The Extent of Evidence Supporting the Effectiveness of Extended Reality Telerehabilitation on Different Qualitative and Quantitative Outcomes in Stroke Survivors: A Systematic Review
Abstract
:1. Introduction
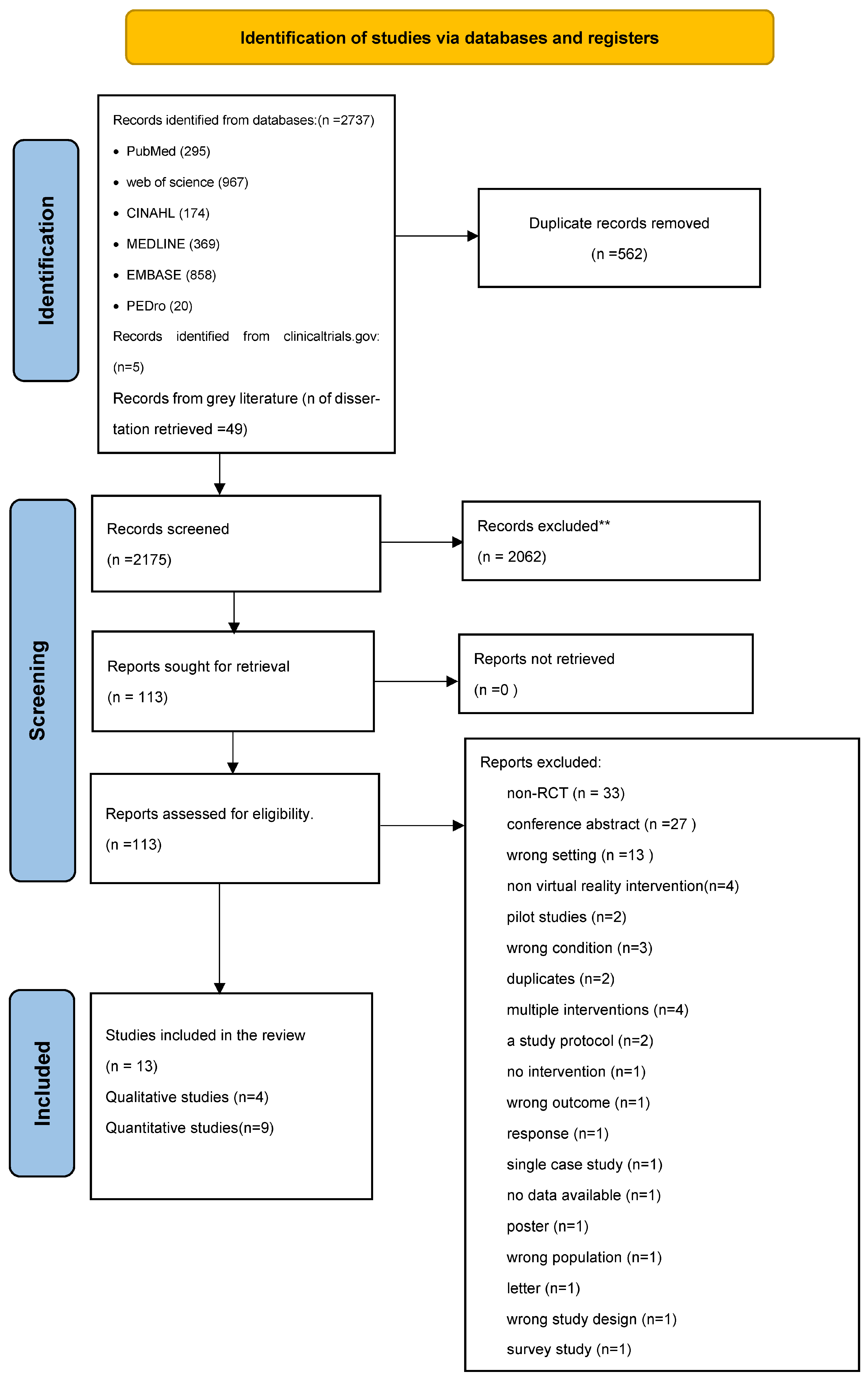
2. Materials and Methods
2.1. Protocol and Registration
2.2. Study Selection
2.3. Search Strategy and Data Sources
2.4. Reviewing Procedures and Study Selection
2.5. Data Extraction
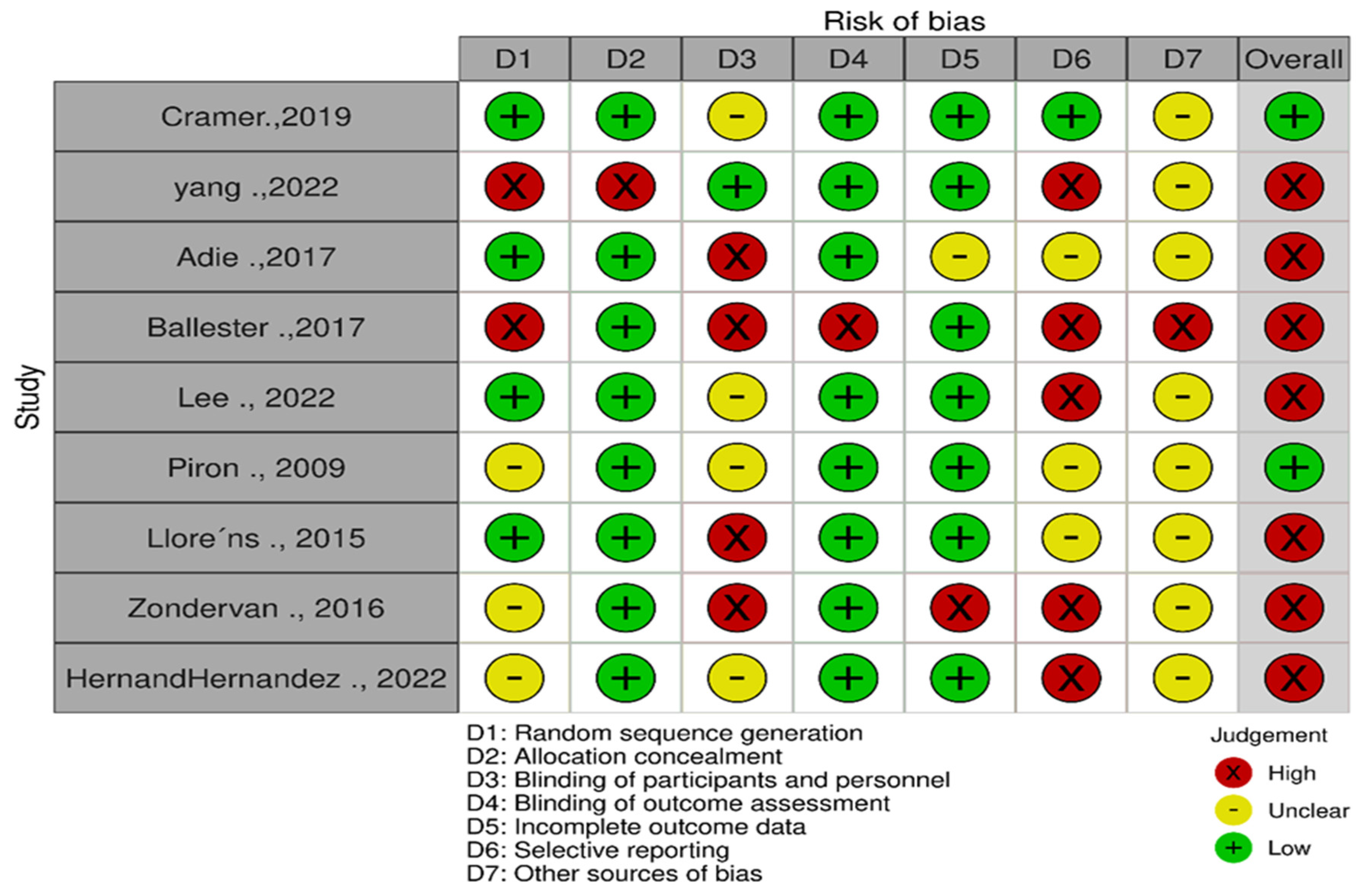
2.6. Methodological Quality
2.7. Data Synthesis and Analysis
3. Results
3.1. Risk of Bias Assessment
3.2. Quantitative Studies
3.2.1. Characteristics of Included Studies
3.2.2. Participants
3.2.3. Types of Exercises and Mode of Delivery
3.2.4. Dose of Exercises
3.2.5. Tracking of the Treatment Plan
3.2.6. Devices Used to Deliver TR Exercises
3.2.7. Main Findings of Motor Outcomes
