Depressed Mood as a Significant Risk Factor for Gynecological Cancer Aggravation
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients Selection and Data Collection
2.2. Statistical Analysis
2.3. Ethics
3. Results
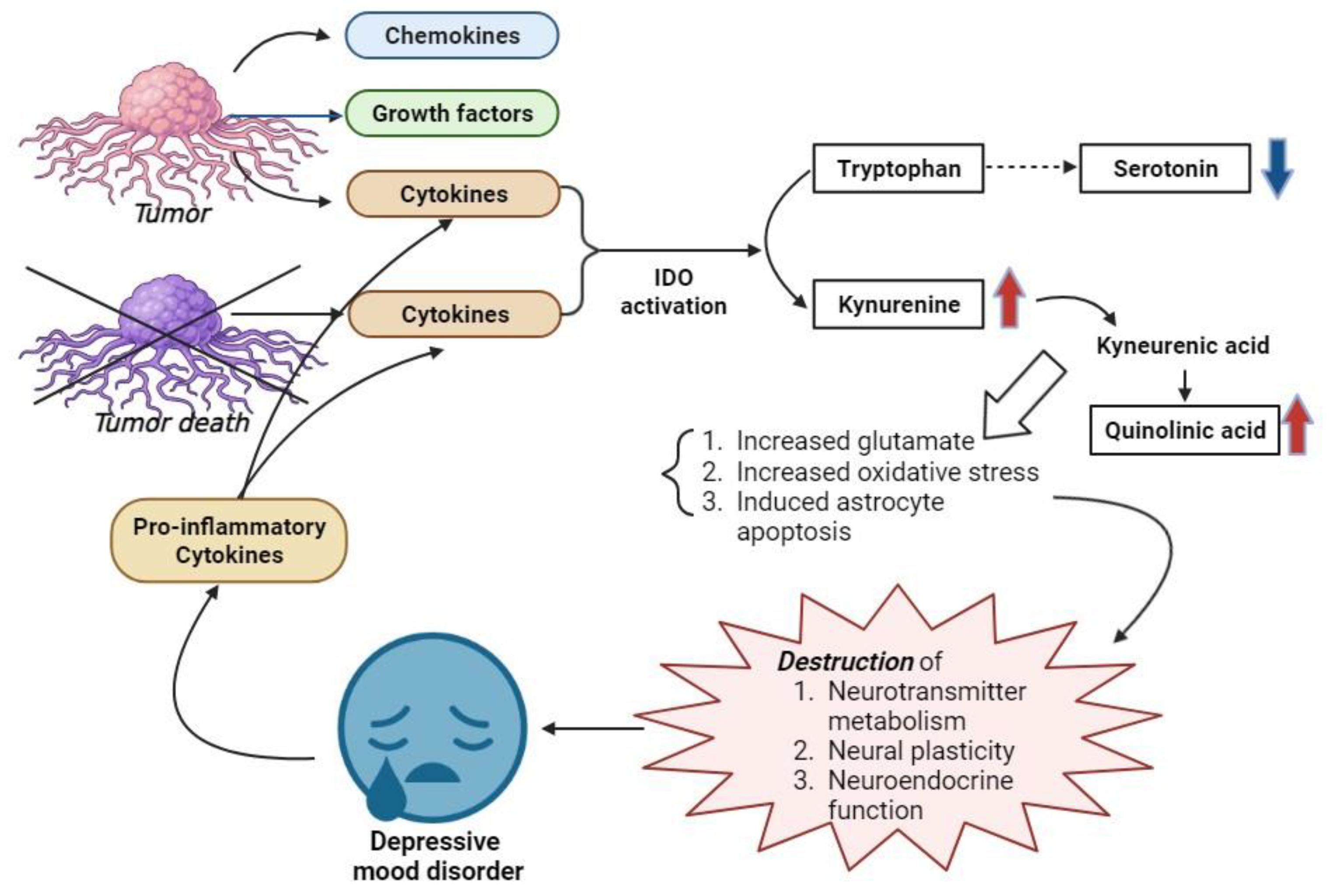
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Cancer and Your Emotions—Macmillan Cancer Support. Available online: https://www.macmillan.org.uk/cancer-information-and-support/treatment/coping-with-treatment/cancer-and-your-emotions (accessed on 3 March 2023).
