The Search for Suitable Habitats for Endangered Species at Their Historical Sites—Conditions for the Success of Salix lapponum and Salix myrtilloides Reintroduction
Abstract
1. Introduction
2. Materials and Methods
2.1. Steps Taken before the Experiment
2.2. Reintroduction Experiment
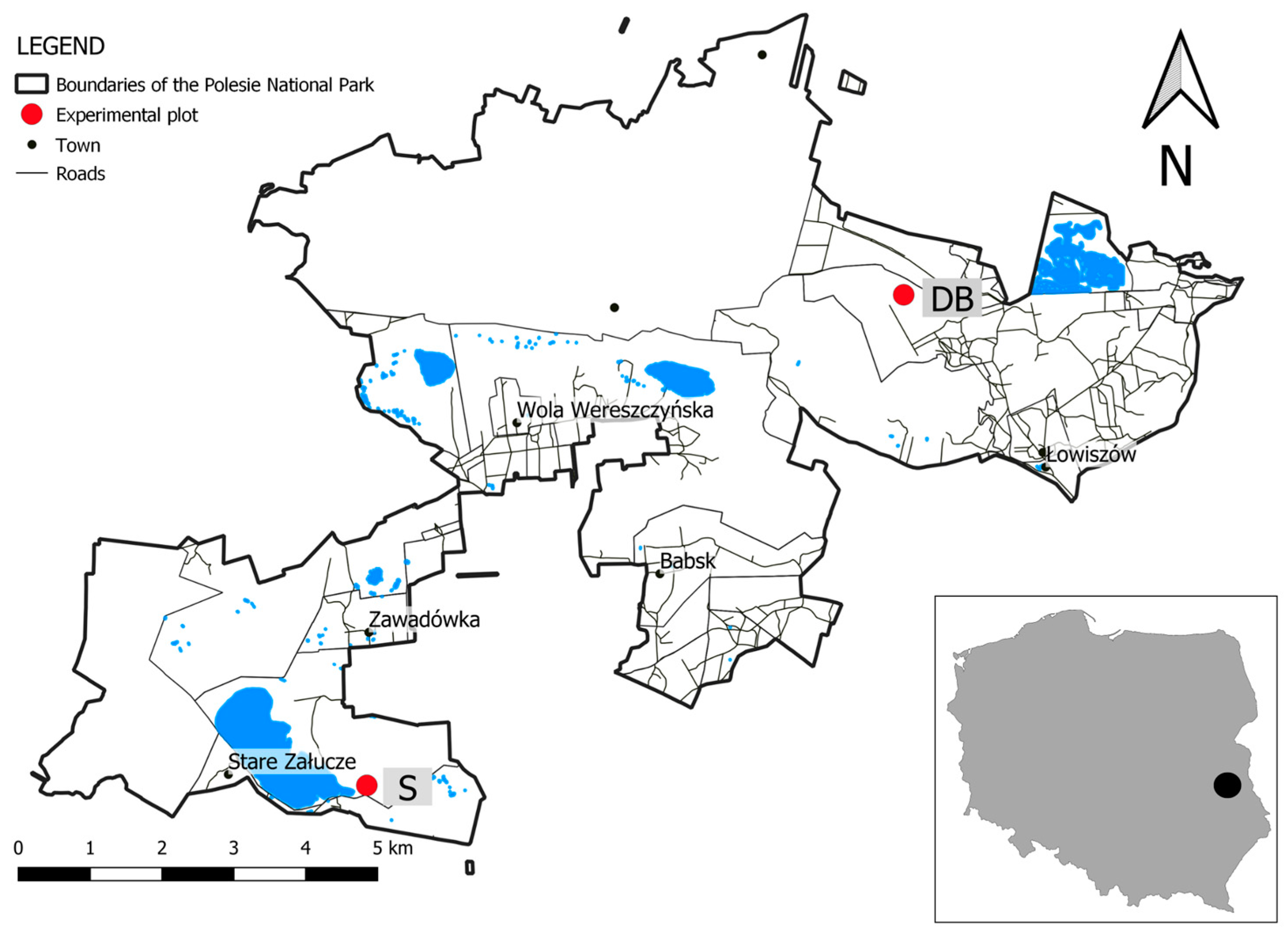
2.3. Assessment of Habitat Conditions at the Reintroduction Sites
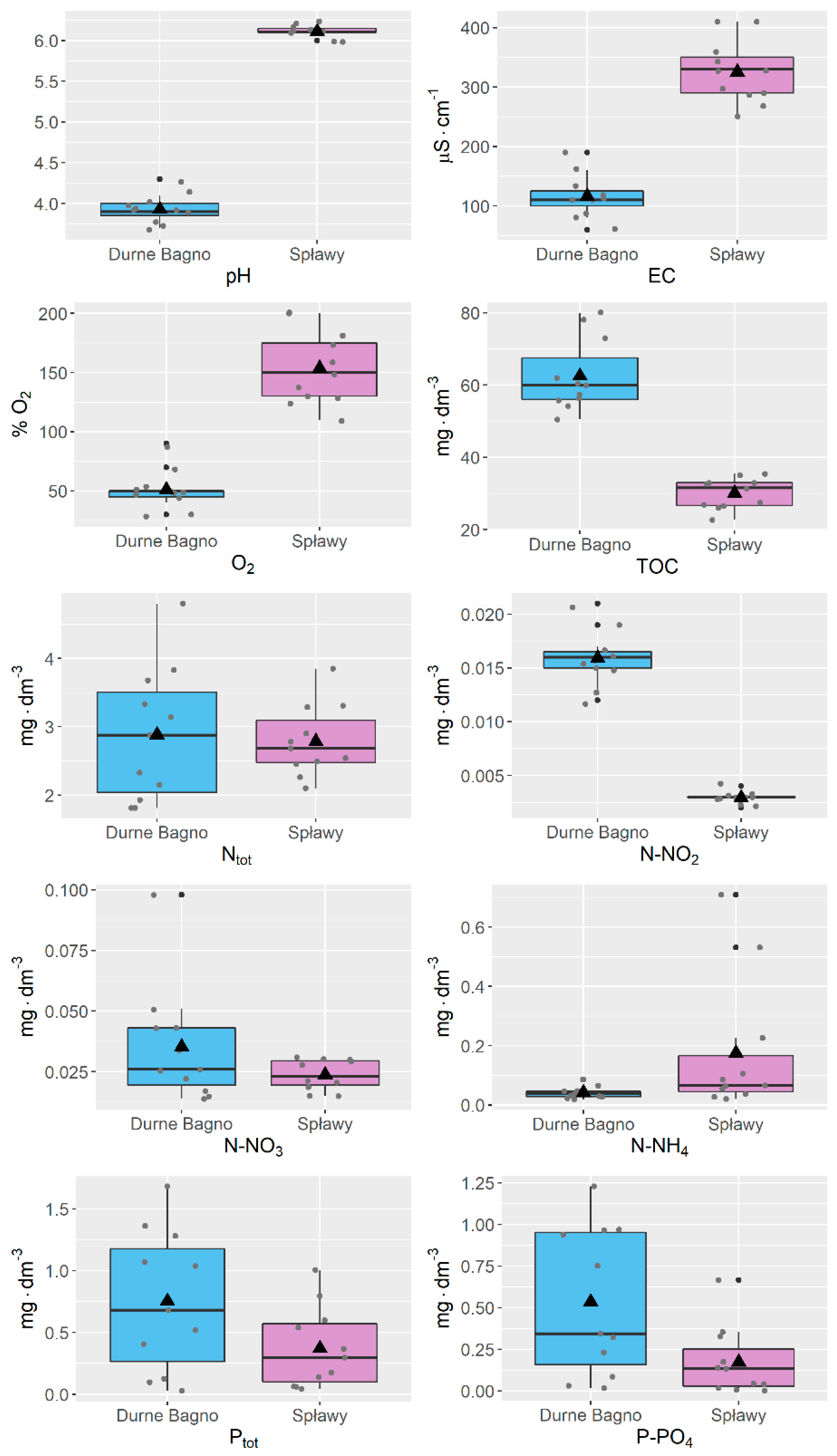
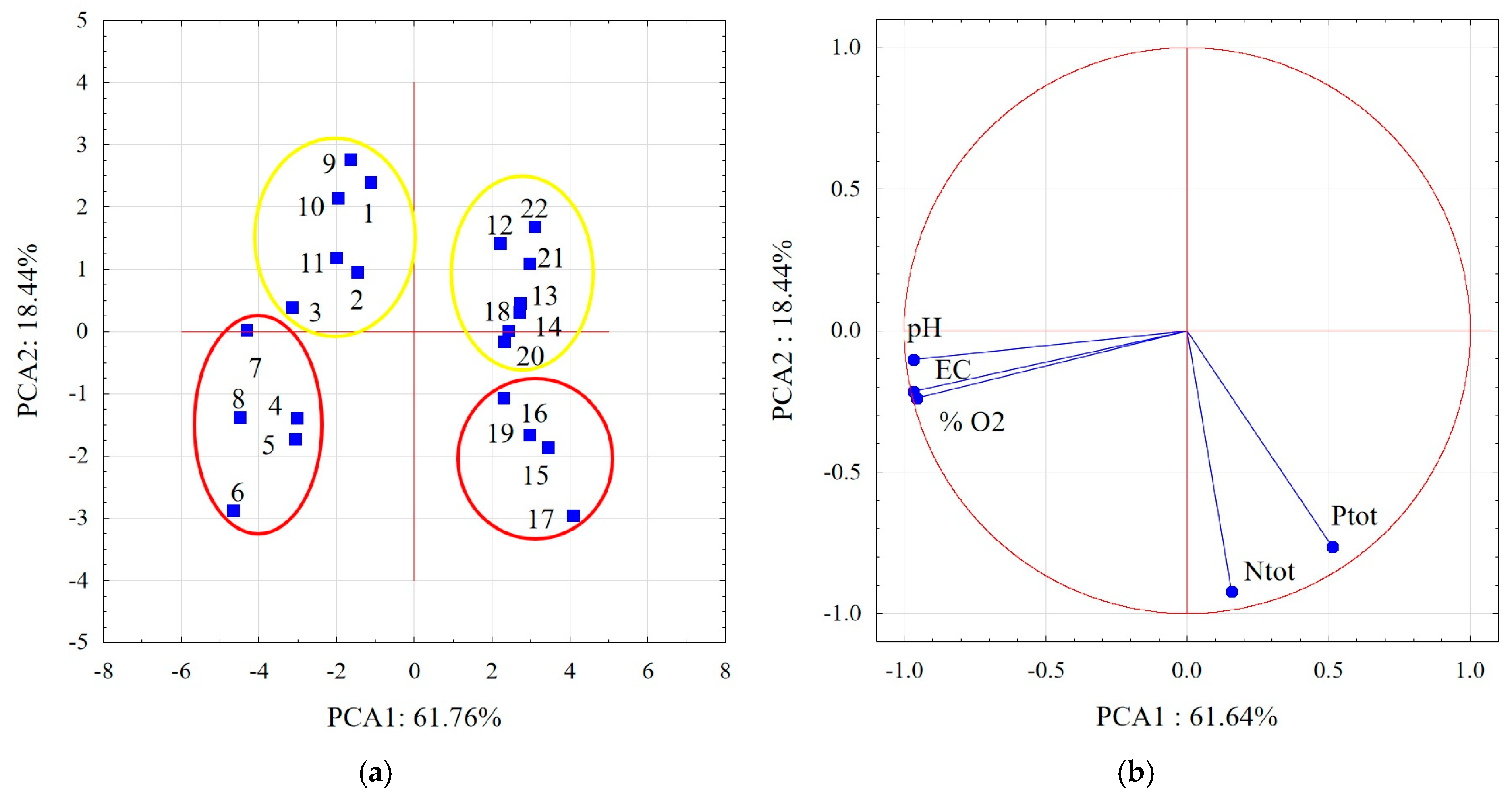
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reiter, N.; Whitfield, J.; Pollard, G.; Bedggood, W.; Argall, M.; Dixon, K.; Davis, B.; Swart, N. Orchid re-introductions: An evaluation of success and ecological considerations using key comparative studies from Australia. Plant Ecol. 2016, 217, 81–95. [Google Scholar] [CrossRef]
- Batson, W.; Gordon, I.; Fletcher, D.; Manning, A. Translocation tactics: A framework to support the IUCN Guidelines for wildlife translocations and improve the quality of applied methods. J. Appl. Ecol. 2015, 52, 1598–1607. [Google Scholar] [CrossRef]
- Abeli, T.; Dixon, K. Translocation ecology: The role of ecological sciences in plant translocation. Plant Ecol. 2016, 2017, 123–125. [Google Scholar] [CrossRef]
- Godefroid, S.; Piazza, C.; Rossi, G.; Buord, S.; Stevens, A.; Aguraiuja, R.; Cowell, C.; Weekley, C.; Vogg, G.; Iriondo, J.; et al. How successful are plant species reintroductions? Biol. Conserv. 2011, 144, 672–682. [Google Scholar] [CrossRef]
- Menendez, R.; Megias, A.; Hill, J.; Braschler, B.; Willis, S.; Collingham, Y.; Fox, R.; Roy, D.; Thomas, C. Species richness changes lag behind climate change. Proc. R. Soc. B 2006, 273, 1465–1470. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.D.; Cameron, A.; Green, R.E.; Bakkenes, M.; Beaumont, L.J.; Collingham, Y.C.; Erasmus, B.F.N.; Ferreira de Siqueira, M.; Grainger, A.; Hannah, L.; et al. Extinction risk from climate change. Nature 2004, 427, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, E.; Brown, A.; Williams, M.; Romero-Severson, J.; Beardmore, T.; Hoban, S. Range shifts in butternut, a rare, endangered tree, in response to past climate and modern conditions. J. Biogeogr. 2022, 49, 866–878. [Google Scholar] [CrossRef]
