Development and Performance Evaluation of an IoT-Integrated Breath Analyzer
Abstract
1. Introduction
1.1. Proposed Solution
1.2. Research Contribution
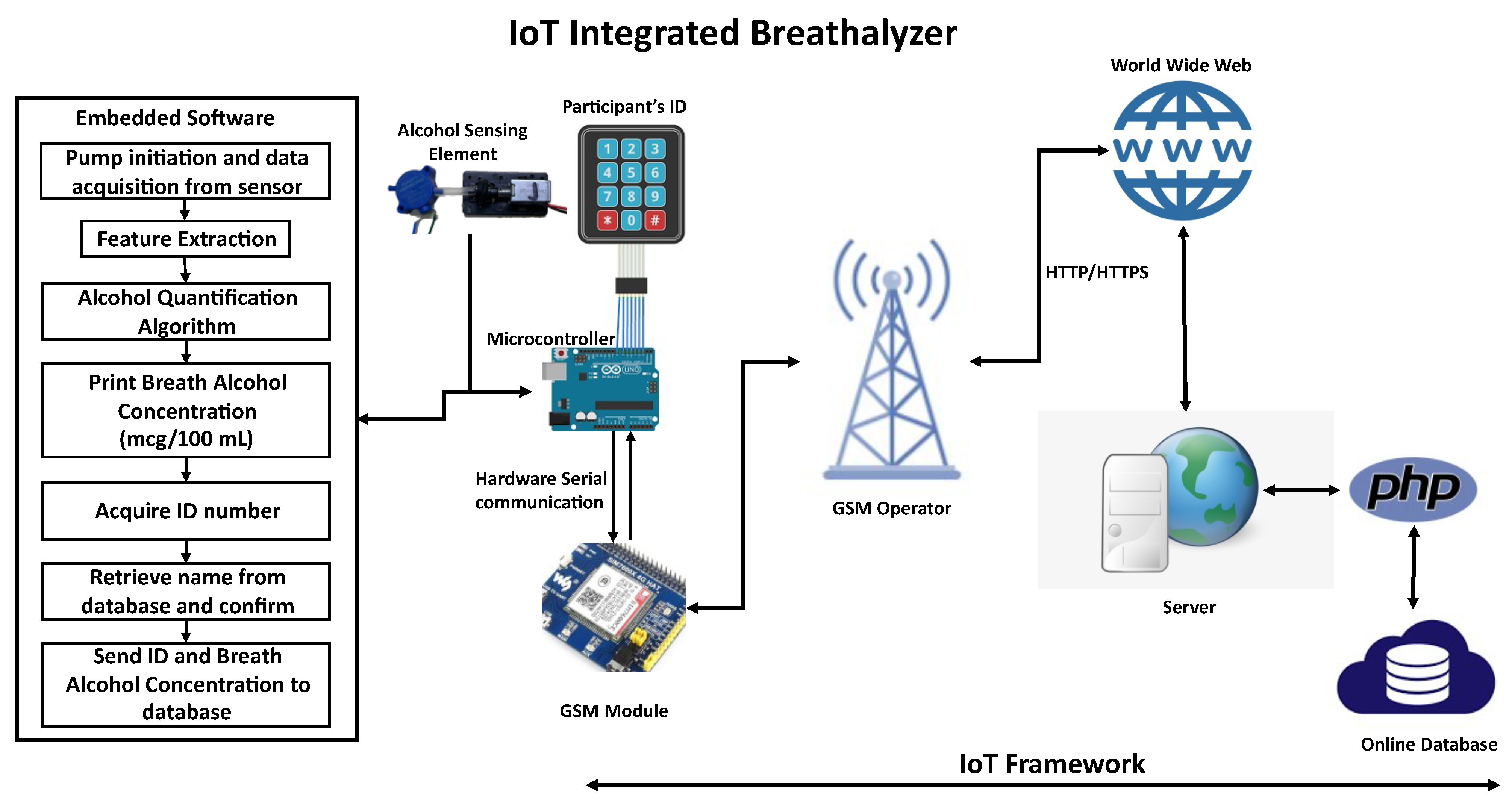
- An implementation of the hypertext transfer protocol (HTTP) to request and post participants’ information and breath-detected alcohol concentrations into a cloud database via cellular IoT technology.
- Quantifying breath alcohol concentration by using a manually developed threshold and linear regression algorithm.
- Performance evaluation of the developed alcohol quantification algorithm.
2. Background
2.1. Breath Analyzers
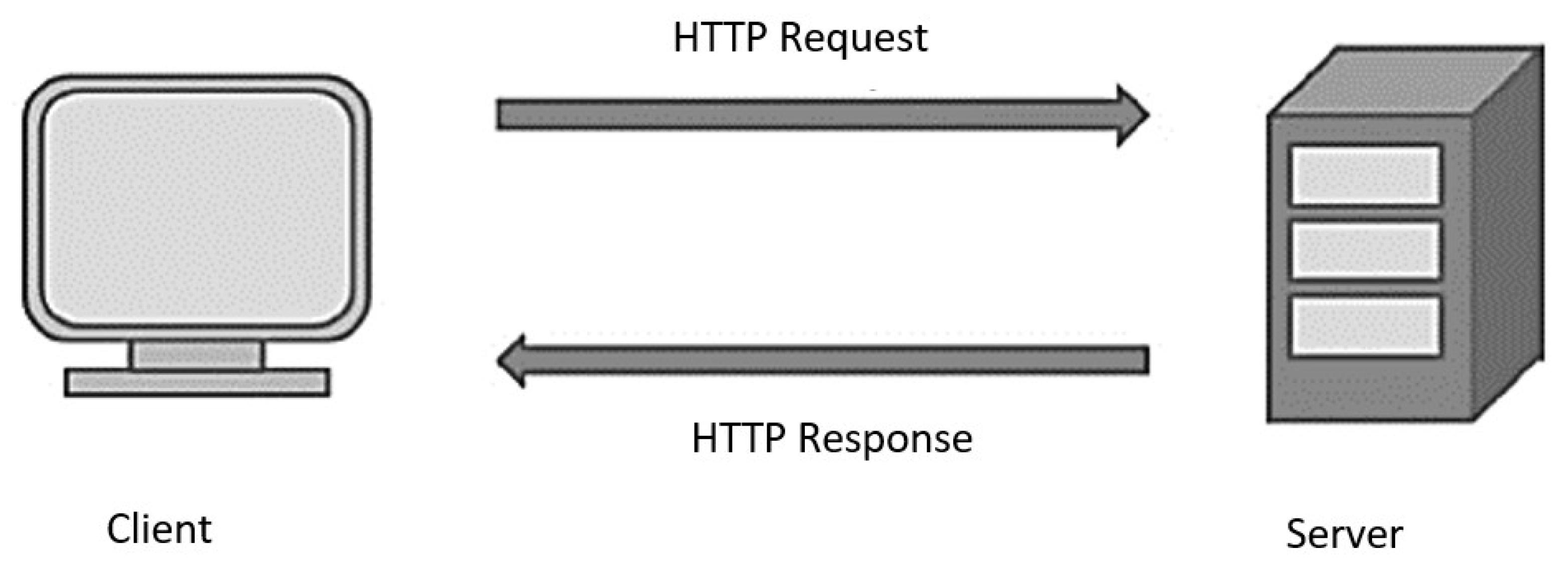
2.2. Cellular IoT and Internet Protocol
3. Methodology
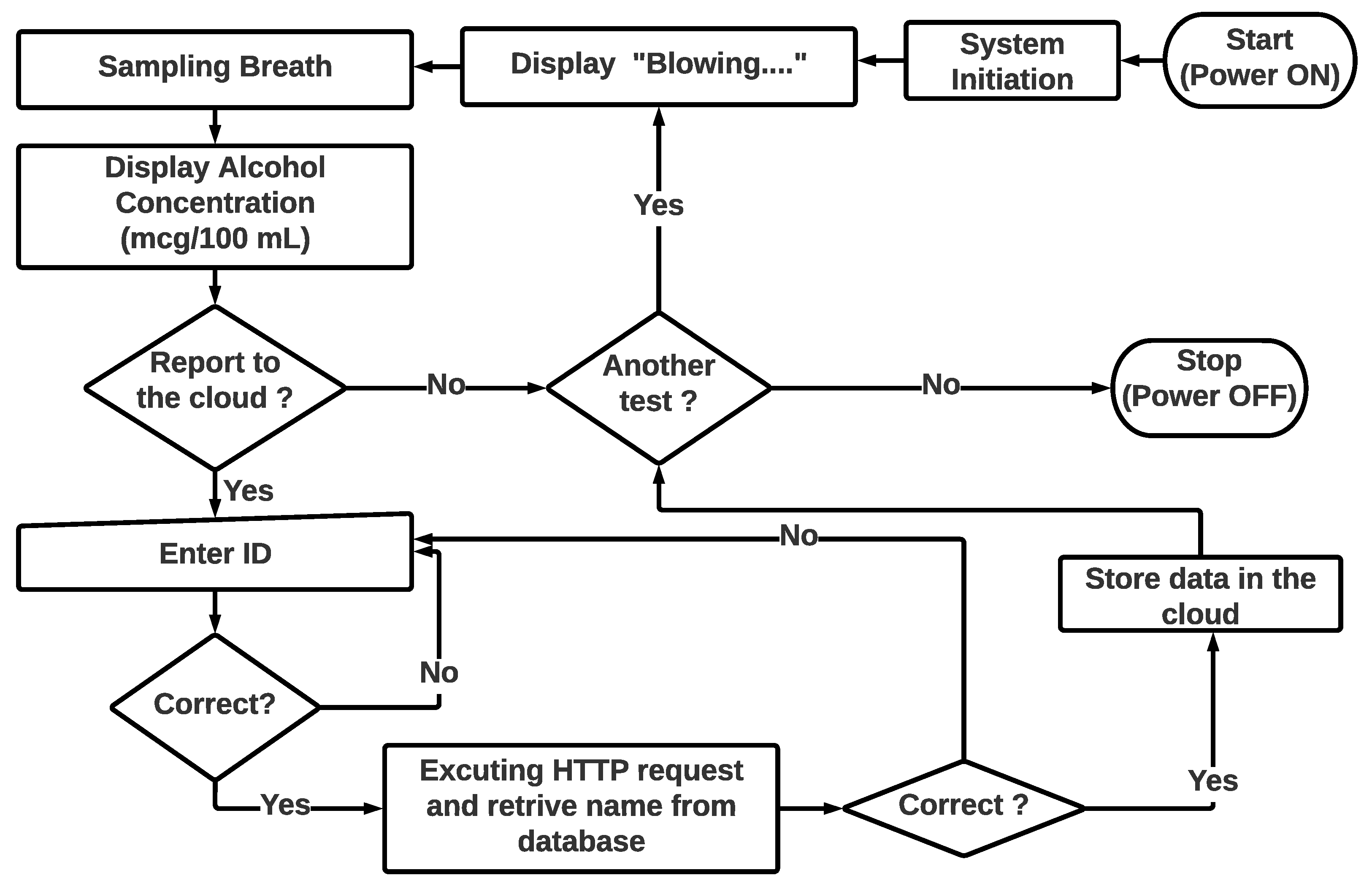
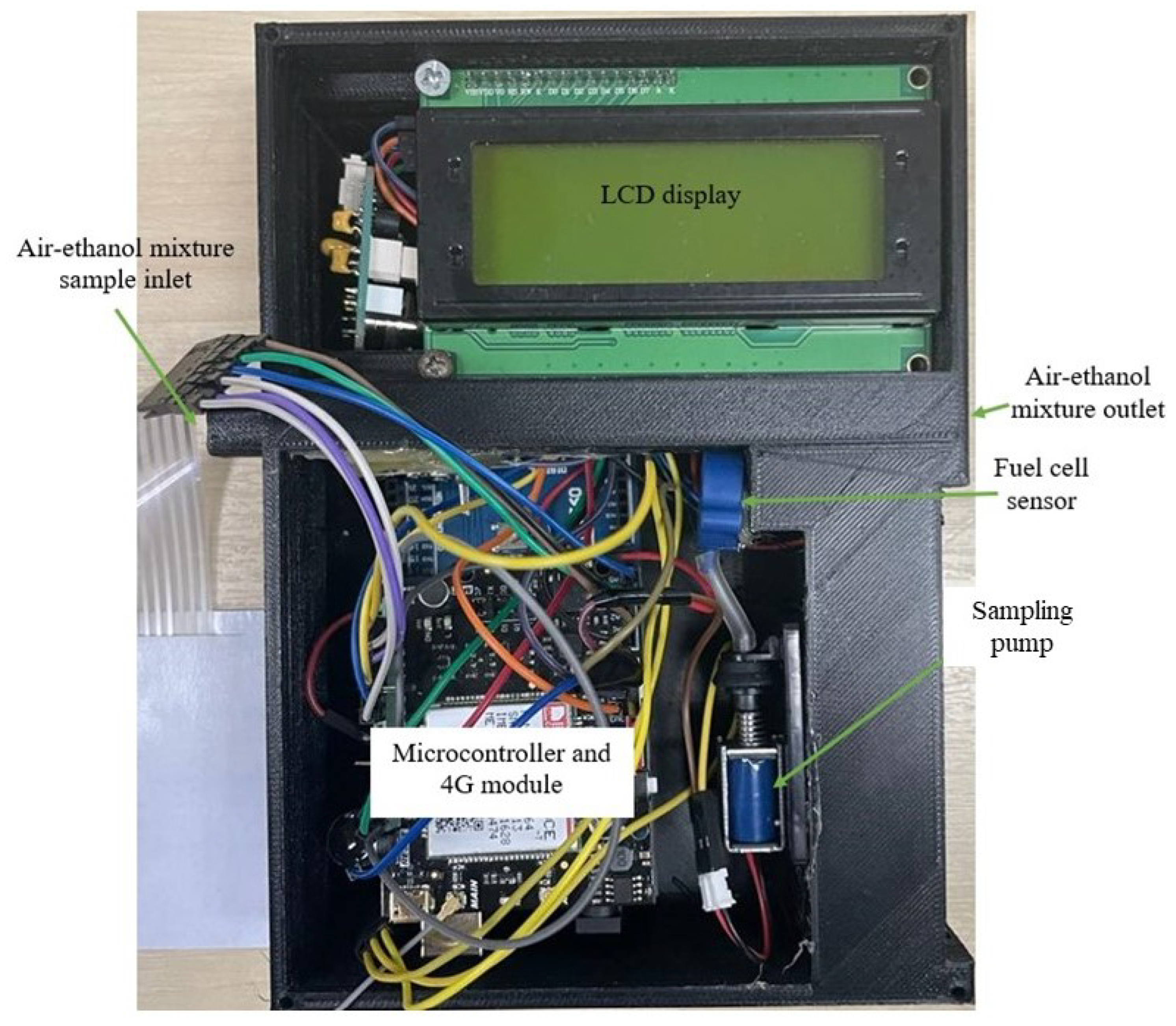
3.1. Conceptual Design
3.2. Data Collection
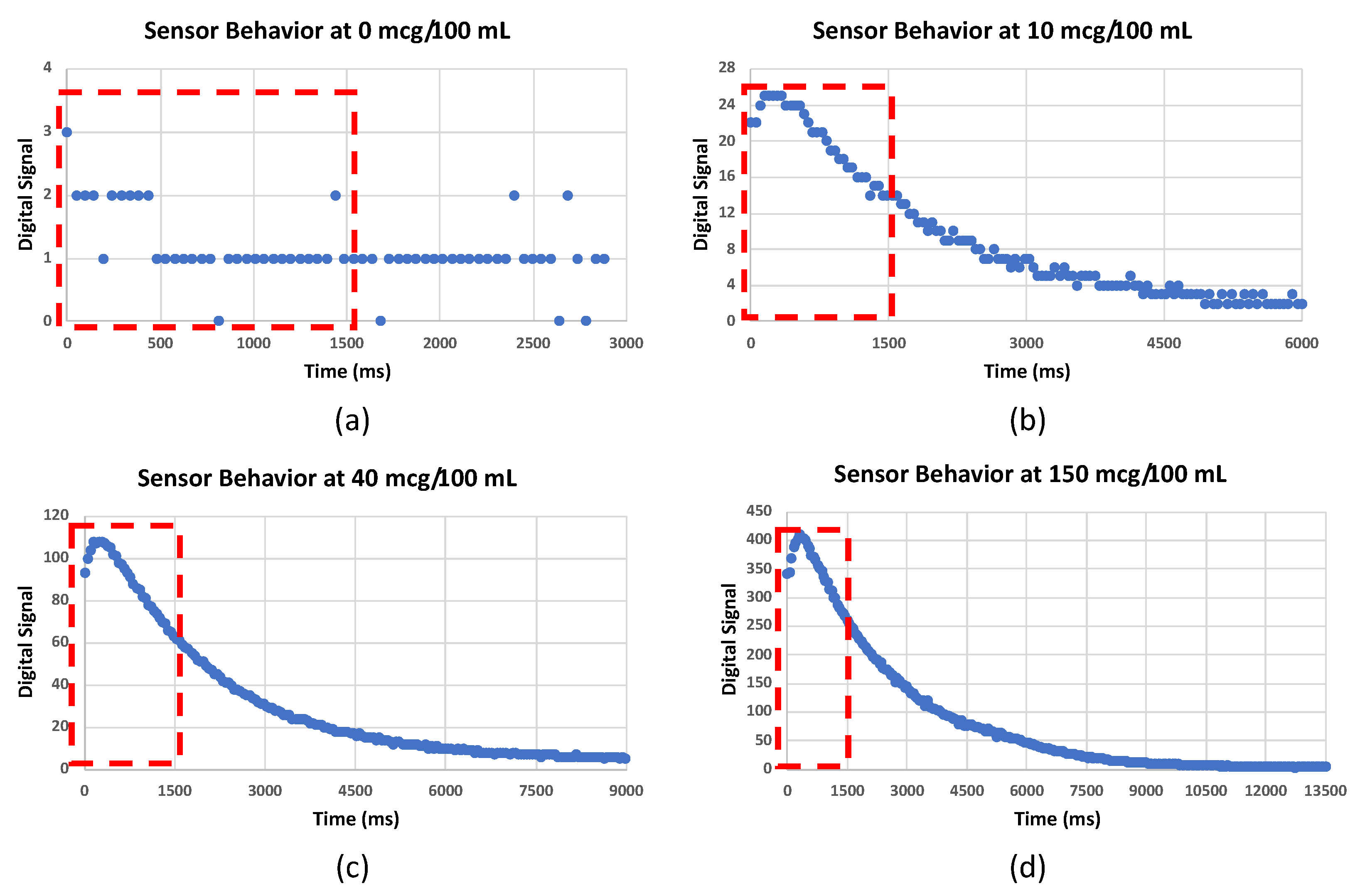
3.3. Feature Extraction
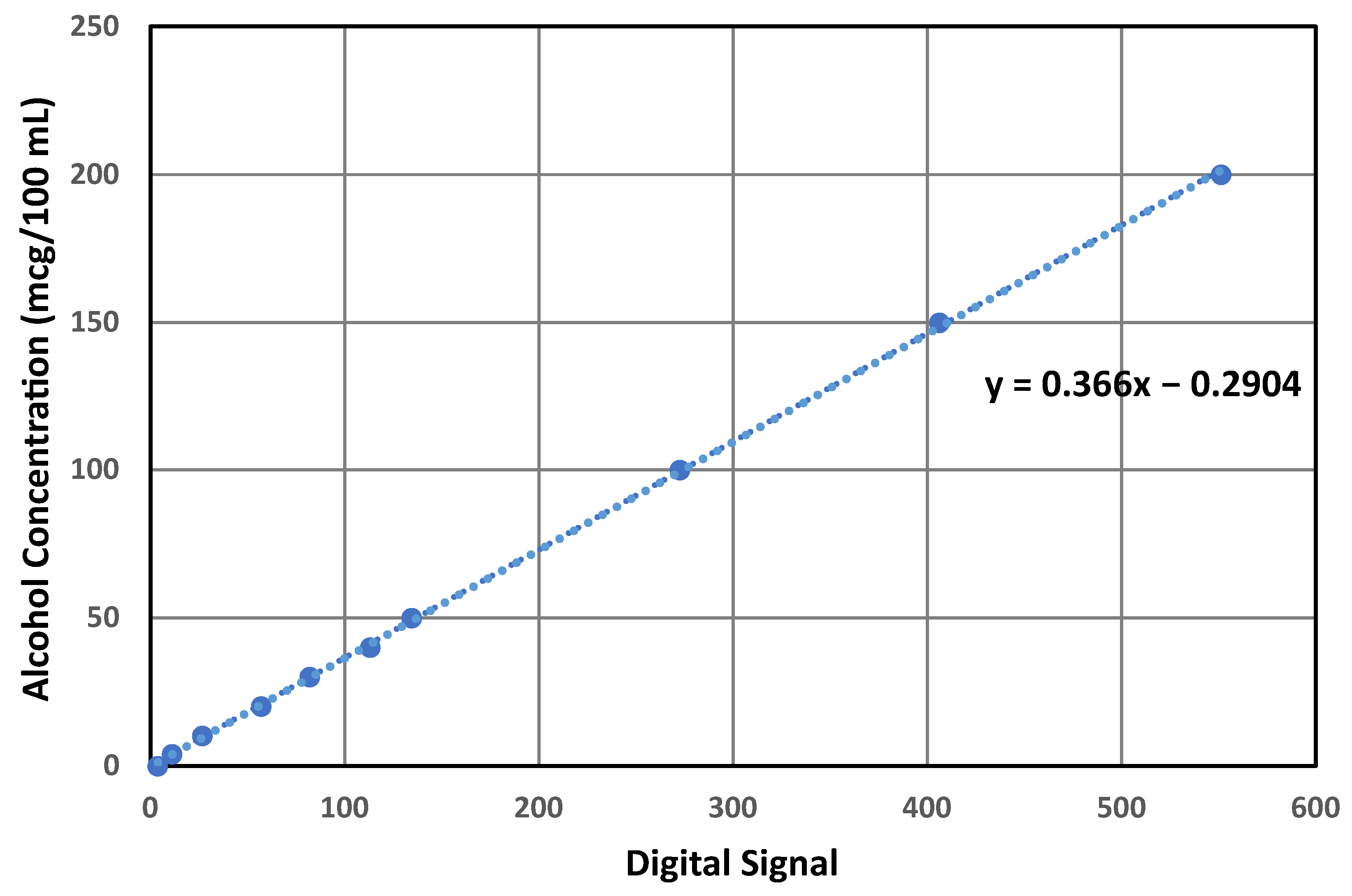
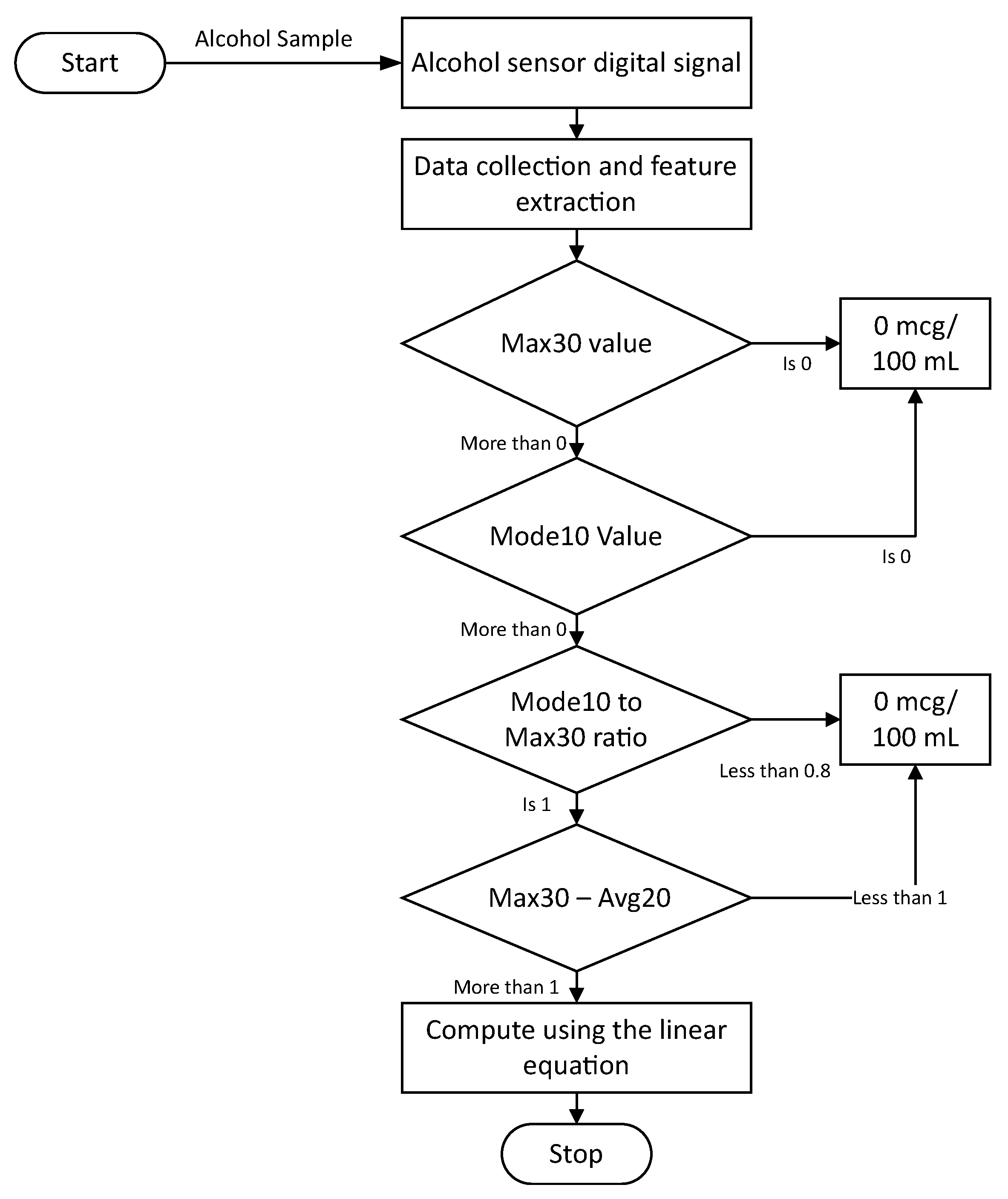
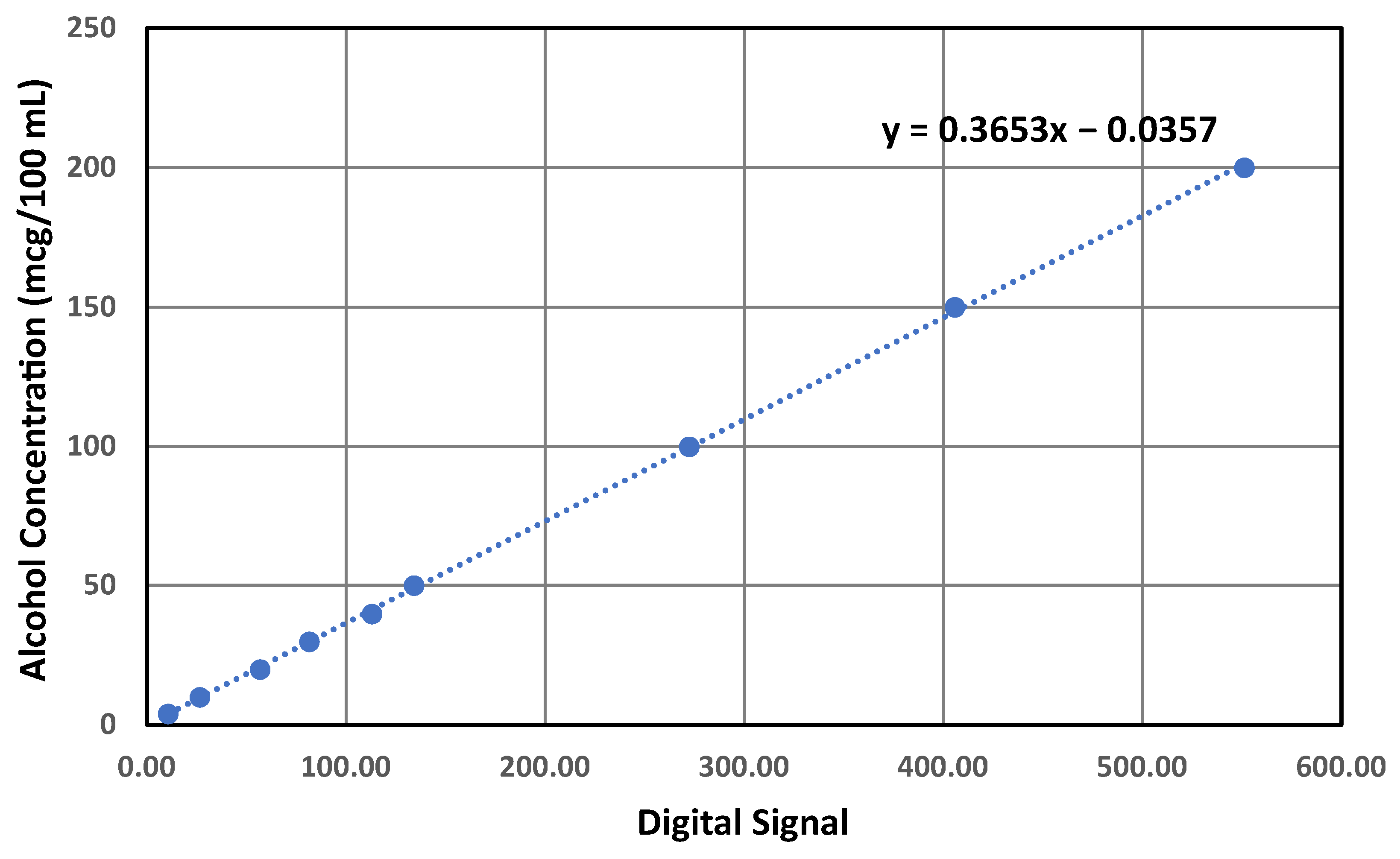
3.4. Alcohol Concentration Quantification Algorithm
3.4.1. Threshold Algorithm
3.5. Experimental Setup and Performance Evaluation
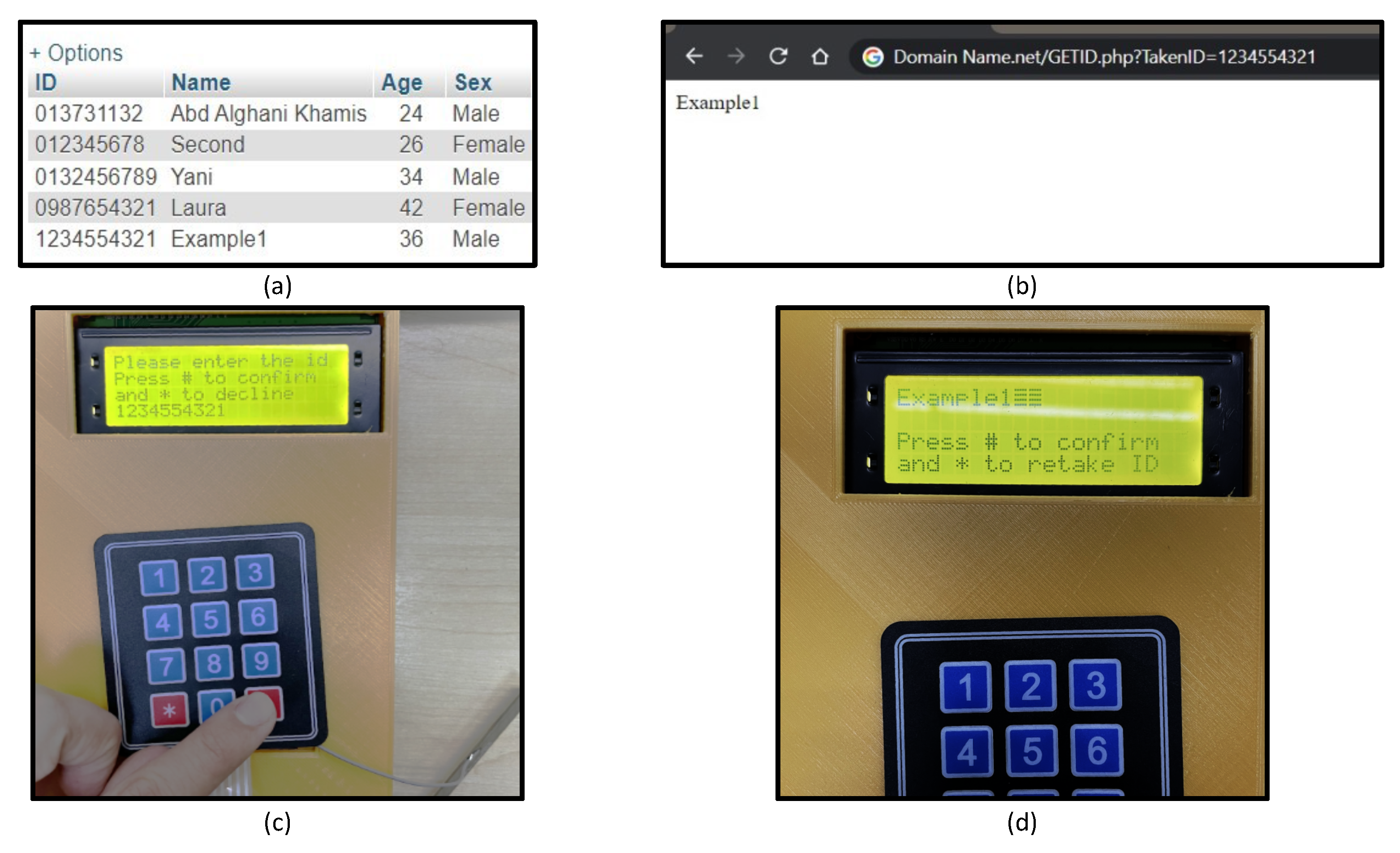
3.6. IoT Integration
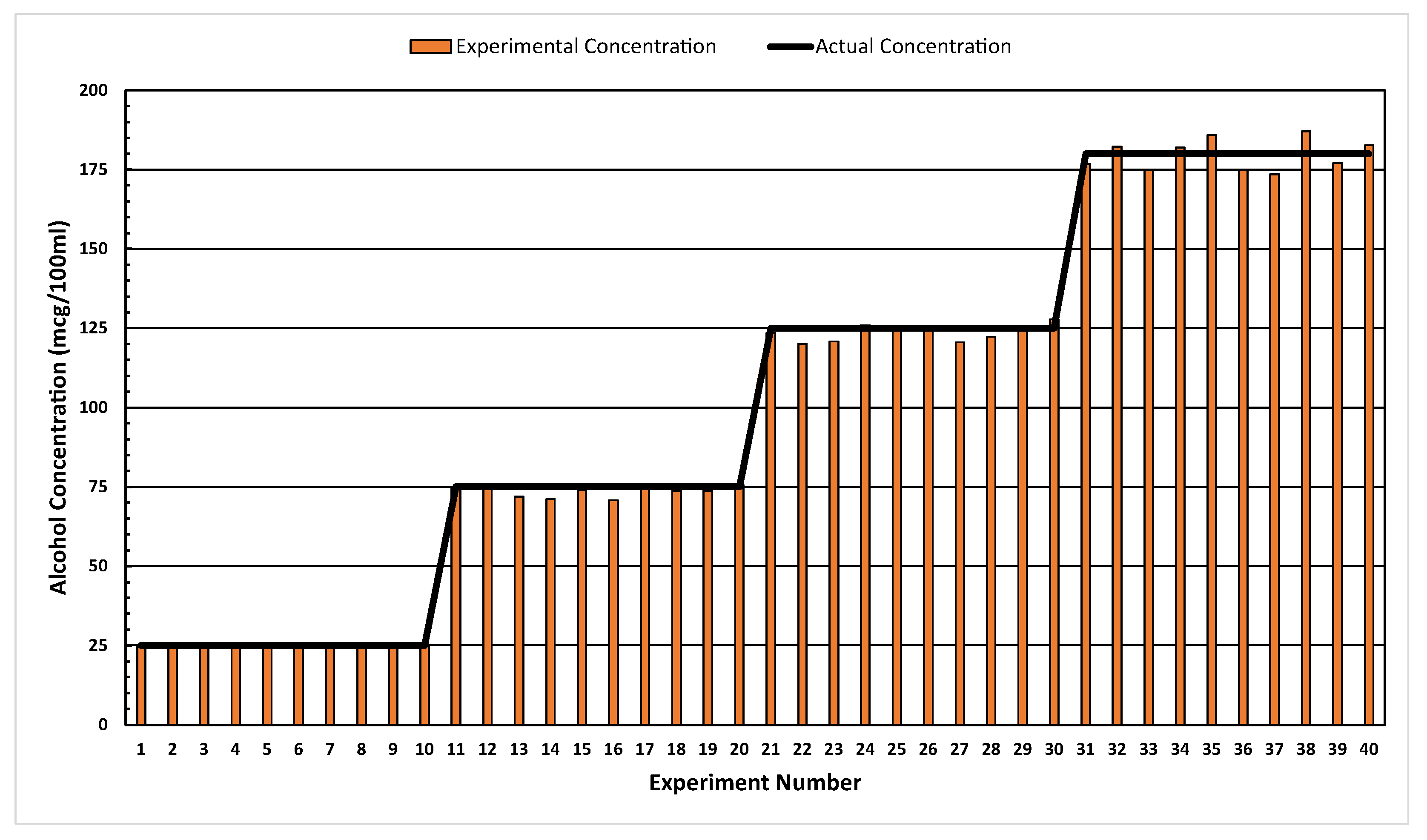
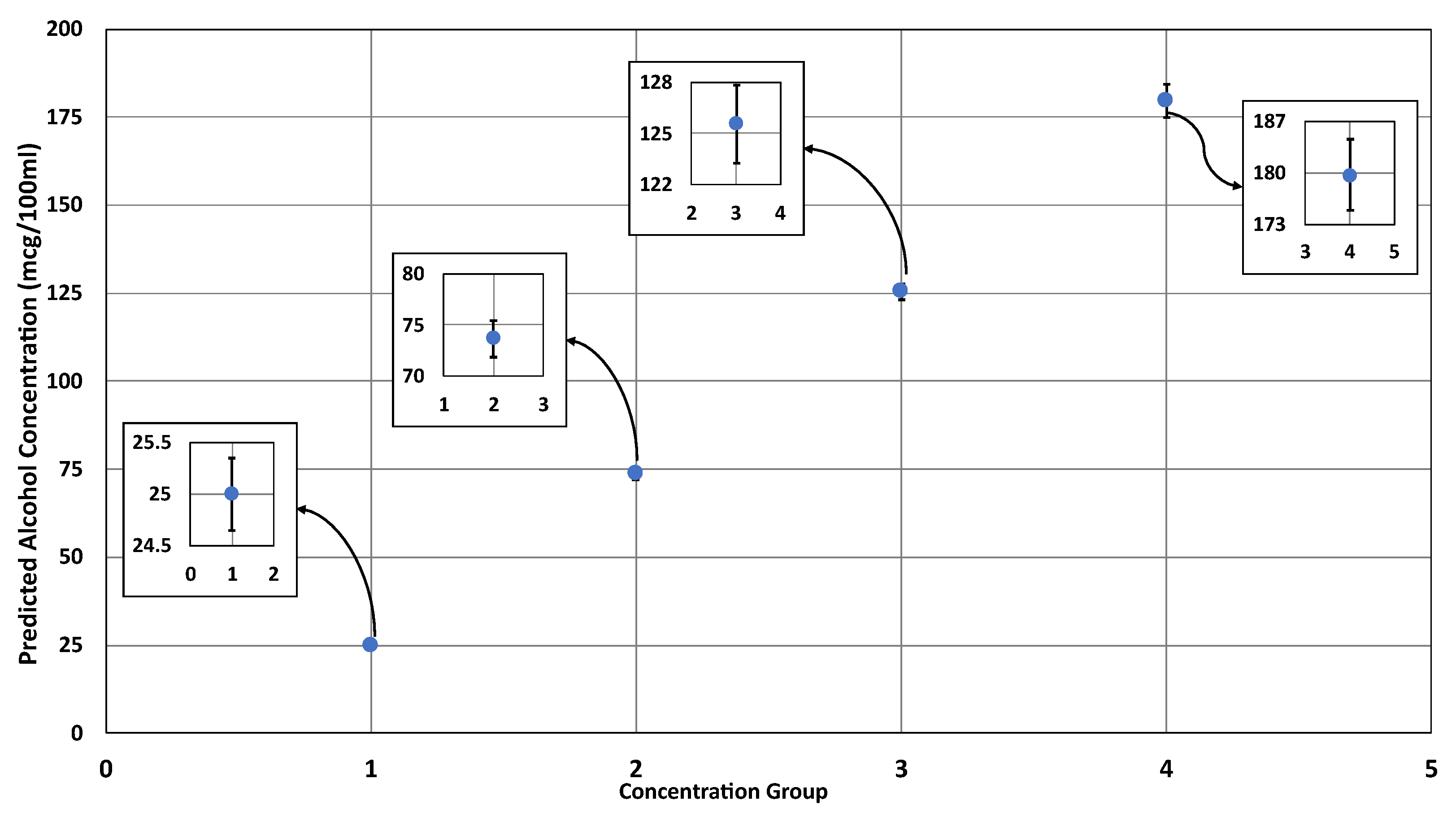
4. Results
Performance Evaluation of Alcohol Quantification
5. Discussion
6. Conclusions
7. Outlook
- The developed device implemented one type of sensing technology (fuel cell). Therefore, future work may investigate different sensing technologies.
- The IoT integration in this work was performed by utilizing cellular technology to enable a wide range of coverage (throughout the country within the local cellular operators’ service areas); however, the energy consumption of this technology was not investigated. Hence, future work may study this aspect when implementing cellular IoT in such applications.
- The Internet protocol used in this work is the HTTP using the GET request due to the small size of data to be transferred between the device and the cloud that does not require a complex protocol to account for connection times; however, HTTP GET may become a limitation when larger applications with larger sets of data need to be sent for each request. Therefore, future research comparing the performances of different protocols in terms of speed in the cellular IoT scope may provide beneficial insights and guidelines for choosing a proper protocol with the cellular IoT.
- The quantification algorithm presented in this research was manually developed; future research may investigate other methods for algorithmic developments, such as machine learning.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IoT | Internet of Things |
| HTTP | Hypertext transfer protocol |
| PHP | Hypertext preprocessor |
| GSM | Global System for Mobile communication |
| BrAC | Breath alcohol content |
| BAC | Blood alcohol content |
| BBR | Breath alcohol to blood alcohol ratio |
| mcg/100 mL | Micrograms of alcohol per 100 milliliters of breath |
| Pt | Platinum |
| Chemical formula of ethanol | |
| Chemical formula of acetaldehyde | |
| H | Hydrogen |
| Electron | |