Extended Reality Telerehabilitation Compared to In-Clinic Rehabilitation
Extended Reality Telerehabilitation Compared to Home Rehabilitation
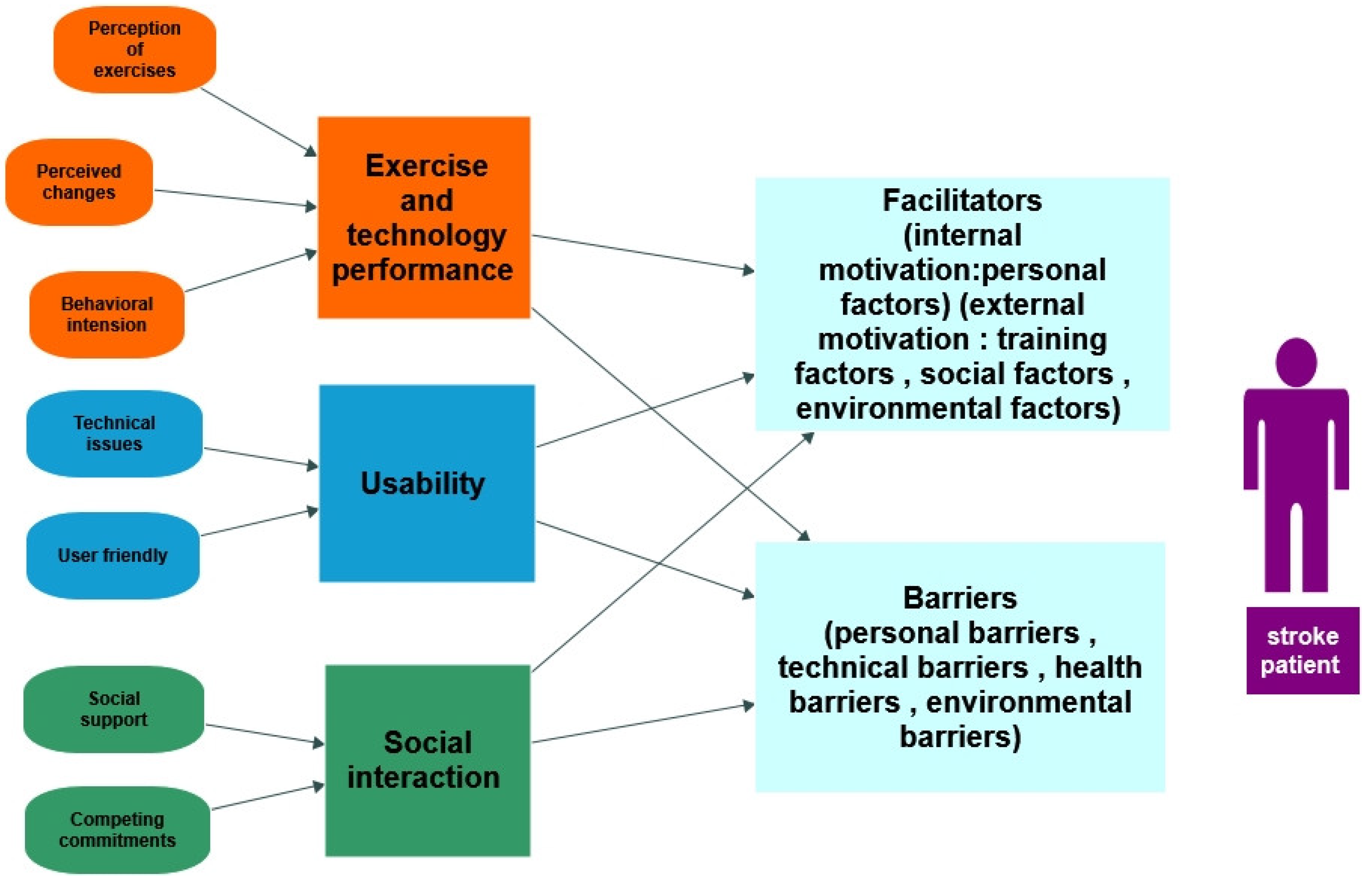
3.3. Qualitative Studies
3.3.1. Characteristics of Included Studies
3.3.2. Participants
3.3.3. Main Findings
Exercises and Technology Performance
Usability
Social Interaction
Facilitators
Barriers
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Donkor, E.S. Stroke in the 21(st) Century: A Snapshot of the Burden, Epidemiology, and Quality of Life. Stroke Res. Treat. 2018, 2018, 3238165. [Google Scholar] [CrossRef] [PubMed]
- Langhorne, P.; Bernhardt, J.; Kwakkel, G. Stroke rehabilitation. Lancet 2011, 377, 1693–1702. [Google Scholar] [CrossRef] [PubMed]
- Norlander, A.; Iwarsson, S.; Jönsson, A.C.; Lindgren, A.; Månsson Lexell, E. Living and ageing with stroke: An exploration of conditions influencing participation in social and leisure activities over 15 years. Brain Inj. 2018, 32, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Johar, M.N.; Mohd Nordin, N.A.; Abdul Aziz, A.F. The effect of game-based in comparison to conventional circuit exercise on functions, motivation level, self-efficacy and quality of life among stroke survivors. Medicine 2022, 101, e28580. [Google Scholar] [CrossRef] [PubMed]
- Hebert, D.; Lindsay, M.P.; McIntyre, A.; Kirton, A.; Rumney, P.G.; Bagg, S.; Bayley, M.; Dowlatshahi, D.; Dukelow, S.; Garnhum, M.; et al. Canadian stroke best practice recommendations: Stroke rehabilitation practice guidelines, update. Int. J. Stroke 2016, 11, 459–484. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Unmet Global Need for Rehabilitation. Available online: https://www.who.int/news-room/fact-sheets/detail/rehabilitation (accessed on 16 June 2023).