- Grzyl, A.; Kiedrzyński, M.; Zielińska, K.M.; Rewicz, A. The relationship between climatic conditions and generative reproduction of a lowland population of Pulsatilla vernalis: The last breath of a relict plant or a fluctuating cycle of regeneration? Plant Ecol. 2014, 215, 457–466. [Google Scholar] [CrossRef]
- Thomas, C.D. Translocation of species, climate change, and the end of trying to recreate past ecological communities. Trends Ecol. Evol. 2011, 26, 216–221. [Google Scholar] [CrossRef]
- Piękoś-Mirkowa, H.; Mirek, Z. Flora Polski. Rośliny Chronione; Multico: Warszawa, Poland, 2006; pp. 1–417. [Google Scholar]
- Kozel, P.; Leong, J.V.; Malenovský, I.; Šumpich, J.; Macek, J.; Michalek, J.; Novakova, N.; Sedio, B.E.; Seifert, C.L.; Volf, M. Specialised chemistry affects insect abundance but not overall community similarity in three rare shrub willows: Salix myrtilloides, S. repens and S. rosmarinifolia. Eur. J. Entomol. 2022, 119, 368–378. [Google Scholar] [CrossRef]
- Kruszelnicki, J.; Gostyńska-Jakuszewska, M. Salix myrtilloides L. Wierzba borówkolistna. In Polska Czerwona Księga Roślin. Paprotniki i Rośliny Kwiatowe, 3rd ed.; Kaźmierczakowa, R., Zarzycki, K., Mirek, Z., Eds.; Instytut Ochrony Przyrody PAN: Kraków, Poland, 2014; pp. 81–83. [Google Scholar]
- Kruszelnicki, J. Salix lapponum L. Wierzba lapońska. In Polska Czerwona Księga Roślin. Paprotniki i Rośliny Kwiatowe, 3rd ed.; Kaźmierczakowa, R., Zarzycki, K., Mirek, Z., Eds.; Instytut Ochrony Przyrody PAN: Kraków, Poland, 2014; pp. 86–88. [Google Scholar]
- Pogorzelec, M.; Głębocka, K.; Hawrylak-Nowak, B.; Bronowicka-Mielniczuk, U. Assessment of chosen reproductive cycle processes and genetic diversity of Salix myrtilloides L. in wetlands of Polesie Lubelskie: The prospects of its survival in the region. Pol. J. Ecol. 2015, 63, 291–303. [Google Scholar]
- Kłosowski, S.; Kłosowski, G. Flora Polski Rośliny Wodne i Bagienne; Multico: Warszawa, Poland, 2006; pp. 1–333. [Google Scholar]