| Chemical formula of water | |
| Chemical formula of acetic acid | |
| Chemical formula of carbon dioxide | |
| OCP | Open circuit potential |
| E | Thermodynamic voltage |
| Reversible voltage standard | |
| T | Temperature |
| R | Ideal gas constant |
| n | Number of transferred electrons |
| F | Faraday constant |
| Partial pressure of the reactants | |
| Partial pressure of the products | |
| 2G | Second generation of cellular network |
| 3G | Third generation of cellular network |
| 4G | Fourth generation of cellular network |
| LTE | Long-term evolution |
| Mbps | Megabits per second |
| kbps | Kilobits per second |
| mL | Milliliter |
| A/D | Analog-to-digital |
| V | Volts |
| mv | Millivolts |
| ms | Milliseconds |
| MSE | Mean squared error |
| RMSE | Root mean squared error |
| MAE | Mean absolute error |
| Coefficient of determination | |
| RSD | Relative standard deviation |
| Standard deviation | |
| Predicted BrAC obtained from the algorithm | |
| Actual BrAC obtained from the algorithm | |
| Average of all the actual BrAC values | |
| Average of the predicted BrAC values at a certain concentration |
Appendix A
| Command | Purpose | Expected Responses and Interpretation |
|---|---|---|
| AT | Checking the readiness of the chip | OK, system is ready |
| ERROR, wiring issue | ||
| AT+HTTPTERM | Termination of HTTP | OK, protocol has been terminated |
| ERROR, protocol is already terminated | ||
| AT+HTTPINIT | Initialization of HTTP | OK, protocol has been initialized |
| ERROR, protocol cannot be initialized | ||
| AT+HTTPPARA = | Sending HTTP parameters: should include the URL for the website (e.g., AT+HTTPPARA = URL, DomainName.net/GETID.php?TakenID=1234554321”) | OK, address is correct, and the status is online |
| ERROR, incorrect address | ||
| AT+HTTPACTION = 0 | Choosing HTTP GET method | +HTTPACTION: 0, 200, 18, HTTP GET is ready and 8 is the length of the content |
| ERROR, Server error | ||
| AT+HTTPREAD = 0, 18 | Reading the content from position 0 to position 18, this code is based on the previous output | Displaying the name, (e.g., Example 1) |
| A response from the cloud: “No ID found” |
References
- Hajek, A.; Bock, J.O.; Weyerer, S.; König, H.H. Correlates of alcohol consumption among Germans in the second half of life. Results of a population-based observational study. BMC Geriatr. 2017, 17, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, H.F. Alcohol and human health: What is the evidence? Annu. Rev. Food Sci. Technol. 2020, 11, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Poli, A.; Visioli, F. Moderate alcohol use and health: An update a Consensus Document. BIO Web Conf. 2015, 5, 04001. [Google Scholar] [CrossRef]
- Martins, J.G.; Guimarães, M.O.; Jorge, K.O.; Silva, C.J.d.P.; Ferreira, R.C.; Pordeus, I.A.; Kawachi, I.; Zarzar, P.M.P.d.A. Binge drinking, alcohol outlet density and associated factors: A multilevel analysis among adolescents in Belo Horizonte, Minas Gerais State, Brazil. Cad. De Saúde Pública 2019, 36, e00052119. [Google Scholar] [CrossRef]
- Joseph, A.; Lasimbang, H.B.; James, S.; Seok, C.B. Effects of Alcohol towards Quality of Life in the Indigenous Groups of the West Coast Division, Sabah, Malaysia. Borneo J. Med Sci. 2020, 14, 19–30. [Google Scholar] [CrossRef]