- Brennan, D.; Tindall, L.; Theodoros, D.; Brown, J.; Campbell, M.; Christiana, D.; Smith, D.; Cason, J.; Lee, A. A blueprint for telerehabilitation guidelines. Int. J. Telerehabilit. 2010, 2, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Levy, C.E.; Silverman, E.; Jia, H.; Geiss, M.; Omura, D. Effects of physical therapy delivery via home video telerehabilitation on functional and health-related quality of life outcomes. J. Rehabil. Res. Dev. 2015, 52, 361–370. [Google Scholar] [CrossRef]
- Qin, P.; Cai, C.; Chen, X.; Wei, X. Effect of home-based interventions on basic activities of daily living for patients who had a stroke: A systematic review with meta-analysis. BMJ Open 2022, 12, e056045. [Google Scholar] [CrossRef]
- Nascimento, L.R.; Gaviorno, L.F.; Brunelli, M.D.; Goncalves, J.V.; Areas, F.Z.D. Home-based is as effective as centre-based rehabilitation for improving upper limb motor recovery and activity limitations after stroke: A systematic review with meta-analysis. Clin. Rehabil. 2022, 36, 1565–1577. [Google Scholar] [CrossRef]
- Wechsler, L.R. Advantages and limitations of teleneurology. JAMA Neurol. 2015, 72, 349–354. [Google Scholar] [CrossRef]
- Edirippulige, S.; Martin-Khan, M.; Beattie, E.; Smith, A.C.; Gray, L.C. A systematic review of telemedicine services for residents in long term care facilities. J. Telemed. Telecare 2013, 19, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, S.; Lim, D.S.Y.; Ho, W.H.H.; Koh, Y.Q.; Cai, V.; Koh, G.C.H.; Legido-Quigley, H. Acceptance of Tele-Rehabilitation by Stroke Patients: Perceived Barriers and Facilitators. Arch. Phys. Med. Rehabil. 2018, 99, 2472–2477.e2472. [Google Scholar] [CrossRef] [PubMed]
- Mulder, M.; Nikamp, C.; Nijland, R.; van Wegen, E.; Prinsen, E.; Vloothuis, J.; Buurke, J.; Kwakkel, G. Can telerehabilitation services combined with caregiver-mediated exercises improve early supported discharge services poststroke? A study protocol for a multicentre, observer-blinded, randomized controlled trial. BMC Neurol. 2022, 22, 29. [Google Scholar] [CrossRef] [PubMed]
- Russell, T. Telerehabilitation: A service delivery strategy for stroke. Int. J. Stroke 2013, 1, 3. [Google Scholar]
- Lohse, K.R.; Lang, C.E.; Boyd, L.A. Is more better? Using metadata to explore dose-response relationships in stroke rehabilitation. Stroke 2014, 45, 2053–2058. [Google Scholar] [CrossRef] [PubMed]
- Lohse, K.R.; Hilderman, C.G.; Cheung, K.L.; Tatla, S.; Van der Loos, H.F. Virtual reality therapy for adults post-stroke: A systematic review and meta-analysis exploring virtual environments and commercial games in therapy. PLoS ONE 2014, 9, e93318. [Google Scholar] [CrossRef] [PubMed]
- Ludlow, B.L. Virtual Reality: Emerging Applications and Future Directions. Rural. Spec. Educ. Q. 2015, 34, 3–10. [Google Scholar] [CrossRef]
- Le Noury, P.; Polman, R.; Maloney, M.; Gorman, A. A Narrative Review of the Current State of Extended Reality Technology and How it can be Utilised in Sport. Sports Med. 2022, 52, 1473–1489. [Google Scholar] [CrossRef]
- Edwards, D.; Williams, J.; Carrier, J.; Davies, J. Technologies used to facilitate remote rehabilitation of adults with deconditioning, musculoskeletal conditions, stroke, or traumatic brain injury: An umbrella review. JBI Evid. Synth. 2022, 20, 1927–1968. [Google Scholar] [CrossRef]
- Buccellato, K.H.; Nordstrom, M.; Murphy, J.M.; Burdea, G.C.; Polistico, K.; House, G.; Kim, N.; Grampurohit, N.; Sorensen, J.; Isaacson, B.M.; et al. A Randomized Feasibility Trial of a Novel, Integrative, and Intensive Virtual Rehabilitation Program for Service Members Post-Acquired Brain Injury. Mil. Med. 2020, 185, e203–e211. [Google Scholar] [CrossRef]