- Pogorzelec, M. Influence of Chosen Environmental Abiotic Factors on Salix lapponum L. Populations in Polesie Lubelskie Region. Pol. J. Environ. Stud. 2008, 17, 581–856. [Google Scholar]
- Stamati, K.; Hollingsworth, P.M.; Russell, J.J.P.S. Patterns of clonal diversity in three species of sub-arctic willow (Salix lanata, Salix lapponum and Salix herbacea). Plant Syst. Evol. 2007, 269, 75–88. [Google Scholar] [CrossRef]
- Finger, A.; Rao, S.; Cowie, N.; MacDonell, T.; Beck, A.; Denny, B. Conservation genetics of montane willow populations in Scotland-limited natural recovery despite long-distance gene flow and high genetic diversity. Environ. Res. Ecol. 2022, 2, 015001. [Google Scholar] [CrossRef]
- Hroneš, M.; Hrachová, S.; Dančák, M.; Vašut, R.J. Vrba laponská (Salix lapponum L.) v Krkonoších. Opera Corcon. 2011, 48, 69–78. [Google Scholar]
- Hroneš, M.; Hrachová Macurová, S.; Hradílek, Z.; Hekera, P.; Duchoslav, M. Habitat conditions, stage structure and vegetation associations of geographically isolated subalpine populations of Salix lapponum L. (Salicaceae) in the Krkonoše Mts (Czech Republic). Biologia 2018, 73, 319–332. [Google Scholar] [CrossRef]
- Hroneš, M.; Hrachová Macurová, S.; Hradílek, Z.; Hekera, P.; Duchoslav, M. Female-biased sex ratio despite the absence of spatial and niche segregation between sexes in alpine populations of dioecious Salix lapponum (Salicaceae). Alp. Bot. 2019, 129, 1–9. [Google Scholar] [CrossRef]
- Fijałkowski, D. Studiem upon distribution and ecology of downy willow (Salix lapponum) in Łęczyńsko-Włodawskie Lakeland. Fragm. Florist. Geobot. 1958, 3, 89–103. [Google Scholar]
- Fijałkowski, D. Study on the distribution and ecology of swamp willow (Salix myrtilloides) in the Łęczna-Włodawa Lakeland. Acta Soc. B 1958, 27, 605–611. [Google Scholar] [CrossRef]
- Urban, D.; Wawer, M. Salix lapponum L. i S. myrtilloides L. w okolicach Sobiboru na Pojezierzu Łęczyńsko-Wlodawskim. Ann. Univ. Mariae Curie-Skłodowska 2001, 56, 83–93. [Google Scholar]
- Churski, M.; Danielewicz, W. Salix myrtilloides in north central Poland. Distribution, threats and conservation. Dendrobiology 2008, 60, 3–9. [Google Scholar]