- Sjödin, L.; Larm, P.; Karlsson, P.; Livingston, M.; Raninen, J. Drinking motives and their associations with alcohol use among adolescents in Sweden. Nord. Stud. Alcohol Drugs 2021, 38, 256–269. [Google Scholar] [CrossRef]
- Khamis, A.A.; Salleh, S.Z.; Ab Karim, M.S.; Mohd Rom, N.A.; Janasekaran, S.; Idris, A.; Abd Rashid, R.B. Alcohol Consumption Patterns: A Systematic Review of Demographic and Sociocultural Influencing Factors. Int. J. Environ. Res. Public Health 2022, 19, 8103. [Google Scholar] [CrossRef]
- Boyd, J.; Bambra, C.; Purshouse, R.C.; Holmes, J. Beyond Behaviour: How Health Inequality Theory Can Enhance Our Understanding of the ‘Alcohol-Harm Paradox’. Int. J. Environ. Res. Public Health 2021, 18, 6025. [Google Scholar] [CrossRef]
- Borrell, C.; Palència, L.; Bosakova, L.; Gotsens, M.; Morrison, J.; Costa, C.; Dzurova, D.; Deboosere, P.; Lustigova, M.; Marí-Dell’Olmo, M.; et al. Socioeconomic Inequalities in Chronic Liver Diseases and Cirrhosis Mortality in European Urban Areas before and after the Onset of the 2008 Economic Recession. Int. J. Environ. Res. Public Health 2021, 18, 8801. [Google Scholar] [CrossRef]
- Anderson, P.; O’Donnell, A.; Kokole, D.; Jané Llopis, E.; Kaner, E. Is Buying and Drinking Zero and Low Alcohol Beer a Higher Socio-Economic Phenomenon? Analysis of British Survey Data, 2015–2018 and Household Purchase Data 2015–2020. Int. J. Environ. Res. Public Health 2021, 18, 10347. [Google Scholar] [CrossRef]
- Dong, J.; Zhao, Q.; Ren, Y. Dark Side or Bright Side: The Impact of Alcohol Drinking on the Trust of Chinese Rural Residents. Int. J. Environ. Res. Public Health 2022, 19, 5924. [Google Scholar] [CrossRef] [PubMed]
- Beksinska, A.; Nyariki, E.; Kabuti, R.; Kungu, M.; Babu, H.; Shah, P.; Champions, T.M.F.S.; Nyabuto, C.; Okumu, M.; Mahero, A.; et al. Harmful Alcohol and Drug Use Is Associated with Syndemic Risk Factors among Female Sex Workers in Nairobi, Kenya. Int. J. Environ. Res. Public Health 2022, 19, 7294. [Google Scholar] [CrossRef] [PubMed]
- Saul, C.; Lange, S.; Probst, C. Employment Status and Alcohol-Attributable Mortality Risk—A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 7354. [Google Scholar] [CrossRef] [PubMed]
- Ahlner, F.; Falk Erhag, H.; Johansson, L.; Mellqvist Fässberg, M.; Rydberg Sterner, T.; Samuelsson, J.; Zettergren, A.; Waern, M.; Skoog, I. Patterns of Alcohol Consumption and Associated Factors in a Population-Based Sample of 70-Year-Olds: Data from the Gothenburg H70 Birth Cohort Study 2014–16. Int. J. Environ. Res. Public Health 2022, 19, 8248. [Google Scholar] [CrossRef]
- Nord, J.H.; Koohang, A.; Paliszkiewicz, J. The Internet of Things: Review and theoretical framework. Expert Syst. Appl. 2019, 133, 97–108. [Google Scholar] [CrossRef]
- Ullah, F.U.M.; Muhammad, K.; Haq, I.U.; Khan, N.; Heidari, A.A.; Baik, S.W.; de Albuquerque, V.H.C. AI-Assisted Edge Vision for Violence Detection in IoT-Based Industrial Surveillance Networks. IEEE Trans. Ind. Inf. 2021, 18, 5359–5370. [Google Scholar] [CrossRef]