- Viñas-Diz, S.; Sobrido-Prieto, M. Virtual reality for therapeutic purposes in stroke: A systematic review. Neurologia 2016, 31, 255–277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, D.; Liu, Y.; Wang, J.; Xiao, Q. Virtual reality for limb motor function, balance, gait, cognition and daily function of stroke patients: A systematic review and meta-analysis. J. Adv. Nurs. 2021, 77, 3255–3273. [Google Scholar] [CrossRef] [PubMed]
- Laver, K.E.; Lange, B.; George, S.; Deutsch, J.E.; Saposnik, G.; Crotty, M. Virtual reality for stroke rehabilitation. Cochrane Database Syst. Rev. 2017, 11, CD008349. [Google Scholar] [CrossRef] [PubMed]
- Dockx, K.; Bekkers, E.M.; Van den Bergh, V.; Ginis, P.; Rochester, L.; Hausdorff, J.M.; Mirelman, A.; Nieuwboer, A. Virtual reality for rehabilitation in Parkinson’s disease. Cochrane Database Syst. Rev. 2016, 12, CD010760. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Kan, L.; Dong, A.; Zhang, J.; Bai, Z.; Xie, Y.; Liu, Q.; Peng, Y. The effects of action observation training on improving upper limb motor functions in people with stroke: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0221166. [Google Scholar] [CrossRef] [PubMed]
- Baus, O.; Bouchard, S. Moving from Virtual Reality Exposure-Based Therapy to Augmented Reality Exposure-Based Therapy: A Review. Front. Hum. Neurosci. 2014, 8, 112. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Hsiang, E.-L.; He, Z.; Zhan, T.; Wu, S.-T. Augmented reality and virtual reality displays: Emerging technologies and future perspectives. Light Sci. Appl. 2021, 10, 216. [Google Scholar] [CrossRef]
- Bevilacqua, R.; Maranesi, E.; Riccardi, G.R.; di Donna, V.; Pelliccioni, P.; Luzi, R.; Lattanzio, F.; Pelliccioni, G. Non-immersive virtual reality for rehabilitation of the older people: A systematic review into efficacy and effectiveness. J. Clin. Med. 2019, 8, 1882. [Google Scholar] [CrossRef]
- Dilmegani, C. Ultimate Guide to Virtual Reality (VR) in 2023: Types & Uses. Available online: https://research.aimultiple.com/virtual-reality/#what-are-the-different-types-of-virtual-reality-vr (accessed on 1 August 2023).
- Patsaki, I.; Dimitriadi, N.; Despoti, A.; Tzoumi, D.; Leventakis, N.; Roussou, G.; Papathanasiou, A.; Nanas, S.; Karatzanos, E. The effectiveness of immersive virtual reality in physical recovery of stroke patients: A systematic review. Front. Syst. Neurosci. 2022, 16, 880447. [Google Scholar] [CrossRef]
- Chang, M.C.; Boudier-Revéret, M. Usefulness of Telerehabilitation for Stroke Patients During the COVID-19 Pandemic. Am. J. Phys. Med. Rehabil. 2020, 99, 582. [Google Scholar] [CrossRef]
- Nikolaev, V.A.; Safonicheva, O.G.; Nikolaev, A.A. Telerehabilitation of Post-Stroke Patients with Motor Function Disorders: A Review. Adv. Gerontol. 2022, 12, 339–346. [Google Scholar] [CrossRef]
- Nikolaev, V.A.; Nikolaev, A.A. Recent trends in telerehabilitation of stroke patients: A narrative review. Neuro Rehabil. 2022, 51, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.Q.; Du, D.; Wei, X.Y.; Tong, R.K. Augmented reality for stroke rehabilitation during COVID-19. J. Neuroeng. Rehabil. 2022, 19, 136. [Google Scholar] [CrossRef] [PubMed]
- Lloréns, R.; Noé, E.; Colomer, C.; Alcañiz, M. Effectiveness, Usability, and Cost-Benefit of a Virtual Reality–Based Telerehabilitation Program for Balance Recovery After Stroke: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2015, 96, 418–425.e412. [Google Scholar] [CrossRef]
- Toh, S.F.M.; Chia, P.F.; Fong, K.N.K. Effectiveness of home-based upper limb rehabilitation in stroke survivors: A systematic review and meta-analysis. Front. Neurol. 2022, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Savović, J.; Page, M.J.; Elbers, R.G.; Sterne, J.A. Assessing risk of bias in a randomized trial. In Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 205–228. [Google Scholar] [CrossRef]