- Husson, O. Mood Disorders in Cancer Patients. Eur. J. Cancer Suppl. 2013, 11, 204. [Google Scholar] [CrossRef][Green Version]
- Adjustment to Cancer: Anxiety and Distress (PDQ®)–Health Professional Version—NCI. Available online: https://www.cancer.gov/about-cancer/coping/feelings/anxiety-distress-hp-pdq (accessed on 3 March 2023).
- Cutillo, A.; O’Hea, E.; Person, S.; Lessard, D.; Harralson, T.; Boudreaux, E. The Distress Thermometer: Cutoff Points and Clinical Use. ONF 2017, 44, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Depression (PDQ®)–Health Professional Version—NCI. Available online: https://www.cancer.gov/about-cancer/coping/feelings/depression-hp-pdq (accessed on 3 March 2023).
- National Institute of Mental Health (NIMH). Major Depression. Available online: https://www.nimh.nih.gov/health/statistics/major-depression (accessed on 3 March 2023).
- Hengrasmee, P.; Padungsutt, P.; Boriboonhirunsarn, D. Depression among Gynecologic Cancer Patients at Siriraj Hospital: Prevalence and Associated Factors. J. Med Assoc. Thail. 2004, 87, S74–S79. [Google Scholar]
- Tosic Golubovic, S.; Binic, I.; Krtinic, D.; Djordjevic, V.; Conic, I.; Gugleta, U.; Apostolovic, M.A.; Stanojevic, M.; Kostic, J. Risk Factors and Predictive Value of Depression and Anxiety in Cervical Cancer Patients. Medicina 2022, 58, 507. [Google Scholar] [CrossRef]
- Zhao, H.; Zhao, Z.; Chen, C. Prevalence, Risk Factors and Prognostic Value of Anxiety and Depression in Cervical Cancer Patients Underwent Surgery. Transl. Cancer Res. 2020, 9, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Breitbart, W. Depression, Hopelessness, and Desire for Hastened Death in Terminally Ill Patients with Cancer. JAMA 2000, 284, 2907. [Google Scholar] [CrossRef]
- Hutton, J.M.; Williams, M. An Investigation of Psychological Distress in Patients Who Have Beentreated for Head and Neck Cancer. Br. J. Oral Maxillofac. Surg. 2001, 39, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Ciaramella, A.; Poli, P. Assessment of Depression among Cancer Patients: The Role of Pain, Cancer Type and Treatment. Psycho-Oncology 2001, 10, 156–165. [Google Scholar] [CrossRef]
- Lansky, S.B.; List, M.A.; Herrmann, C.A.; Ets-Hokin, E.G.; DasGupta, T.K.; Wilbanks, G.D.; Hendrickson, F.R. Absence of Major Depressive Disorder in Female Cancer Patients. JCO 1985, 3, 1553–1560. [Google Scholar] [CrossRef]