- Dang, L.M.; Piran, M.J.; Han, D.; Min, K.; Moon, H. A survey on internet of things and cloud computing for healthcare. Electronics 2019, 8, 768. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, Z.; Guan, C.; Wolter, K.; Xu, M. Collaborate edge and cloud computing with distributed deep learning for smart city internet of things. IEEE Internet Things J. 2020, 7, 8099–8110. [Google Scholar] [CrossRef]
- Javaid, M.; Khan, I.H. Internet of Things (IoT) enabled healthcare helps to take the challenges of COVID-19 Pandemic. J. Oral Biol. Craniofacial Res. 2021, 11, 209–214. [Google Scholar] [CrossRef]
- R, D.K.; R, A.K. A comprehensive review on usage of Internet of Things (IoT) in healthcare system. In Proceedings of the 2015 International Conference on Emerging Research in Electronics, Computer Science and Technology (ICERECT), Mandya, India, 17–19 December 2015; pp. 132–136. [Google Scholar] [CrossRef]
- Islam, S.R.; Kwak, D.; Kabir, M.H.; Hossain, M.; Kwak, K.S. The internet of things for health care: A comprehensive survey. IEEE Access 2015, 3, 678–708. [Google Scholar] [CrossRef]
- Greco, L.; Percannella, G.; Ritrovato, P.; Tortorella, F.; Vento, M. Trends in IoT based solutions for health care: Moving AI to the edge. Pattern Recognit. Lett. 2020, 135, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Mikołajczyk, M.; Patrzyk, S.; Nieniewski, M.; Woźniacka, A. Evaluation of a smartphone application for diagnosis of skin diseases. Adv. Dermatol. Allergol. Dermatol. I Alergol. 2021, 38, 761–766. [Google Scholar] [CrossRef]
- Shamsa, A.B.; Raihanb, M.M.S.; Khanc, M.M.U.; Monjurd, O.; Bin, R. Telehealthcare and Telepathology in Pandemic: A Noninvasive, Low-Cost Micro-Invasive and Multimodal Real-Time Online Application for Early Diagnosis of COVID-19 Infection. arXiv 2022, arXiv:2109.07846. [Google Scholar]
- Satpathy, S.; Mohan, P.; Das, S.; Debbarma, S. A new healthcare diagnosis system using an IoT-based fuzzy classifier with FPGA. J. Supercomput. 2020, 76, 5849–5861. [Google Scholar] [CrossRef]
- Abdel-Basset, M.; Manogaran, G.; Gamal, A.; Chang, V. A Novel Intelligent Medical Decision Support Model Based on Soft Computing and IoT. IEEE Internet Things J. 2020, 7, 4160–4170. [Google Scholar] [CrossRef]
- Xiao, P. Designing Embedded Systems and the Internet of Things (IoT) with the ARM® MbedTM; John Wiley & Sons: Hoboken, NJ, USA, 2016; Volume 4. [Google Scholar]
- Stewart, K.; Kelley-Baker, T. The History of Impaired Driving and the Role of the Transportation Research Board Committee on Alcohol, Other Drugs, and Transportation; Centennial Papers; Transportation Research Board: Washington, DC, USA, 2020. [Google Scholar]
- Jones, A.W.; Cowan, J.M. Reflections on variability in the blood-breath ratio of ethanol and its importance when evidential breath-alcohol instruments are used in law enforcement. Forensic. Sci. Res. 2020, 5, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Swartz, J.A. Research Methods in the Study of Substance Abuse; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Alcohol Measurement Units. Available online: https://www.lionlaboratories.com/alcohol-science/alcohol-measurement-units/ (accessed on 23 September 2022).