- Pogorzelec, M. Salix lapponum L. (Downy willow) in stands under anthropopressure in the Łęczna-Włodawa Lakeland. Acta Agrobot. 2010, 63, 47–53. [Google Scholar] [CrossRef]
- Pogorzelec, M.; Bronowicka-Mielniczuk, U.; Banach, B.; Szczurowska, A.; Serafin, A. Relict boreal willows (Salix lapponum and Salix myrtilloides) as an element of phytocoenoses overgrowing the water bodies in Eastern Poland. Appl. Ecol. Environ. Res. 2014, 12, 441–456. [Google Scholar] [CrossRef]
- Pogorzelec, M.; Serafin, A.; Banach-Albińska, B.; Szczurowska, A.; Parzymies, M.; Bronowicka-Mielniczuk, U. Pollen viability of an endangered species in Poland—Salix myrtilloides L. Acta Agrobot. 2016, 69, 1679. [Google Scholar] [CrossRef]
- Serafin, A.; Pogorzelec, M.; Banach, B.; Szczurowska, A.; Mielniczuk, J. Physico-chemical groundwater conditions at Salix lapponum stands in Eastern Poland. Dendrobiology 2015, 73, 65–74. [Google Scholar] [CrossRef]
- Serafin, A.; Pogorzelec, M.; Banach, B.; Mielniczuk, J. Habitat conditions of the endangered species Salix myrtilloides in Eastern Poland. Dendrobiology 2015, 73, 55–64. [Google Scholar] [CrossRef]
- Pogorzelec, M.; Parzymies, M.; Banach-Albińska, B.; Serafin, A.; Szczurowska, A. Experimental reintroduction of the boreal species Salix lapponum L. to refuges at the southern limit of its range—Short-term results. Boreal Environ. Res. 2020, 25, 161–169. [Google Scholar]
- Pogorzelec, M.; Hawrylak-Nowak, B.; Banach-Albińska, B.; Szczurowska, A.; Parzymies, M.; Spólna, K. From ex situ cultivation to stands in natural habitats: Critical periods for plants during the reintroduction of Salix lapponum L. in Eastern Poland. J. Nat. Conserv. 2022, 67, 126172. [Google Scholar] [CrossRef]
- Parzymies, M.; Pogorzelec, M.; Głębocka, K.; Śliwińska, E. Genetic Stability of the Endangered Species Salix lapponum L. Regenerated In Vitro during the Reintroduction Process. Biology 2020, 9, 378. [Google Scholar] [CrossRef]
- Fijałkowski, D. Flora of lakes situated between Łęczna and Włodawa and of the peat-bogs adjacent to those lakes. Ann. Univ. Mariae Curie-Sklodowska 1959, 14, 131–206. [Google Scholar]
