Exploring the Mediating Role of Parental Anxiety in the Link between Children’s Mental Health and Glycemic Control in Type 1 Diabetes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measurements
2.3. Statistical Data Analysis
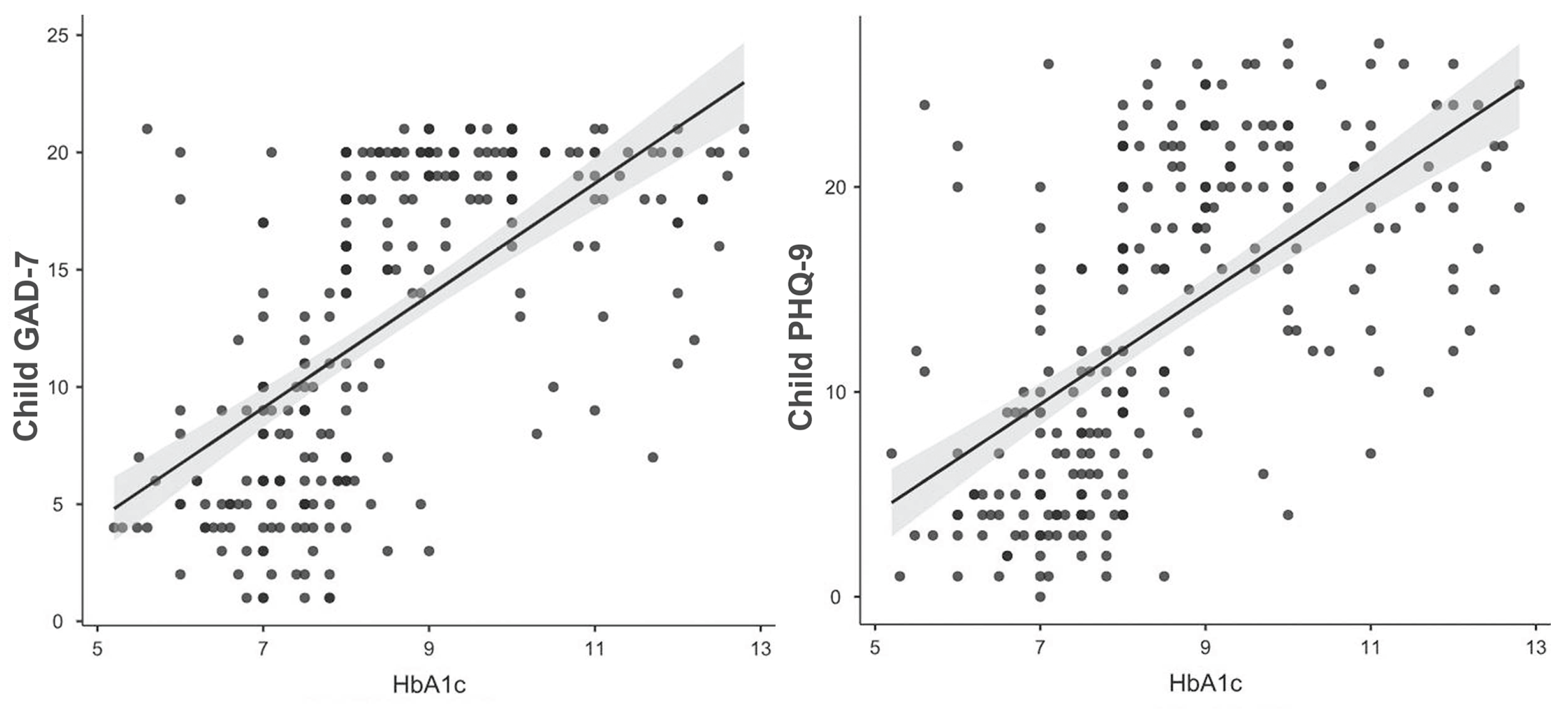
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Characteristics of the Individual Factors of Adolescents with T1D (Inner Child Environment) | |
|---|---|
| Duration of T1D | less than a year |
| 1–5 years | |
| 6–10 years | |
| more than 10 years | |
| Diabetes control level | optimal (HbA1C < 7%) |
| suboptimal (HbA1C = 7–9%) | |
| poor (HbA1C > 9%) | |
| Type of glycemic control | with a glucometer |
| with continuous glucose monitoring | |
| Method of insulin administration | with insulin pen |
| with insulin pump | |
| Mocking from the child’s peers | yes |
| no | |
| Age (years) | 12–15 |
| 15.1–18 | |
| Gender | female |
| male | |
| Characteristics of the Surrounding Factors (Parental and Socio-Economic Environment) | |
| Parental factors | |
| Parents’ anxiety level (GAD-7 score) | |
| Parents’ depression level (PHQ-9 score) | |
| Parents’ self-assessment of health status | excellent |
| good | |
| satisfactory | |
| unsatisfactory | |
| Parents’ life satisfaction | fully satisfied |
| rather satisfied | |
| rather dissatisfied | |
| completely unsatisfied | |
| Self-assessment of family relationship | good and mutually supportive |
| variables could be better | |
| problematic | |
| Marital status | unmarried |
| married | |
| divorced | |
| widow | |
| other | |
| Socio-economic factors | |
| Number of children in family | 1 |
| 2 | |
| 3 and more | |
| Number of persons in the household | 2 |
| 3 | |
| 4 | |
| 5 and more | |
| Parents’ education | primary |
| secondary | |
| bachelor’s | |
| master’s | |
| other | |
| Parents’ employment | paid full-time work |
| self-employed | |
| several places of work | |
| unemployed | |
| Income per family member per month (EUR) | less than 128.07 |
| 128.07–300 | |
| 301–500 | |
| 501–700 | |
| 701 and more | |
References