- Butler, A.; Hall, H.; Copnell, B. A Guide to Writing a Qualitative Systematic Review Protocol to Enhance Evidence-Based Practice in Nursing and Health Care. Worldviews Evid. Based Nurs. 2016, 13, 241–249. [Google Scholar] [CrossRef]
- Lipsey, M.W.; Wilson, D.B. Practical Meta-Analysis; Sage Publications, Inc: Thousand Oaks, CA, USA, 2001; p. 247. [Google Scholar]
- Cramer, S.C.; Dodakian, L.; Le, V.; See, J.; Augsburger, R.; McKenzie, A.; Zhou, R.J.; Chiu, N.L.; Heckhausen, J.; Cassidy, J.M.; et al. Efficacy of Home-Based Telerehabilitation vs. In-Clinic Therapy for Adults After Stroke: A Randomized Clinical Trial. JAMA Neurol. 2019, 76, 1079–1087. [Google Scholar] [CrossRef]
- Piron, L.; Turolla, A.; Agostini, M.; Zucconi, C.; Cortese, F.; Zampolini, M.; Zannini, M.; Dam, M.; Ventura, L.; Battauz, M.; et al. Exercises for Paretic Upper Limb after Stroke: A Combined Virtual-Reality and Telemedicine Approach. J. Rehabil. Med. 2009, 41, 1016–1020. [Google Scholar] [CrossRef]
- Adie, K.; Schofield, C.; Berrow, M.; Wingham, J.; Humfryes, J.; Pritchard, C.; James, M.; Allison, R. Does the use of Nintendo Wii Sports(TM) improve arm function? Trial of Wii(TM) in Stroke: A randomized controlled trial and economics analysis. Clin. Rehabil. 2017, 31, 173–185. [Google Scholar] [CrossRef]
- Ballester, B.; Nirme, J.; Camacho, I.; Duarte, E.; Rodriguez, S.; Cuxart, A.; Duff, A.; Verschure, P.; Rubio Ballester, B.; Nirme, J.; et al. Domiciliary VR-Based Therapy for Functional Recovery and Cortical Reorganization: Randomized Controlled Trial in Participants at the Chronic Stage Post Stroke. JMIR Serious Games 2017, 5, e15. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.I.; Park, J.; Koo, J.; Son, M.; Hwang, J.H.; Lee, J.Y.; Chang, W.H. Effects of the home-based exercise program with an augmented reality system on balance in patients with stroke: A randomized controlled trial. Disabil. Rehabil. 2022, 45, 1705–1712. [Google Scholar] [CrossRef] [PubMed]
- Zondervan, D.; Friedman, N.; Chang, E.; Zhao, X.; Augsburger, R.; Reinkensmeyer, D.; Cramer, S.; Zondervan, D.K.; Friedman, N.; Chang, E.; et al. Home-based hand rehabilitation after chronic stroke: Randomized, controlled single-blind trial comparing the MusicGlove with a conventional exercise program. J. Rehabil. Res. Dev. 2016, 53, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, A.; Bubyr, L.; Archambault, P.; Higgins, J.; Levin, M.; Kairy, D.; Hernandez, A.; Bubyr, L.; Archambault, P.S.; Higgins, J.; et al. Virtual Reality-Based Rehabilitation as a Feasible and Engaging Tool for the Management of Chronic Poststroke Upper-Extremity Function Recovery: Randomized Controlled Trial. JMIR Serious Games 2022, 10, e37506. [Google Scholar] [CrossRef] [PubMed]
- Allegue, D.R.; Sweet, S.N.; Higgins, J.; Archambault, P.S.; Michaud, F.; Miller, W.C.; Tousignant, M.; Kairy, D. Lessons Learned From Clinicians and Stroke Survivors about Using Telerehabilitation Combined With Exergames: Multiple Case Study. JMIR Rehabil. Assist. Technol. 2022, 9, e31305. [Google Scholar] [CrossRef] [PubMed]
- Wingham, J.; Adie, K.; Turner, D.; Schofield, C.; Pritchard, C. Participant and caregiver experience of the Nintendo Wii SportsTM after stroke: Qualitative study of the trial of WiiTM in stroke (TWIST). Clin. Rehabil. 2015, 29, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Standen, P.; Threapleton, K.; Connell, L.; Richardson, A.; Brown, D.; Battersby, S.; Sutton, C.; Platts, F.; Standen, P.; Threapleton, K.; et al. Patients’ Use of a Home-Based Virtual Reality System to Provide Rehabilitation of the Upper Limb Following Stroke. Phys. Ther. 2015, 95, 350–359. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.; Zheng, K.; Dodakian, L.; See, J.; Zhou, R.; Chiu, N.; Augsburger, R.; McKenzie, A.; Cramer, S.C. A qualitative study on user acceptance of a home-based stroke telerehabilitation system. Top. Stroke Rehabil. 2020, 27, 81–92. [Google Scholar] [CrossRef]