- Abrie, A.Á.; Van Staden, J. Micropropagation of the endangered Aloe polyphylla. Plant Growth Regul. 2001, 33, 19–23. [Google Scholar] [CrossRef]
- Chokheli, V.A.; Dmitriev, P.A.; Rajput, V.D.; Bakulin, S.D.; Azarov, A.S.; Varduni, T.V.; Stepanenko, V.V.; Tarigholizadeh, S.; Singh, R.K.; Verma, K.K.; et al. Recent Development in Micropropagation Techniques for Rare Plant Species. Plants 2020, 9, 1733. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Aceves, L.; Portillo, L.; Folgado, R.; de Jesús Romo-Paz, F.; González-Arnao, M.T. New approaches for micropropagation and cryopreservation of Agave peacockii, an endangered species. Plant Cell Tissue Organ Cult. (PCTOC) 2022, 150, 85–95. [Google Scholar] [CrossRef]
- Głębocka, K.; Pogorzelec, M. Genetic diversity of the Salix lapponum L. population intended as a source of material for reintroduction. Dendrobiology 2017, 78, 136–145. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 5 September 2022).
- Braun-Blanquet, J. Plant Sociology. The Study of Plant Communities, 1st ed.; McGraw-Hill: New York, NY, USA, 1932; p. 439. [Google Scholar]
- Matuszkiewicz, W.; Sikorski, P.; Szwed, W.; Wierzba, M. Zbiorowiska Roślinne Polski, Ilustrowany Przewodnik-Lasy i Zarośla; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2012. [Google Scholar]
- Zarzycki, K.; Trzcińska-Tacik, H.; Różański, W.; Szeląg, Z.; Wołek, J.; Korzeniak, U. Ekologiczne Liczby Wskaźnikowe Roślin Naczyniowych Polski; Instytut Botaniki im. Władysława Szafera PAN: Kraków, Poland, 2002. [Google Scholar]
- Maschinski, J.; Haskins, K.E. Plant Reintroduction in a Changing Climate: Promises and Perils; Island Press: Washington, DC, USA, 2012; p. 432. [Google Scholar]
- Ren, H.; Zhang, Q.; Lu, H.; Liu, H.; Guo, Q.; Wang, J.; Jian, S.; Bao, H.O. Wild plant species with extremely small populations require conservation and reintroduction in China. Ambio 2012, 41, 913–917. [Google Scholar] [CrossRef] [PubMed]
- Silcock, J.L.; Simmons, C.L.; Monks, L.; Dillon, R.; Reiter, N.; Jusaitis, M.; Vesk, P.A.; Byrne, M.; Coates, D.J. Threatened plant translocation in Australia: A review. Biol. Conserv. 2019, 236, 211–222. [Google Scholar] [CrossRef]