- Gale, E.A. The rise of childhood type 1 diabetes in the 20th century. Diabetes 2002, 51, 3353–3361. [Google Scholar] [CrossRef] [PubMed]
- IDF Diabetes Atlas. Estimating the Incidence and Prevalence of Type 1 Diabetes in Children and Adolescents, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Galler, A.; Tittel, S.R.; Baumeister, H.; Reinauer, C.; Brosig, B.; Becker, M.; Haberland, H.; Hilgard, D.; Jivan, M.; Mirza, J.; et al. Worse glycemic control, higher rates of diabetic ketoacidosis, and more hospitalizations in children, adolescents, and young adults with type 1 diabetes and anxiety disorders. Pediatr. Diabetes 2021, 22, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Benton, M.; Cleal, B.; Prina, M.; Baykoca, J.; Willaing, I.; Price, H.; Ismail, K. Prevalence of mental disorders in people living with type 1 diabetes: A systematic literature review and meta-analysis. Gen. Hosp. Psychiatry 2023, 80, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Henríquez-Tejo, R.; Cartes-Velásquez, R. Impacto psicosocial de la diabetes mellitus tipo 1 en niños, adolescentes y sus familias. Revisión de la literatura. Rev. Chil. Pediatría 2018, 89, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Sildorf, S.M.; Breinegaard, N.; Lindkvist, E.B.; Tolstrup, J.S.; Boisen, K.A.; Teilmann, G.K.; Skovgaard, A.M.; Svensson, J. Poor Metabolic Control in Children and Adolescents with Type 1 Diabetes and Psychiatric Comorbidity. Diabetes Care 2018, 41, 2289–2296. [Google Scholar] [CrossRef]
- Hagger, V.; Hendrieckx, C.; Cameron, F.; Pouwer, F.; Skinner, T.C.; Speight, J. Diabetes distress is more strongly associated with HbA1c than depressive symptoms in adolescents with type 1 diabetes: Results from Diabetes MILES Youth—Australia. Pediatr. Diabetes 2018, 19, 840–847. [Google Scholar] [CrossRef]
- Johnson, B.; Eiser, C.; Young, V.; Brierley, S.; Heller, S. Prevalence of depression among young people with Type 1 diabetes: A systematic review. Diabet. Med. 2013, 30, 199–208. [Google Scholar] [CrossRef]