- Hong, J.S.; Tian, J. Prevalence of Anxiety and Depression and Their Risk Factors in Chinese Cancer Patients. Support. Care Cancer 2014, 22, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Conversano, C.; Giuseppe, M.D.; Miccoli, M.; Ciacchini, R.; Silvestre, A.D.; Sterzo, R.L.; Gemignani, A.; Orrù, G. Retrospective analyses of psychological distress and defense style among cancer patients. Clin. Neuropsychiatry 2020, 17, 217. [Google Scholar] [PubMed]
- Vodermaier, A.; Linden, W.; Rnic, K.; Young, S.N.; Ng, A.; Ditsch, N.; Olson, R. Prospective associations of depression with survival: A population-based cohort study in patients with newly diagnosed breast cancer. Breast Cancer Res Treat. 2014, 143, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Hjerl, K.; Andersen, E.W.; Keiding, N.; Mouridsen, H.T.; Mortensen, P.B.; Jørgensen, T. Depression as a Prognostic Factor for Breast Cancer Mortality. Psychosomatics 2003, 44, 24–30. [Google Scholar] [CrossRef]
- Sharma, A.; Sharp, D.M.; Walker, L.G.; Monson, J.R.T. Predictors of Early Postoperative Quality of Life after Elective Resection for Colorectal Cancer. Ann. Surg. Oncol. 2007, 14, 3435–3442. [Google Scholar] [CrossRef]
- Sullivan, D.R.; Forsberg, C.W.; Ganzini, L.; Au, D.H.; Gould, M.K.; Provenzale, D.; Slatore, C.G. Longitudinal Changes in Depression Symptoms and Survival Among Patients with Lung Cancer: A National Cohort Assessment. J. Clin. Oncol. 2016, 34, 3984–3991. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Margolis, K.L.; Hendryx, M.; Reeves, K.; Wassertheil-Smoller, S.; Weitlauf, J.; Danhauer, S.C.; Chlebowski, R.T.; Caan, B.; Qi, L.; et al. Effect of Depression before Breast Cancer Diagnosis on Mortality among Postmenopausal Women: Depression and Breast Cancer Mortality. Cancer 2017, 123, 3107–3115. [Google Scholar] [CrossRef]
- Young, K.; Singh, G. Biological Mechanisms of Cancer-Induced Depression. Front. Psychiatry 2018, 9, 299. [Google Scholar] [CrossRef]
- Jehn, C.F.; Kuehnhardt, D.; Bartholomae, A.; Pfeiffer, S.; Krebs, M.; Regierer, A.C.; Schmid, P.; Possinger, K.; Flath, B.C. Biomarkers of Depression in Cancer Patients. Cancer 2006, 107, 2723–2729. [Google Scholar] [CrossRef]