- Seddon, P.J.; Armstrong, D.P.; Maloney, R.F. Developing the science of reintroduction biology. Conserv. Biol. 2007, 21, 303–312. [Google Scholar] [CrossRef]
- Pence, V.C. Evaluating costs for the in vitro propagation and preservation of endangered plants. In Vitro Cell. Dev. Biol.-Plant 2011, 47, 176–187. [Google Scholar] [CrossRef]
- Perullo, N.; Determann, R.O.; Cruse-Sanders, J.M.; Pullman, G.S. Seed cryopreservation and micropropagation of the critically endangered species swamp pink (Helonias bullata L.). In Vitro Cell. Dev. Biol.-Plant 2015, 51, 284–293. [Google Scholar] [CrossRef]
- Pogorzelec, M.; Serafin, A.; Banach-Albińska, B.; Parzymies, M.; Szczurowska, A. Czynna ochrona zagrożonych gatunków flory torfowiskowej Polesia Lubelskiego na przykładzie Salix lapponum (wierzby lapońskiej) i Salix mytilloides (wierzby borówkolistnej). In Polesie-Środowisko, Melioracje; Mażajski, J.A., Rokoczyński, A.N., Wołczek, A.A., Mieszyk, O.P., Jeznach, J., Eds.; SGGW: Warszawa, Poland, 2020; pp. 314–325. [Google Scholar]

| Vegetation Stratum | Species | Experimental Plot S | Experimental Plot DB |
|---|---|---|---|
| B | Alnus glutinosa L. | 3 | |
| Betula humilis Schrank. | 2 | ||
| Betula pubescens Ehrh. | 3 | 1 | |
| Pinus sylvestris L. | 1 | ||
| Salix cinerea L. | 2 | ||
| Salix pentandra L. | 2 | ||
| C | Carex limosa L. | 2 | 2 |
| Cirsium palustre L. | 2 | ||
| Comarum palustre L. | 3 | ||
| Drosera rotundifolia L. | 1 | ||
| Eriophorum vaginatum L. | + | 4 | |
| Geranium palustre L. | + | ||
| Juncus conglomeratus L. | 2 | ||
| Lysimachia thyrsiflora L. | 2 | ||
| Lythrum salicaria L. | 2 | ||
| Menyanthes trifoliata L. | 2 | ||
| Oxycoccus palustris L. | 3 | 4 | |
| Peucedanum palustre L. | + | ||
| Ranunculus acris L. | + | ||
| Rhododendron tomentosum L. | 2 | ||
| Silene flos-cuculi L. | + | ||
| Thelypteris palustris Schott | 3 | 1 | |
| Typha angustifolia L. | 2 | 2 | |
| D | Sphagnum spp. | 4 | 5 |
| Experimental Plot | Indicator Value | L (%) | W (%) | Tr (%) | R (%) | H(%) |
|---|---|---|---|---|---|---|
| Spławy (S) | 1 | - | - | 4.42 | 4.17 | 1.50 |