- Vesco, A.T.; Howard, K.R.; Anderson, L.M.; Papadakis, J.L.; Hood, K.K.; Weissberg-Benchell, J. Examining Indirect Effects of Anxiety on Glycated Hemoglobin via Automatic Negative Thinking and Diabetes-Specific Distress in Adolescents with Type 1 Diabetes. Can. J. Diabetes 2021, 45, 473–480. [Google Scholar] [CrossRef]
- Rechenberg, K.; Whittemore, R.; Grey, M. Anxiety in Youth With Type 1 Diabetes. J. Pediatr. Nurs. 2017, 32, 64–71. [Google Scholar] [CrossRef]
- Buchberger, B.; Huppertz, H.; Krabbe, L.; Lux, B.; Mattivi, J.T.; Siafarikas, A. Symptoms of depression and anxiety in youth with type 1 diabetes: A systematic review and meta-analysis. Psychoneuroendocrinology 2016, 70, 70–84. [Google Scholar] [CrossRef]
- Ilonen, J.; Lempainen, J.; Veijola, R. The heterogeneous pathogenesis of type 1 diabetes mellitus. Nat. Rev. Endocrinol. 2019, 15, 635–650. [Google Scholar] [CrossRef]
- Fritsch, S.L.; Overton, M.W.; Robbins, D.R. The interface of child mental health and juvenile diabetes mellitus. Child Adolesc. Psychiatr. Clin. N. Am. 2010, 19, 335–352. [Google Scholar] [CrossRef]
- Chapman, D.P.; Perry, G.S.; Strine, T.W. The vital link between chronic disease and depressive disorders. Prev. Chronic Dis. 2005, 2, A14. [Google Scholar] [PubMed]
- Cunningham, N.R.; Vesco, A.T.; Dolan, L.M.; Hood, K.K. From caregiver psychological distress to adolescent glycemic control: The mediating role of perceived burden around diabetes management. J. Pediatr. Psychol. 2011, 36, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Fryt, J.; Pilecka, W.; Smoleń, T. Importance of symptom control: Self-regulation in children with diabetes type 1 and asthma. Stud. Psychol. 2013, 51, 5–18. [Google Scholar]
- Spitzer, R.L.; Kroenke, K.; Williams, J.B.W.; Löwe, B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch. Intern. Med. 2006, 166, 1092–1097. [Google Scholar] [CrossRef]
- Vrublevska, J.; Trapencieris, M.; Rancans, E. Adaptation and validation of the Patient Health Questionnaire-9 to evaluate major depression in a primary care sample in Latvia. Nord. J. Psychiatry 2018, 72, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Ryan, T.A.; Bailey, A.; Fearon, P.; King, J. Factorial invariance of the Patient Health Questionnaire and Generalized Anxiety Disorder Questionnaire. Br. J. Clin. Psychol. 2013, 52, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B.W. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef]