- Maes, M.; Bosmans, E.; De Jongh, R.; Kenis, G.; Vandoolaeghe, E.; Neels, H. Increased Serum Il-6 and Il-1 Receptor Antagonist Concentrations in Major Depression and Treatment Resistant Depression. Cytokine 1997, 9, 853–858. [Google Scholar] [CrossRef]
- Moreau, M.; André, C.; O’Connor, J.C.; Dumich, S.A.; Woods, J.A.; Kelley, K.W.; Dantzer, R.; Lestage, J.; Castanon, N. Inoculation of Bacillus Calmette-Guerin to Mice Induces an Acute Episode of Sickness Behavior Followed by Chronic Depressive-like Behavior. Brain Behav. Immun. 2008, 22, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.H.; Maletic, V.; Raison, C.L. Inflammation and Its Discontents: The Role of Cytokines in the Pathophysiology of Major Depression. Biol. Psychiatry 2009, 65, 732–741. [Google Scholar] [CrossRef] [PubMed]
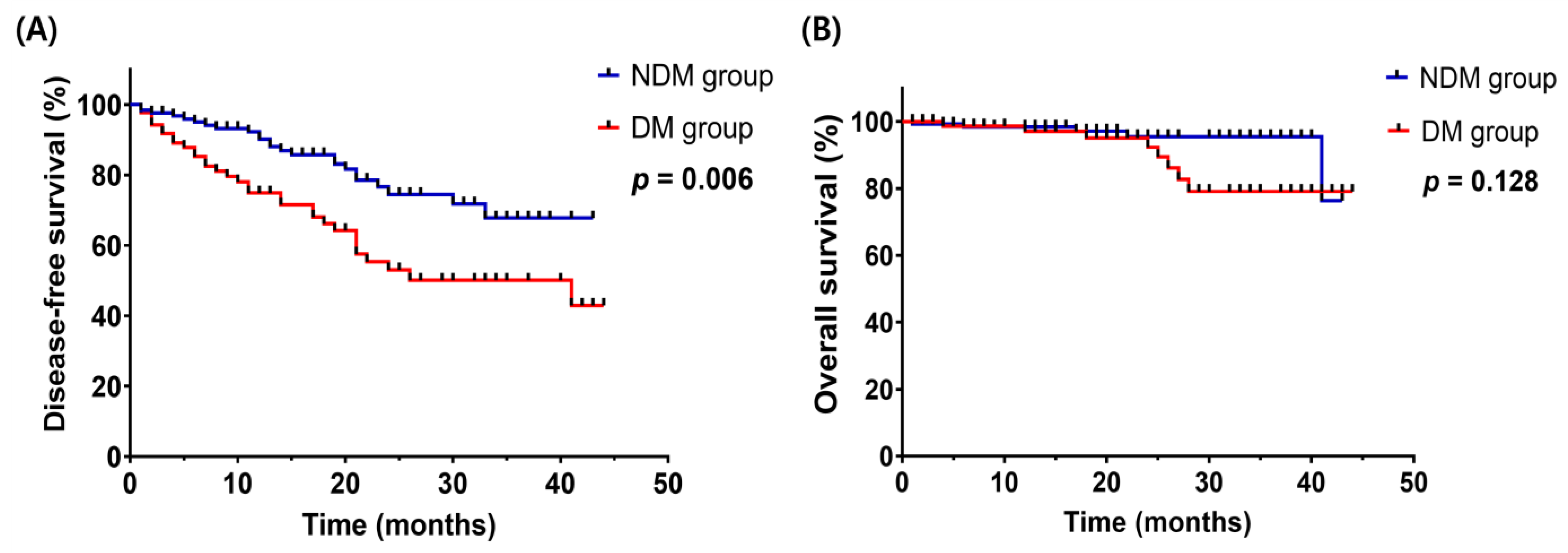
| Non-DepressedMood (N = 129) | Depressed Mood (N = 88) | p-Value | Risk Factors for DFS | |||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | ||||
| Age (years), M ± SD | 57.57 ± 14.23 | 57.83 ± 14.73 | 0.898 | 1.01 | (0.99, 1.03) | 0.171 |
| Marital status, N (%) | 0.635 | |||||
| Unmarried | 19 (14.7%) | 16 (18.2%) | 1.00 | Reference | ||
| Married | 100 (77.5%) | 66 (75.0%) | 1.99 | (0.84, 4.69) | 0.116 | |
| Divorced | 5 (3.9%) | 1 (1.1%) | 2.47 | (0.50, 12.32) | 0.270 | |
| Widowed | 5 (3.9%) | 5 (5.7%) | 1.52 | (0.30, 7.58) | 0.661 | |
| Occupational status, N (%) | 0.186 | |||||
| Inoccupation | 30 (23.3%) | 21 (23.9%) | 1.00 | Reference | ||
| Housewife | 86 (66.7%) | 51 (58.0%) | 1.00 | (0.55, 1.82) | 0.994 | |
| Employee | 10 (7.8%) | 10 (11.4%) | 0.44 | (0.13, 1.50) | 0.188 | |
| Profession | 3 (2.2%) | 2 (2.3%) | 2.05 | (0.47, 8.99) | 0.340 | |
| Others | 0 (0.0%) | 4 (4.4%) | 0.00 | (0.00, 1.43) | 0.972 | |
| Health insurance status, N (%) | 1.000 | |||||
| Health insurance | 124 (96.1%) | 84 (95.5%) | 1.00 | Reference | ||
| Medical benefit | 5 (3.9%) | 4 (4.5%) | 0.43 | (0.06, 3.13) | 0.406 | |
| Economic support status, N (%) | 0.688 | |||||
| No | 126 (97.7%) | 85 (96.6%) | 1.00 | Reference | ||
| Yes | 3 (2.3%) | 3 (3.4%) | 0.68 | (0.09, 4.92) | 0.703 | |
| Religious status, N (%) | 0.482 | |||||
| Atheism | 62 (48.0%) | 44 (50.0%) | 1.00 | Reference | ||
| Christianism | 33 (25.6%) | 18 (20.5%) | 0.77 | (0.39, 1.54) | 0.456 | |
| Buddhism | 21 (16.3%) | 15 (17.0%) | 1.01 | (0.47, 2.17) | 0.987 | |
| Catholicism | 11 (8.5%) | 6 (6.8%) | 0.77 | (0.26, 2.23) | 0.629 | |