- Abbott, H.D. Breathalyzer Coaching and Setup Methodology. United States Patent US20180317838A1, 8 November 2018. [Google Scholar]
- Hamalainen, M.; Andersson, K. Method and Device for Estimating a Risk of Relapse of Addictive Behaviour. United States Patent Application 20180140241, 29 April 2016. [Google Scholar]
- Hamalainen, M.; Zetterström, A.; Winkvist, M.; Söderquist, M.; Karlberg, E.; Ohagen, P.; Andersson, K.; Nyberg, F. Real-time monitoring using a breathalyzer-based ehealth system can identify lapse/relapse patterns in alcohol use disorder patients. Alcohol Alcohol. 2018, 53, 368–375. [Google Scholar] [CrossRef]
- Min, A.; Gao, G.; Shih, P.C.; Lee, D.; Jeong, S. Design and assessment of a personal breathalyzer intervention to support responsible drinking. Artic. Int. J.-Hum.-Comput. Stud. 2020, 14, 102382. [Google Scholar] [CrossRef]
- Oluwoye, O.; Reneau, H.; Herron, J.; Alcover, K.C.; McPherson, S.; Roll, J.; McDonell, M.G. Pilot study of an integrated smartphone and breathalyzer contingency management intervention for alcohol use. J. Addict. Med. 2020, 14, 193–198. [Google Scholar] [CrossRef]
- Aschbacher, K.; Hendershot, C.S.; Tison, G.; Hahn, J.A.; Avram, R.; Olgin, J.E.; Marcus, G.M. Machine learning prediction of blood alcohol concentration: A digital signature of smart-breathalyzer behavior. NPJ Digit. Med. 2021, 4, 1–10. [Google Scholar] [CrossRef]
- Gao, W.; Emaminejad, S.; Nyein, H.Y.Y.; Challa, S.; Chen, K.; Peck, A.; Fahad, H.M.; Ota, H.; Shiraki, H.; Kiriya, D.; et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 2016, 529, 509–514. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Brazaca, L.C.; García-Carmona, L.; Bolat, G.; Campbell, A.S.; Martin, A.; Tang, G.; Shah, R.; Mishra, R.K.; Kim, J.; et al. Eyeglasses-based tear biosensing system: Non-invasive detection of alcohol, vitamins and glucose. Biosens. Bioelectron. 2019, 137, 161–170. [Google Scholar] [CrossRef]
- Mohan, A.V.; Windmiller, J.R.; Mishra, R.K.; Wang, J. Continuous minimally-invasive alcohol monitoring using microneedle sensor arrays. Biosens. Bioelectron. 2017, 91, 574–579. [Google Scholar] [CrossRef]
- Wang, Y.; Fridberg, D.J.; Leeman, R.F.; Cook, R.L.; Porges, E.C. Wrist-worn alcohol biosensors: Strengths, limitations, and future directions. Alcohol 2019, 81, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Lansdorp, B.; Ramsay, W.; Hamid, R.; Strenk, E. Wearable Enzymatic Alcohol Biosensor. Sensors 2019, 19, 2380. [Google Scholar] [CrossRef] [PubMed]
- AWARE: A Wearable Awareness with Real-time Exposure, for monitoring alcohol consumption impact through ethyl glucuronide detection. Alcohol 2019, 81, 93–99. [CrossRef]
- Kinnamon, D.; Muthukumar, S.; Panneer Selvam, A.; Prasad, S. Portable Chronic Alcohol Consumption Monitor in Human Sweat through Square-Wave Voltammetry. SLAS Technol. 2018, 23, 144–153. [Google Scholar] [CrossRef]
- Thepchuay, Y.; Sonsa-ard, T.; Ratanawimarnwong, N.; Auparakkitanon, S.; Sitanurak, J.; Nacapricha, D. Paper-based colorimetric biosensor of blood alcohol with in-situ headspace separation of ethanol from whole blood. Anal. Chim. Acta 2020, 1103, 115–121. [Google Scholar] [CrossRef]