- EGL. HbA1c. Available online: https://www.egl.lv/en/tests/ (accessed on 4 August 2023).
- Rewers, M.; Pihoker, C.; Donaghue, K.; Hanas, R.; Swift, P.; Klingensmith, G.J. Assessment and monitoring of glycemic control in children and adolescents with diabetes. Pediatr. Diabetes 2009, 10, 71–81. [Google Scholar] [CrossRef]
- Cook, R.D. Detection of influential observation in linear regression. Technometrics 1977, 19, 15–18. [Google Scholar]
- Silina, E.; Taube, M.; Zolovs, M. Relationship between Glycated Hemoglobin (HbA1c) in Adolescents with Type 1 Diabetes Mellitus (T1DM) and Parental Anxiety and Depression. IJMRHS 2023, 17, 09. [Google Scholar] [CrossRef]
- Turin, A.; Drobnič Radobuljac, M. Psychosocial factors affecting the etiology and management of type 1 diabetes mellitus: A narrative review. World J. Diabetes 2021, 12, 1518–1529. [Google Scholar] [CrossRef]
- Bassi, G.; Mancinelli, E.; Di Riso, D.; Salcuni, S. Parental Stress, Anxiety and Depression Symptoms Associated with Self-Efficacy in Paediatric Type 1 Diabetes: A Literature Review. Int. J. Environ. Res. Public Health 2020, 18, 152. [Google Scholar] [CrossRef]
- Akbarizadeh, M.; Naderi Far, M.; Ghaljaei, F. Prevalence of depression and anxiety among children with type 1 and type 2 diabetes: A systematic review and meta-analysis. World J. Pediatr. 2022, 18, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Tsiouli, E.; Alexopoulos, E.C.; Stefanaki, C.; Darviri, C.; Chrousos, G.P. Effects of diabetes-related family stress on glycemic control in young patients with type 1 diabetes: Systematic review. Can. Fam. Physician 2013, 59, 143–149. [Google Scholar]
- Frye, S.S.; Perfect, M.M.; Silva, G.E. Diabetes management mediates the association between sleep duration and glycemic control in youth with type 1 diabetes mellitus. Sleep Med. 2019, 60, 132–138. [Google Scholar] [CrossRef]