| Others | 2 (1.6%) | 5 (5.7%) | 3.52 | (0.65, 18.99) | 0.143 | |
| Menopause status, N (%) | 0.566 | |||||
| No | 35 (27.3%) | 21 (23.9%) | 1.00 | Reference | ||
| Yes | 93 (72.7%) | 67 (76.1%) | 2.47 | (1.12, 5.44) | 0.025 | |
| Cancer types, N (%) | 0.003 | |||||
| Ovarian cancer | 44 (34.1%) | 40 (45.5%) | 1.00 | Reference | ||
| Cervical cancer | 36 (27.9%) | 34 (38.6%) | 0.34 | (0.17, 0.68) | 0.002 | |
| Endometrial cancer | 48 (37.2%) | 14 (15.9%) | 0.32 | (0.16, 0.64) | 0.001 | |
| Uterine sarcoma | 1 (0.8%) | 0 (0.0%) | 0.00 | (0.00, 0.00) | 0.976 | |
| Cancer stage, N (%) | 0.001 | |||||
| Stage 1 | 72 (55.8%) | 27 (30.7%) | 1.00 | Reference | ||
| Stage 2 | 20 (15.5%) | 17 (19.3%) | 0.03 | (0.00, 0.26) | 0.001 | |
| Stage 3 | 25 (19.4%) | 22 (25.0%) | 0.05 | (0.01, 0.42) | 0.006 | |
| Stage 4 | 12 (9.3%) | 22 (25.0%) | 0.17 | (0.02, 1.34) | 0.093 | |
| RTx. status, N (%) | 0.033 | |||||
| No | 124 (96.1%) | 78 (88.6%) | 1.00 | Reference | ||
| Yes | 5 (3.9%) | 10 (11.4%) | 0.77 | (0.24, 2,48) | 0.666 | |
| Number of CTx. | 5.96 ± 8.20 | 3.97 ± 5.44 | 0.032 | 1.07 | (1.04, 1.09) | <0.001 |
| Categorization of number of CTx. | 0.047 | |||||
| CTx. < 5, N (%) | 63 (48.8%) | 55 (62.5%) | 1.00 | Reference | ||
| CTx. ≥ 5, N (%) | 66 (51.2%) | 33 (37.5%) | 2.80 | (1.59, 4.92) | <0.001 | |
| Side effect after CTx., N (%) | 0.001 | |||||
| No | 122 (94.6%) | 71 (80.7%) | 1.00 | Reference | ||
| Yes | 7 (5.4%) | 17 (19.3%) | 2.38 | (1.28, 4.43) | 0.006 | |
| Timing of cancer w/u, N (%) | <0.001 | |||||
| No | 42 (32.6%) | 60 (68.2%) | 1.00 | Reference | ||
| Yes | 87 (67.4%) | 28 (31.8%) | 0.60 | (0.35, 1.01) | 0.053 | |
| Timing of cancer operation, N (%) | 0.036 | |||||
| No | 100 (77.5%) | 78 (88.6%) | 1.00 | Reference | ||
| Yes | 29 (22.5%) | 10 (11.4%) | 0.55 | (0.25, 1.20) | 0.132 | |
| Timing of CTx. start, N (%) | 0.197 | |||||
| No | 123 (95.3%) | 80 (90.9%) | 1.00 | Reference | ||
| Yes | 6 (4.7%) | 8 (9.1%) | 0.96 | (0.30, 3.10) | 0.951 | |
| Cancer aggravation, N (%) | 0.008 | |||||
| No | 103 (79.8%) | 56 (63.6%) | ||||
| Yes | 26 (20.2%) | 32 (36.4%) | ||||
| Death, N (%) | 0.105 | |||||
| No | 124 (96.1%) | 80 (90.7%) | ||||
| Yes | 5 (3.9%) | 8 (9.3%) | ||||
| Depressed mood | ||||||
| No | 1.00 | Reference | ||||
| Yes | 2.05 | (1.22, 3.45) | 0.007 | |||
| Risk Factors for DFS | |||