- Li, B.; Scott Downen, R.; Dong, Q.; Tran, N.; LeSaux, M.; Meltzer, A.C.; Li, Z. A Discreet Wearable IoT Sensor for Continuous Transdermal Alcohol Monitoring—Challenges and Opportunities. IEEE Sens. J. 2021, 21, 5322–5330. [Google Scholar] [CrossRef] [PubMed]
- Iamudomchai, P.; Seelaso, P.; Pattanasak, S.; Piyawattanametha, W. Deep learning technology for drunks detection with infrared camera. In Proceedings of the 2020 6th International Conference on Engineering, Applied Sciences and Technology, ICEAST 2020, Chiang Mai, Thailand, 1–4 July 2020; pp. 5–8. [Google Scholar] [CrossRef]
- Maheswari, K.; Madhumitha, U.; Madhusurya, S.; Divya, T. Alcohol Consumption Detection Using Smart Helmet System. Int. J. Sci. Res. Sci. Eng. Technol. 2020, 7, 167–173. [Google Scholar] [CrossRef]
- P, M.A.; Krishnaji, N.; P, S.S.; J, K. Smart Helmet Alcohol Detection and Sleep Alert. Int. J. Trend Sci. Res. Dev. 2020, 4, 517–520. [Google Scholar]
- Tapadar, S.; Ray, S.; Saha, H.N.; Saha, A.K.; Karlose, R. Accident and alcohol detection in bluetooth enabled smart helmets for motorbikes. In Proceedings of the 2018 IEEE 8th Annual Computing and Communication Workshop and Conference, CCWC 2018, Las Vegas, NV, USA, 8–10 January 2018; pp. 584–590. [Google Scholar] [CrossRef]
- Anil Kumar, M.; Venkata Suman, M.; Misra, Y.; Geetha Pratyusha, M. Intelligent vehicle black box using IoT. Int. J. Eng. Technol. 2018, 7, 215–218. [Google Scholar] [CrossRef]
- Nirosha, M.K.; Priyanka, C.; Kishore, K.A. Alcohol detection in vehicles. Int. Res. J. Eng. Technol. 2017. [Google Scholar]
- Win, D.K.Z. Design and Implementation of Alcohol Detector and Accident Detection System using GSM Modem. Int. J. Trend Sci. Res. Dev. 2019, 3, 1086–1090. [Google Scholar] [CrossRef]
- Das, R.; Chattopadhyay, O.; Biswas, P.; Das, M.; Chatterjee, S.; Paul, A. Intelligent Alcohol Detection System for Car Drivers. In Proceedings of Industry Interactive Innovations in Science, Engineering & Technology (I3SET2K19). 2020. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3526008 (accessed on 27 December 2022).
- Wakana, H.; Yamada, M.; Sakairi, M. Development of Breath-Alcohol-Detection System. In Proceedings of the SAE Technical Papers; SAE International: Warrendale, PA, USA, 2016. [Google Scholar] [CrossRef]
- Ljungblad, J.; Hök, B.; Allalou, A.; Pettersson, H. Passive in-vehicle driver breath alcohol detection using advanced sensor signal acquisition and fusion. Traffic Inj. Prev. 2017, 18, S31–S36. [Google Scholar] [CrossRef]
- Manu, M.A.; Jaijan, D.; Nissa, S.N.; Jesna, S.; Shukoor, A.; Shamna, A.R. A Novel Approach to Detect Driver Drowsiness and Alcohol Intoxication using Haar Algorithm with Raspberry Pi. Int. J. Res. Eng. Sci. Manag. 2020, 3, 48–51. [Google Scholar] [CrossRef]
- Abu Al-Haija, Q.; Krichen, M. A Lightweight In-Vehicle Alcohol Detection Using Smart Sensing and Supervised Learning. Computers 2022, 11, 121. [Google Scholar] [CrossRef]
- Celaya-Padilla, J.M.; Romero-González, J.S.; Galvan-Tejada, C.E.; Galvan-Tejada, J.I.; Luna-García, H.; Arceo-Olague, J.G.; Gamboa-Rosales, N.K.; Sifuentes-Gallardo, C.; Martinez-Torteya, A.; De la Rosa, J.I.; et al. In-Vehicle Alcohol Detection Using Low-Cost Sensors and Genetic Algorithms to Aid in the Drinking and Driving Detection. Sensors 2021, 21, 7752. [Google Scholar] [CrossRef]