- Jung, H.-Y. Rehabilitation in subacute and chronic stage after stroke. In Stroke Revisited: Diagnosis and Treatment of Ischemic Stroke; Springer: Singapore, 2017; pp. 351–360. [Google Scholar]
- Cramer, S.C. Repairing the human brain after stroke: I. Mechanisms of spontaneous recovery. Ann. Neurol. 2008, 63, 272–287. [Google Scholar] [CrossRef]
- Hao, J.; Xie, H.; Harp, K.; Chen, Z.; Siu, K.-C. Effects of Virtual Reality Intervention on Neural Plasticity in Stroke Rehabilitation: A Systematic Review. Arch. Phys. Med. Rehabil. 2022, 103, 523–541. [Google Scholar] [CrossRef]
- Maier, M.; Rubio Ballester, B.; Duff, A.; Duarte Oller, E.; Verschure, P. Effect of Specific over Nonspecific VR-Based Rehabilitation on Poststroke Motor Recovery: A Systematic Meta-analysis. Neurorehabil. Neural Repair. 2019, 33, 112–129. [Google Scholar] [CrossRef] [PubMed]
- Laver, K.E.; Adey-Wakeling, Z.; Crotty, M.; Lannin, N.A.; George, S.; Sherrington, C. Telerehabilitation services for stroke. Cochrane Database Syst. Rev. 2020, 1, Cd010255. [Google Scholar] [CrossRef] [PubMed]
- Amorim, P.; Santos, B.S.; Dias, P.; Silva, S.; Martins, H. Serious Games for Stroke Telerehabilitation of Upper Limb—A Review for Future Research. Int. J. Telerehabilit. 2020, 12, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Schroder, J.; van Criekinge, T.; Embrechts, E.; Celis, X.; Van Schuppen, J.; Truijen, S.; Saeys, W. Combining the benefits of tele-rehabilitation and virtual reality-based balance training: A systematic review on feasibility and effectiveness. Disabil. Rehabil. Assist. Technol. 2019, 14, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Pu, Y.; Chen, Z.; Siu, K.C. Effects of virtual reality-based telerehabilitation for stroke patients: A systematic review and meta-analysis of randomized controlled trials. J. Stroke Cerebrovasc. Dis. 2023, 32, 106960. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, M.; Robinson, L.R.; Mayo, A. Practical Considerations for Implementing Virtual Care in Physical Medicine and Rehabilitation: For the Pandemic and Beyond. Am. J. Phys. Med. Rehabil. 2020, 99, 464–467. [Google Scholar] [CrossRef] [PubMed]
- French, B.; Thomas, L.H.; Coupe, J.; McMahon, N.E.; Connell, L.; Harrison, J.; Sutton, C.J.; Tishkovskaya, S.; Watkins, C.L. Repetitive task training for improving functional ability after stroke. Cochrane Database Syst. Rev. 2016, 11, Cd006073. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.F.; Demers, M. Motor learning in neurological rehabilitation. Disabil. Rehabil. 2021, 43, 3445–3453. [Google Scholar] [CrossRef]
- Charles, D.; Holmes, D.; Charles, T.; McDonough, S. Virtual Reality Design for Stroke Rehabilitation. Adv. Exp. Med. Biol. 2020, 1235, 53–87. [Google Scholar] [CrossRef]
- Holmes, D.; Charles, D.; Morrow, P.; McClean, S.; McDonough, S. Rehabilitation Game Model for Personalised Exercise. In Proceedings of the 2015 International Conference on Interactive Technologies and Games, Nottingham, UK, 22–23 October 2015; pp. 41–48. [Google Scholar] [CrossRef]
- Moore, G.F.; Audrey, S.; Barker, M.; Bond, L.; Bonell, C.; Hardeman, W.; Moore, L.; O’Cathain, A.; Tinati, T.; Wight, D. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015, 350, h1258. [Google Scholar] [CrossRef]
- Pollock, A.; Farmer, S.E.; Brady, M.C.; Langhorne, P.; Mead, G.E.; Mehrholz, J.; van Wijck, F. Interventions for improving upper limb function after stroke. Cochrane Database Syst Rev. 2014, 12, CD010820. [Google Scholar] [CrossRef]
- Frenkel-Toledo, S.; Ofir-Geva, S.; Mansano, L.; Granot, O.; Soroker, N. Stroke Lesion Impact on Lower Limb Function. Front Hum Neurosci. 2021, 15, 592975. [Google Scholar] [CrossRef] [PubMed]