- Thomas, D.M.; Lipsky, L.M.; Liu, A.Y.; Nansel, T.R. Income Relates to Adherence in Youth with Type 1 Diabetes Through Parenting Constructs. J. Dev. Behav. Pediatr. 2018, 39, 508–515. [Google Scholar] [CrossRef]
- Hilliard, M.E.; Holmes, C.S.; Chen, R.; Maher, K.; Robinson, E.; Streisand, R. Disentangling the roles of parental monitoring and family conflict in adolescents’ management of type 1 diabetes. Health Psychol. 2013, 32, 388–396. [Google Scholar] [CrossRef]

| Study Population (N = 502; 251 Child–Parent Dyads) | |
|---|---|
| Adolescents with T1D (N = 251) | Parents (N = 251) |
| Methods | |
| Generalized Anxiety Disorder 7-item (GAD-7) Scale ↓ Symptoms of anxiety | Patient Health Questionnaire 9 (PHQ-9) Scale ↓ Symptoms of depression |
| Predictor | Estimate | 95% CI | t | p |
|---|---|---|---|---|
| Intercept | 7.04 | 6.35–7.73 | 20.12 | <0.001 |
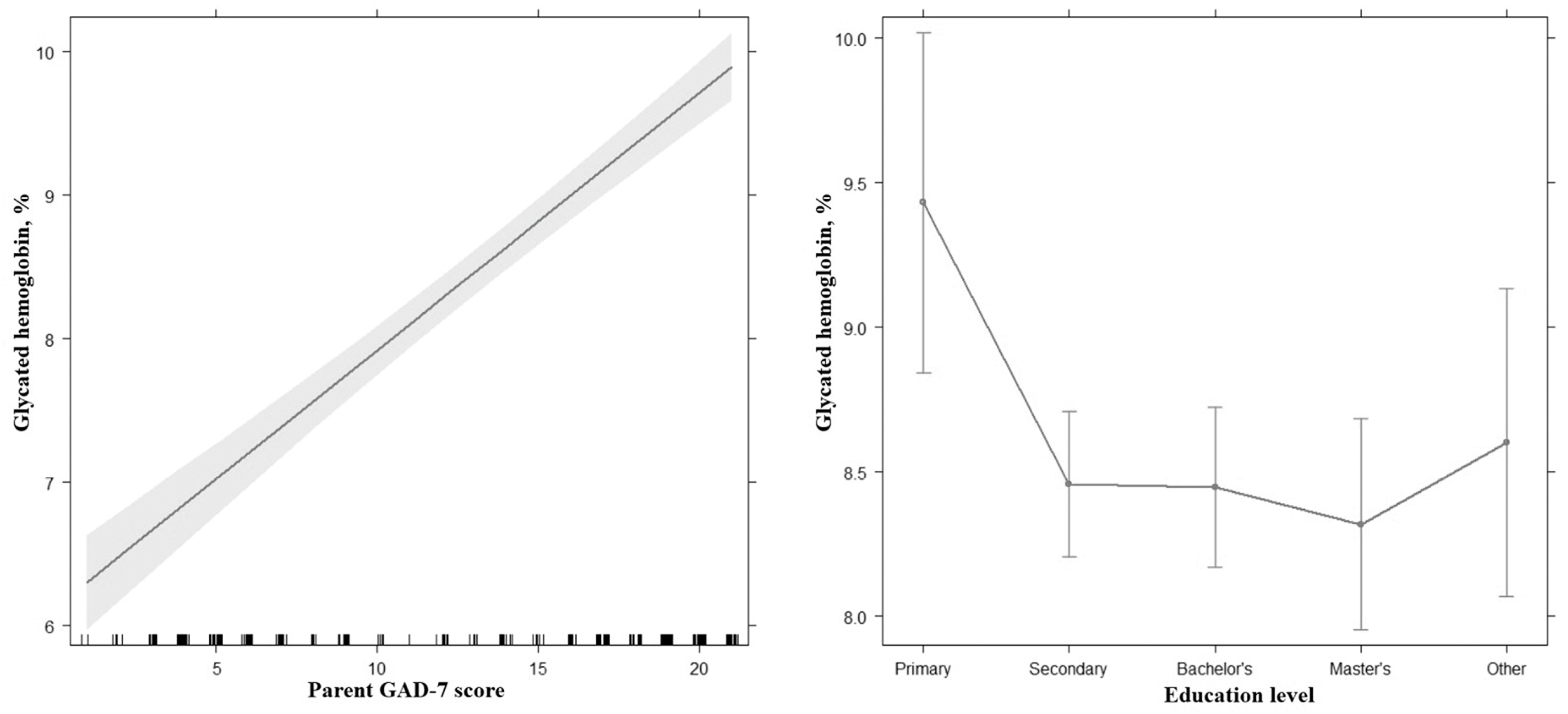
| Parent’s GAD-7 score | 0.18 | 0.16–0.20 | 14.86 | <0.001 |
| Parental education: | ||||
| Secondary—Primary | −0.97 | 0.33–1.61 | −3.00 | 0.003 |
| Bachelor’s—Primary | −0.98 | 0.33–1.64 | −2.97 | 0.003 |
| Master’s—Primary | −1.11 | 0.42–1.81 | −3.16 | 0.002 |
| Other—Primary | −0.83 | 0.04–1.62 | −2.07 | 0.040 |
| Type | Effect | Estimate | 95% CI of Estimate | β | z | p | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
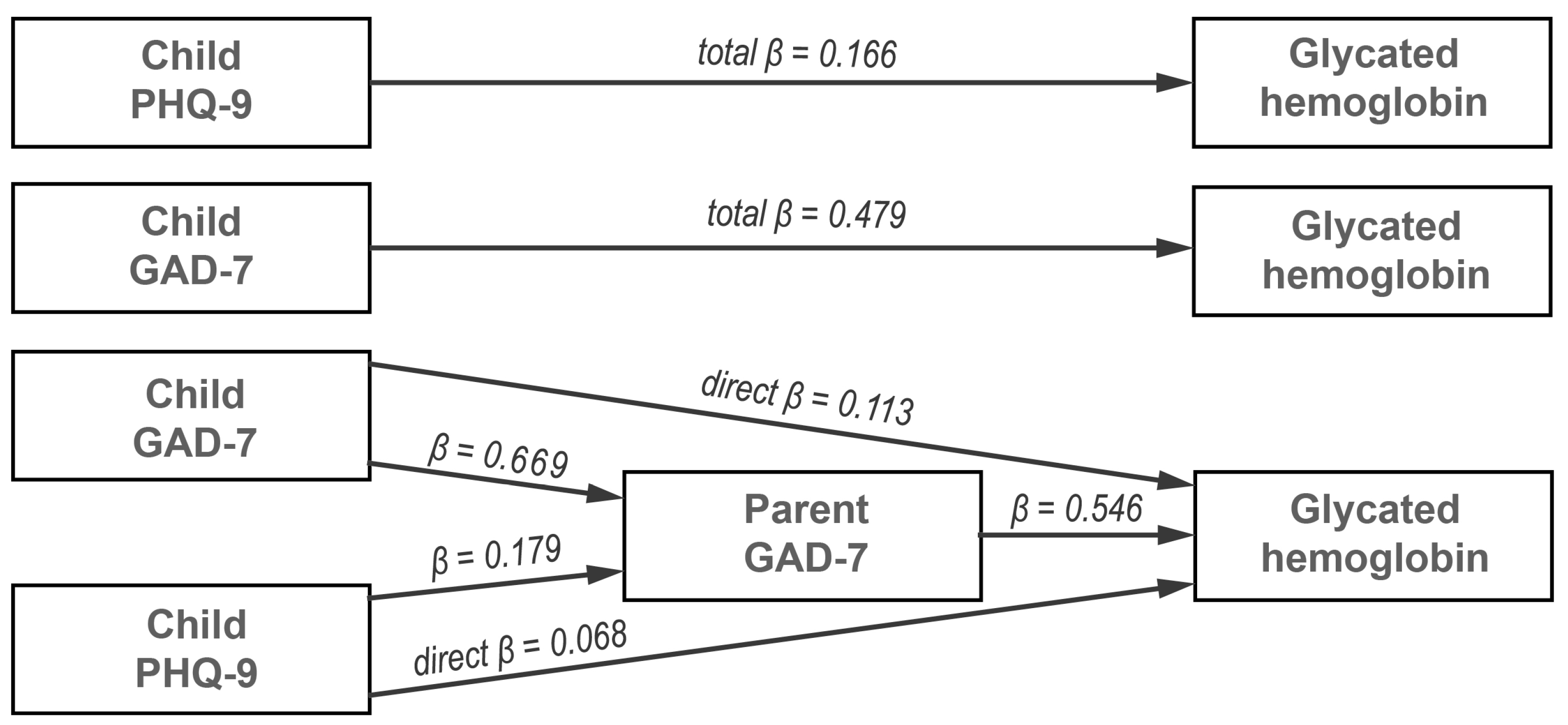
| Indirect | Child’s GAD-7 → Parent’s GAD-7 → Glycated hemoglobin | 0.096 | 0.052 | 0.139 | 0.366 | 4.319 | <0.001 |
| Child’s PHQ-9 → Parent’s GAD-7 → Glycated hemoglobin | 0.022 | 0.004 | 0.038 | 0.098 | 2.565 | 0.010 | |
| Component | Child’s GAD-7 → Parent’s GAD-7 | 0.665 | 0.519 | 0.815 | 0.669 | 8.828 | <0.001 |
| Parent’s GAD-7 → Glycated hemoglobin | 0.144 | 0.093 | 0.194 | 0.546 | 5.612 | <0.001 | |
| Child’s PHQ-9 → Parent’s GAD-7 | 0.151 | 0.035 | 0.263 | 0.179 | 2.607 | 0.009 | |
| Direct | Child’s GAD-7 → Glycated hemoglobin | 0.029 | −0.029 | 0.089 | 0.113 | 0.982 | 0.326 |
| Child’s PHQ-9 → Glycated hemoglobin | 0.015 | −0.025 | 0.055 | 0.068 | 0.742 | 0.458 | |
| Total | Child’s GAD-7 → Glycated hemoglobin | 0.126 | 0.068 | 0.183 | 0.479 | 4.300 | <0.001 |