|---|---|---|---|
| HR * | 95% CI | p-Value | |
| Cancer type | |||
| Ovarian cancer | 1.00 | Reference | |
| Cervical cancer | 0.37 | (0.19, 0.76) | 0.006 |
| Endometrial cancer | 0.36 | (0.18, 0.71) | 0.003 |
| Uterine sarcoma | 0.00 | (0.00, 0.00) | 0.977 |
| Cancer stage | |||
| Stage 1 | 1.00 | Reference | |
| Stage 2 | 1.50 | (0.54, 4.13) | 0.436 |
| Stage 3 | 5.43 | (2.51, 11.72) | <0.001 |
| Stage 4 | 9.11 | (4.13, 20.09) | <0.001 |
| Number of CTx. | 1.07 | (1.04, 1.09) | <0.001 |
| CTx. < 5 | 1.00 | Reference | |
| CTx. ≥ 5 | 2.72 | (1.53, 4.86) | 0.001 |
| Side effect= after CTx. | |||
| No | 1.00 | Reference | |
| Yes | 2.07 | (1.09, 3.90) | 0.025 |
| Timing of cancer w/u | |||
| No | 1.00 | Reference | |
| Yes | 0.63 | (0.37, 1.08) | 0.092 |
| Timing of cancer operation | |||
| No | 1.00 | Reference | |
| Yes | 0.59 | (0.27, 1.30) | 0.188 |
| Timing of CTx. start | |||
| No | 1.00 | Reference | |
| Yes | 0.81 | (0.25, 2.63) | 0.729 |
| Depressed mood | |||
| No | 1.00 | Reference | |
| Yes | 2.09 | (1.24, 3.54) | 0.006 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-M.; Song, J.-Y.; Seol, A.; Lee, S.; Cho, H.-W.; Min, K.-J.; Hong, J.-H.; Lee, J.-K.; Lee, N.-W. Depressed Mood as a Significant Risk Factor for Gynecological Cancer Aggravation. Int. J. Environ. Res. Public Health 2023, 20, 6874. https://doi.org/10.3390/ijerph20196874
Lee S-M, Song J-Y, Seol A, Lee S, Cho H-W, Min K-J, Hong J-H, Lee J-K, Lee N-W. Depressed Mood as a Significant Risk Factor for Gynecological Cancer Aggravation. International Journal of Environmental Research and Public Health. 2023; 20(19):6874. https://doi.org/10.3390/ijerph20196874
Chicago/Turabian StyleLee, Seon-Mi, Jae-Yun Song, Aeran Seol, Sanghoon Lee, Hyun-Woong Cho, Kyung-Jin Min, Jin-Hwa Hong, Jae-Kwan Lee, and Nak-Woo Lee. 2023. "Depressed Mood as a Significant Risk Factor for Gynecological Cancer Aggravation" International Journal of Environmental Research and Public Health 20, no. 19: 6874. https://doi.org/10.3390/ijerph20196874
APA StyleLee, S.-M., Song, J.-Y., Seol, A., Lee, S., Cho, H.-W., Min, K.-J., Hong, J.-H., Lee, J.-K., & Lee, N.-W. (2023). Depressed Mood as a Significant Risk Factor for Gynecological Cancer Aggravation. International Journal of Environmental Research and Public Health, 20(19), 6874. https://doi.org/10.3390/ijerph20196874