- Pollock, A.S.; Durward, B.R.; Rowe, P.J.; Paul, J.P. What is balance? Clin Rehabil. 2000, 14, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Suchomel, T.J.; Nimphius, S.; Bellon, C.R.; Stone, M.H. The Importance of Muscular Strength: Training Considerations. Sports Med. 2018, 48, 765–785. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, H.; Barthelemy, M.; Ghoshal, G.; James, C.R.; Lenormand, M.; Louail, T.; Tomasini, M. Human mobility: Models and applications. Phys. Rep. 2018, 734, 1–74. [Google Scholar] [CrossRef]
- Montero-Odasso, M.; van der Velde, N.; Martin, F.C.; Petrovic, M.; Tan, M.P.; Ryg, J.; Aguilar-Navarro, S.; Alexander, N.B.; Becker, C.; Blain, H.; et al. World guidelines for falls prevention and management for older adults: A global initiative. Age Ageing 2022, 51, 205. [Google Scholar] [CrossRef] [PubMed]
- Mirelman, A.; Shema, S.; Maidan, I.; Hausdorff, J.M. Gait. Handb. Clin. Neurol. 2018, 159, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Oben, P. Understanding the Patient Experience: A Conceptual Framework. J. Patient Exp. 2020, 7, 906–910. [Google Scholar] [CrossRef]
| CASP Critical Appraisal Tool | Allegue, 2022 [49] | Wingham, 2015 [50] | Standen, 2014 [51] | Chen, 2020 [52] |
|---|---|---|---|---|
| Was there a clear statement of the aims of the research? | Yes | Yes | Yes | Yes |
| Is a qualitative methodology appropriate? | Yes | Yes | Yes | Yes |
| Was the research design appropriate to address the aims of the research? | Yes | Yes | Cannot tell | Cannot tell |
| Was the recruitment strategy appropriate to the aims of the research? | Yes | Yes | Cannot tell | Yes |
| Was the data collected in a way that addressed the research issue? | Yes | Cannot tell | Cannot tell | Cannot tell |
| Has the relationship between researcher and participants been adequately considered? | Cannot tell | Cannot tell | Cannot tell | Cannot tell |
| Have ethical issues been taken into consideration? | Cannot tell | Cannot tell | Cannot tell | no |
| Was the data analysis sufficiently rigorous? | Yes | Yes | No | Yes |
| Is there a clear statement of findings? | Cannot tell | Cannot tell | No | Yes |
| How valuable is the research? | Yes | yes | Cannot tell | yes |
| Score: Yes: 1 point; Cannot tell: 0.5 point; No: 0 point. If 9–10 (high quality) If 7.5–9 (moderate quality) If less than 7.5 (low quality) | 8.5 (moderate) | 8 (moderate) | 5 (low quality) | 7.5 (moderate) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazem, H.; Hall, A.; Gomaa, Y.; Mansoubi, M.; Lamb, S.; Dawes, H. The Extent of Evidence Supporting the Effectiveness of Extended Reality Telerehabilitation on Different Qualitative and Quantitative Outcomes in Stroke Survivors: A Systematic Review. Int. J. Environ. Res. Public Health 2023, 20, 6630. https://doi.org/10.3390/ijerph20176630
Lazem H, Hall A, Gomaa Y, Mansoubi M, Lamb S, Dawes H. The Extent of Evidence Supporting the Effectiveness of Extended Reality Telerehabilitation on Different Qualitative and Quantitative Outcomes in Stroke Survivors: A Systematic Review. International Journal of Environmental Research and Public Health. 2023; 20(17):6630. https://doi.org/10.3390/ijerph20176630
Chicago/Turabian StyleLazem, Hatem, Abi Hall, Yasmine Gomaa, Maedeh Mansoubi, Sallie Lamb, and Helen Dawes. 2023. "The Extent of Evidence Supporting the Effectiveness of Extended Reality Telerehabilitation on Different Qualitative and Quantitative Outcomes in Stroke Survivors: A Systematic Review" International Journal of Environmental Research and Public Health 20, no. 17: 6630. https://doi.org/10.3390/ijerph20176630
APA StyleLazem, H., Hall, A., Gomaa, Y., Mansoubi, M., Lamb, S., & Dawes, H. (2023). The Extent of Evidence Supporting the Effectiveness of Extended Reality Telerehabilitation on Different Qualitative and Quantitative Outcomes in Stroke Survivors: A Systematic Review. International Journal of Environmental Research and Public Health, 20(17), 6630. https://doi.org/10.3390/ijerph20176630