| 2 | 1.81 | - | 12.71 | 13.54 | 10.53 | |
| 3 | 10.9 | 1.07 | 36.5 | 18.95 | 87.97 * | |
| 4 | 87.2 * | 22.78 | 46.41 * | 52.63 * | - | |
| 5 | - | 67.62 * | - | 10.53 | - | |
| 6 | - | 8.54 | - | - | - | |
| Durne Bagno (DB) | 1 | - | - | 15.52 | 13.1 | 1.85 |
| 2 | 2.86 | 1.90 | 51.72 * | 26.19 | 3.70 | |
| 3 | 12.86 | 2.86 | 25.86 | 35.71 * | 94.44 * | |
| 4 | 77.14 * | 19.05 | 6.90 | 19.05 | - | |
| 5 | 7.14 | 70.07 * | - | 5.95 | - | |
| 6 | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arciszewski, M.; Pogorzelec, M.; Bronowicka-Mielniczuk, U.; Niedźwiecki, M.; Parzymies, M.; Serafin, A. The Search for Suitable Habitats for Endangered Species at Their Historical Sites—Conditions for the Success of Salix lapponum and Salix myrtilloides Reintroduction. Int. J. Environ. Res. Public Health 2023, 20, 1133. https://doi.org/10.3390/ijerph20021133
Arciszewski M, Pogorzelec M, Bronowicka-Mielniczuk U, Niedźwiecki M, Parzymies M, Serafin A. The Search for Suitable Habitats for Endangered Species at Their Historical Sites—Conditions for the Success of Salix lapponum and Salix myrtilloides Reintroduction. International Journal of Environmental Research and Public Health. 2023; 20(2):1133. https://doi.org/10.3390/ijerph20021133
Chicago/Turabian StyleArciszewski, Michał, Magdalena Pogorzelec, Urszula Bronowicka-Mielniczuk, Michał Niedźwiecki, Marzena Parzymies, and Artur Serafin. 2023. "The Search for Suitable Habitats for Endangered Species at Their Historical Sites—Conditions for the Success of Salix lapponum and Salix myrtilloides Reintroduction" International Journal of Environmental Research and Public Health 20, no. 2: 1133. https://doi.org/10.3390/ijerph20021133
APA StyleArciszewski, M., Pogorzelec, M., Bronowicka-Mielniczuk, U., Niedźwiecki, M., Parzymies, M., & Serafin, A. (2023). The Search for Suitable Habitats for Endangered Species at Their Historical Sites—Conditions for the Success of Salix lapponum and Salix myrtilloides Reintroduction. International Journal of Environmental Research and Public Health, 20(2), 1133. https://doi.org/10.3390/ijerph20021133