- Mcvean, A. Before the Breathalyzer There Was the Drunkometer. 2019. Available online: https://www.mcgill.ca/oss/article/did-you-know-history/breathalyzer-there-was-drunkometer#:~:text=The%20first%20breathalyzer%20as%20we,subject’s%20breath%20and%20potassium%20dichromate. (accessed on 27 December 2022).
- McVean, A. From Bottle to Blood to Breath: How Breathalyzers Work. 2019. Available online: https://www.mcgill.ca/oss/article/did-you-know/did-you-know-breathalyzers-dont-directly-measure-your-blood-alcohol-concentration (accessed on 27 December 2022).
- Wigmore, J.; Langille, R. Six generations of breath alcohol testing instruments: Changes in the detection of breath alcohol since 1930. An historical overview. Can. Soc. Forensic Sci. J. 2009, 42, 276–283. [Google Scholar] [CrossRef]
- Workman, T.E., Jr. The science behind breath testing for ethanol. Univ. Mass. Law Rev. 2012, 7, 110. [Google Scholar]
- Yang, G.; Zhang, Q.; Yu, H.; Peng, F. Platinum-based ternary catalysts for the electrooxidation of ethanol. Particuology 2021, 58, 169–186. [Google Scholar] [CrossRef]
- Bay, H.; Blurton, K.; Lieb, H.; Oswin, H. Electrochemical measurements of blood alcohol levels. Nature 1972, 240, 52–53. [Google Scholar] [CrossRef] [PubMed]
- Rizo, R.; Pérez-Rodríguez, S.; Garcia, G. Well-defined platinum surfaces for the ethanol oxidation reaction. ChemElectroChem 2019, 6, 4725–4738. [Google Scholar] [CrossRef]
- Rizo, R.; Ferre-Vilaplana, A.; Herrero, E.; Feliu, J.M. Ethanol Electro-oxidation Reaction Selectivity on Platinum in Aqueous Media. ACS Sustain. Chem. Eng. 2022. [Google Scholar] [CrossRef]
- Hitmi, H.; Belgsir, E.; Léger, J.M.; Lamy, C.; Lezna, R. A kinetic analysis of the electro-oxidation of ethanol at a platinum electrode in acid medium. Electrochim. Acta 1994, 39, 407–415. [Google Scholar] [CrossRef]
- U.S. Department of Energy Office of Fossil Energy. Morgantown, West Virginia. In Fuel Cell Handbook, 7th ed.; U.S. Department of Energy Office of Fossil Energy: Morgantown, WV, USA, 2004. [Google Scholar]
- Prest, L. Fundamental Investigation of Fuel Cell-Based Breath Alcohol Sensors and the Cause of Sensor Degradation in Low-Humidity Conditions. Ph.D. Thesis, University of Ontario Institute of Technology, Oshawa, ON, USA, 2011. [Google Scholar]
- Liberg, O.; Sundberg, M.; Eric Wang, Y.P.; Bergman, J.; Sachs, J.; Wikström, G. Cellular Internet of Things: From Massive Deployments to Critical 5G Applications; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Malaysia Wireless Frequency Bands and Carriers. Available online: https://www.frequencycheck.com/countries/malaysia (accessed on 27 September 2022).
- Dart Sensors Ltd. Available online: https://www.dart-sensors.com/ (accessed on 23 September 2022).
- Reference, A. Analogread(), 2019. Available online: https://www.arduino.cc/reference/en/language/functions/analog-io/analogread (accessed on 2 December 2022).
- Understanding the Dangers of Alcohol Overdose. 2022. Available online: https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/understanding-dangers-of-alcohol-overdose (accessed on 27 December 2022).