| Child’s PHQ-9 → Glycated hemoglobin | 0.037 | −0.011 | 0.086 | 0.166 | 1.496 | 0.135 | |
| Study Authors (Year) | Study Population | Independent Variable | Dependent Variable | Mediator |
|---|---|---|---|---|
| Vesco et al. (2021) [9] | Adolescents with T1D, N = 264 | Anxiety | HbA1c | Diabetes-specific distress |
| Frye et al. (2019) [29] | 10–16-year-old children, N = 111 | Sleep duration | Diabetes management | |
| Hagger et al. (2018) [7] | Adolescents with T1D, N = 450 | Depressive symptoms | Diabetes-specific distress | |
| Thomas et al. (2018) [30] | Youths and their parents, N = 390 | Family income | Parenting constructs | |
| Hilliard et al. (2013) [31] | Adolescent–parent dyads, N = 257 | Parental monitoring, family conflict | Diabetes self-care | |
| Cunningham et al. (2011) [15] | Adolescents and their caregivers, N = 147 | Caregiver depression | Diabetes-specific burden |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silina, E.; Taube, M.; Zolovs, M. Exploring the Mediating Role of Parental Anxiety in the Link between Children’s Mental Health and Glycemic Control in Type 1 Diabetes. Int. J. Environ. Res. Public Health 2023, 20, 6849. https://doi.org/10.3390/ijerph20196849
Silina E, Taube M, Zolovs M. Exploring the Mediating Role of Parental Anxiety in the Link between Children’s Mental Health and Glycemic Control in Type 1 Diabetes. International Journal of Environmental Research and Public Health. 2023; 20(19):6849. https://doi.org/10.3390/ijerph20196849
Chicago/Turabian StyleSilina, Evija, Maris Taube, and Maksims Zolovs. 2023. "Exploring the Mediating Role of Parental Anxiety in the Link between Children’s Mental Health and Glycemic Control in Type 1 Diabetes" International Journal of Environmental Research and Public Health 20, no. 19: 6849. https://doi.org/10.3390/ijerph20196849
APA StyleSilina, E., Taube, M., & Zolovs, M. (2023). Exploring the Mediating Role of Parental Anxiety in the Link between Children’s Mental Health and Glycemic Control in Type 1 Diabetes. International Journal of Environmental Research and Public Health, 20(19), 6849. https://doi.org/10.3390/ijerph20196849






