 0.610 | 0.250 | 0.660 | 0.630 | 0.040 | 0.280 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diwakar, R.; Bucci, R.; Kaushik, A.; Bansal, A.; Bucci, P.; Kochhar, A.S.; Spagnuolo, G. Three-Dimensional Assessment of Temporomandibular Joint Morphology and Facial Asymmetry in Individuals with Different Vertical Skeletal Growth Patterns. Int. J. Environ. Res. Public Health 2023, 20, 1437. https://doi.org/10.3390/ijerph20021437
Diwakar R, Bucci R, Kaushik A, Bansal A, Bucci P, Kochhar AS, Spagnuolo G. Three-Dimensional Assessment of Temporomandibular Joint Morphology and Facial Asymmetry in Individuals with Different Vertical Skeletal Growth Patterns. International Journal of Environmental Research and Public Health. 2023; 20(2):1437. https://doi.org/10.3390/ijerph20021437
Chicago/Turabian StyleDiwakar, Rohan, Rosaria Bucci, Ankur Kaushik, Anubhav Bansal, Paolo Bucci, Anuraj Singh Kochhar, and Gianrico Spagnuolo. 2023. "Three-Dimensional Assessment of Temporomandibular Joint Morphology and Facial Asymmetry in Individuals with Different Vertical Skeletal Growth Patterns" International Journal of Environmental Research and Public Health 20, no. 2: 1437. https://doi.org/10.3390/ijerph20021437
APA StyleDiwakar, R., Bucci, R., Kaushik, A., Bansal, A., Bucci, P., Kochhar, A. S., & Spagnuolo, G. (2023). Three-Dimensional Assessment of Temporomandibular Joint Morphology and Facial Asymmetry in Individuals with Different Vertical Skeletal Growth Patterns. International Journal of Environmental Research and Public Health, 20(2), 1437. https://doi.org/10.3390/ijerph20021437