- Hingson, R.W.; Zha, W.; White, A.M. Drinking beyond the binge threshold: Predictors, consequences, and changes in the US. Am. J. Prev. Med. 2017, 52, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Van Voorhis, C.W.; Morgan, B.L. Understanding power and rules of thumb for determining sample sizes. Tutor. Quant. Methods Psychol. 2007, 3, 43–50. [Google Scholar] [CrossRef]
- Bruce, P.; Bruce, A.; Gedeck, P. Practical Statistics for Data Scientists: 50+ Essential Concepts Using R and Python; O’Reilly Media: Sebastopol, CA, USA, 2020. [Google Scholar]
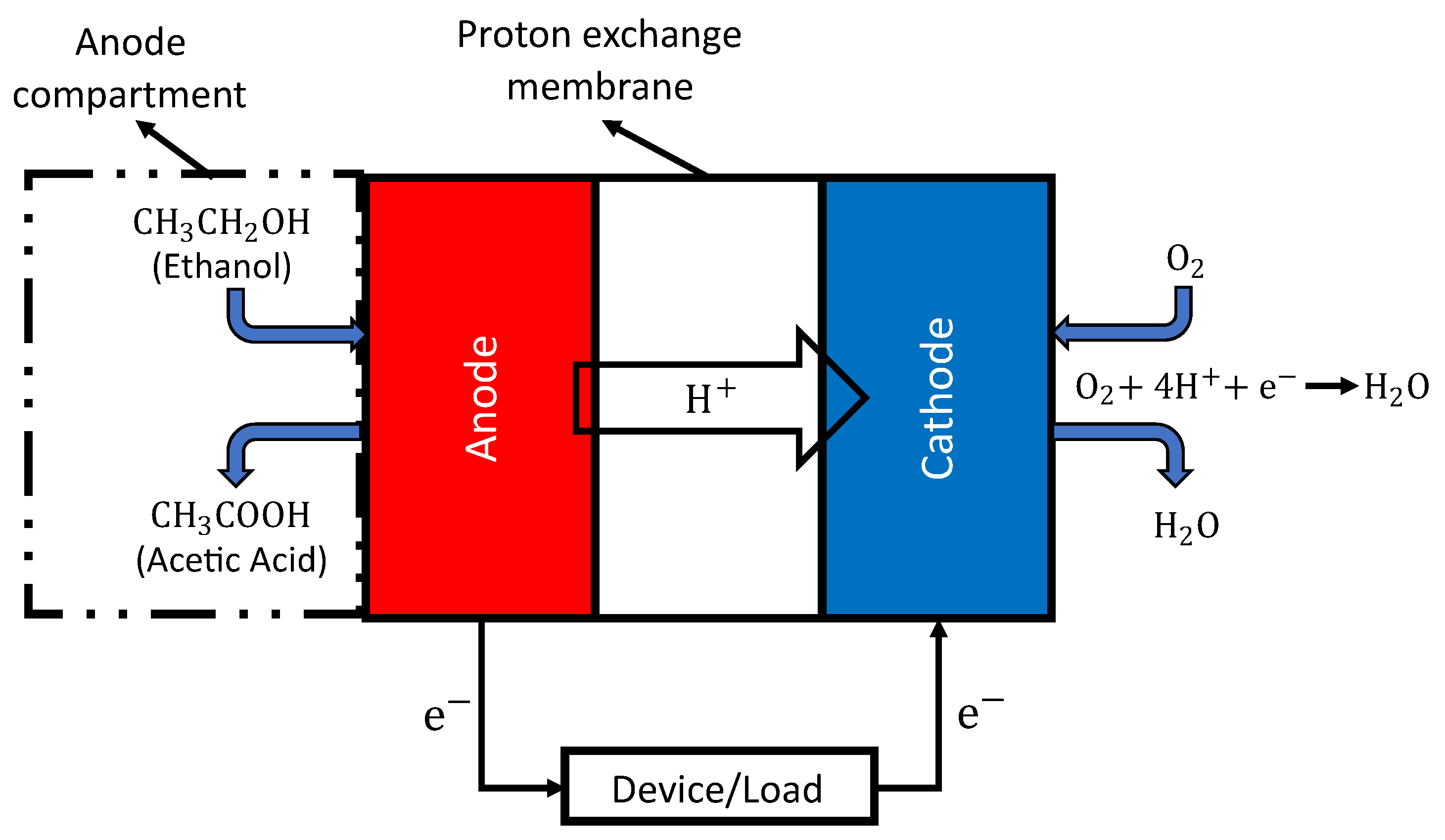
- Bharti, A.; Natarajan, R. Proton exchange membrane testing and diagnostics. In PEM Fuel Cells; Elsevier: Amsterdam, The Netherlands, 2022; pp. 137–171. [Google Scholar]
- Luo, Y.; Shi, Y.; Cai, N. Hybrid Systems and Multi-Energy Networks for the Future Energy Internet; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Wilkinson, D.P.; Zhang, J.; Hui, R.; Fergus, J.; Li, X. Proton Exchange Membrane Fuel Cells: Materials Properties and Performance; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Rightmire, R.; Rowland, R.; Boos, D.; Beals, D. Ethyl alcohol oxidation at platinum electrodes. J. Electrochem. Soc. 1964, 111, 242. [Google Scholar] [CrossRef]
- Okonkwo, P.C.; Ige, O.O.; Barhoumi, E.M.; Uzoma, P.C.; Emori, W.; Benamor, A.; Abdullah, A.M. Platinum degradation mechanisms in proton exchange membrane fuel cell (PEMFC) system: A review. Int. J. Hydrogen Energy 2021, 46, 15850–15865. [Google Scholar] [CrossRef]
| Technology | Criteria | ||||
|---|---|---|---|---|---|
| Portability | Online Connectivity | Wide-Scale Usability | Continuous Monitoring | Independence | |
| Wearable devices | x | x | x | x | |
| Regular breath analyzers | x | x | |||
| Personalized IoT-integrated breath analyzers | x | x | |||
| Vehicle-integrated device | x | x | |||
| Indirect detection | x | ||||
| Proposed solution (cellular IoT breath analyzer) | x | x | x | x | |
| Frequency | Interface | Operators |
|---|---|---|
| 900 (E-GSM) | GSM | Celcom, Digi, Maxis, U-mobile |
| 1800 (DCS) | GSM | Celcom, Digi, Maxis, U-mobile |
| B1 (2100) | UMTS | Celcom, Digi, Maxis, U-mobile |
| B8 (900 GSM) | UMTS | Maxis |
| B3 (1800+) | LTE | Celcom, Maxis |
| B7 (2600) | LTE | Celcom, Digi, Maxis, U-mobile |
| Actual Concentration (mcg/100 mL) | Average Predicted Concentration (mcg/100 mL) | Performance Metrics | ||||||
|---|---|---|---|---|---|---|---|---|
| Average Accuracy (%) | MSE (mcg/100 mL) | MAE (mcg/100 mL) | RMSE (mcg/100 mL) | R2 | Standard Deviation (mcg/100 mL) | RSD (%) | ||
| 0 | 0 | 100 | 0 | 0 | 0 | - | 0 | 0 |
| 4 | 3.98 | 99.5 | 0 | 0.02 | 0 | - | 0 | 0 |
| 10 | 9.28 | 92.8 | 0.55 | 0.72 | 0.74 | - | 0.25 | 2.69 |
| 20 | 19.69 | 98.45 | 0.1 | 0.31 | 0.32 | - | 0 | 0 |
| 30 | 29.01 | 96.68 | 1.83 | 1 | 1.35 | - | 1.29 | 4.45 |
| 40 | 39.9 | 96.8 | 2.77 | 1.28 | 1.66 | - | 1.81 | 4.41 |
| 50 | 49.96 | 96.34 | 3.87 | 1.83 | 1.97 | - | 0.57 | 1.16 |
| 100 | 99.97 | 98.6 | 2.52 | 1.41 | 1.59 | - | 1.04 | 1.05 |
| 150 | 150.15 | 98.61 | 5.58 | 2.09 | 2.36 | - | 1 | 0.68 |
| 200 | 199.51 | 99.37 | 1.63 | 1.28 | 1.28 | - | 1.8 | 0.9 |
| Overall | 97.71 | 1.88 | 0.99 | 1.37 | 0.9995 | - | - | |
| Actual Concentration (mcg/100 mL) | Average Predicted Concentration (mcg/100 mL) | Performance Metrics | ||||||
|---|---|---|---|---|---|---|---|---|
| Average Accuracy (%) | MSE (mcg/100 mL) | MAE (mcg/100 mL) | RMSE (mcg/100 mL) | R2 | Standard Deviation (mcg/100 mL) | RSD (%) | ||
| 25 | 24.8 | 98.64 | 0.15 | 0.34 | 0.39 | - | 0.35 | 1.41 |
| 75 | 73.68 | 97.78 | 4.67 | 1.67 | 2.16 | - | 1.81 | 2.46 |
| 125 | 125.55 | 98.58 | 4.93 | 1.77 | 2.22 | - | 2.27 | 1.81 |
| 180 | 179.69 | 97.65 | 21.16 | 4.24 | 4.6 | - | 4.84 | 2.69 |
| Overall | 97.71 | 7.73 | 1.32 | 2.78 | 0.9977 | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khamis, A.A.; Idris, A.; Abdellatif, A.; Mohd Rom, N.A.; Khamis, T.; Ab Karim, M.S.; Janasekaran, S.; Abd Rashid, R.B. Development and Performance Evaluation of an IoT-Integrated Breath Analyzer. Int. J. Environ. Res. Public Health 2023, 20, 1319. https://doi.org/10.3390/ijerph20021319
Khamis AA, Idris A, Abdellatif A, Mohd Rom NA, Khamis T, Ab Karim MS, Janasekaran S, Abd Rashid RB. Development and Performance Evaluation of an IoT-Integrated Breath Analyzer. International Journal of Environmental Research and Public Health. 2023; 20(2):1319. https://doi.org/10.3390/ijerph20021319
Chicago/Turabian StyleKhamis, Abd Alghani, Aida Idris, Abdallah Abdellatif, Noor Ashikin Mohd Rom, Taha Khamis, Mohd Sayuti Ab Karim, Shamini Janasekaran, and Rusdi Bin Abd Rashid. 2023. "Development and Performance Evaluation of an IoT-Integrated Breath Analyzer" International Journal of Environmental Research and Public Health 20, no. 2: 1319. https://doi.org/10.3390/ijerph20021319
APA StyleKhamis, A. A., Idris, A., Abdellatif, A., Mohd Rom, N. A., Khamis, T., Ab Karim, M. S., Janasekaran, S., & Abd Rashid, R. B. (2023). Development and Performance Evaluation of an IoT-Integrated Breath Analyzer. International Journal of Environmental Research and Public Health, 20(2), 1319. https://doi.org/10.3390/ijerph20021319







