Algerian Workers’ Exposure to Mycotoxins—A Biomonitoring Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Standard Solutions
2.2. Sample Collection
2.3. LC-MS/MS Conditions
2.4. Extraction and Cleanup
2.5. Method Validation
2.6. Statistical Analysis
3. Results and Discussion
3.1. Cleanup Optimization
3.2. Analytical Validation
3.3. Sample Results
3.3.1. Levels of the Mycotoxins and Their Metabolites
| Mycotoxin | Algeria | Chile [24] | Ivory Coast [34] | Nigeria [30] | Portugal [13,25] | Rwanda [28] | South Africa [32] | Spain [29] | |
|---|---|---|---|---|---|---|---|---|---|
| Mycotoxin Prevalence (%) | DON | 0 | 55 | 21 | 0.8 | 30 | 19 | 87 | 23 |
| DON-3-G | 46 | - | - | 5 | 24 | 48 | - | - | |
| DOM-1 | 76 | - | 0 | - | 32 | 24 | - | 53 | |
| ZEN | 91 | 1 | 37 | 0.8 | 57 | 30 | 100 | 40 | |
| α-ZEL | 25 | 8 | - | - | 5 | - | 92 | 43 | |
| OTA | 86 | 1 | - | 28 | 27 | 71 | 96 | 3 | |
| T-2 | 93 | - | - | - | ND | - | - | - | |
| HT-2 | 77 | - | - | - | ND | - | - | - | |
| AFB1 | 19 | 8 | - | - | 2 | 8 | - | - | |
| AFB2 | 10 | - | - | - | 0 | - | - | - | |
| FB1 | 37 | - | 27 | 13.3 | - | 30 | - | - | |
| Mycotoxin Average (µg/L) | DON | - | 60.70 | 10.00 | 2.00 | 0.38 | 18.80 | 4.94 | 9.07 |
| DON-3-G | 13.28 | - | - | 3.50 | 0.25 | 5.88 | - | - | |
| DOM-1 | 47.97 | - | - | - | 0.23 | 35.00 | - | 20.28 | |
| ZEN | 28.59 | 1.10 | - | 0.30 | 1.30 | 1.58 | 0.20 | 6.70 | |
| α-ZEL | 0.43 | 41.80 | - | - | 2.70 | - | 0.25 | 27.44 | |
| OTA | 0.82 | 1.30 | 0.42 | 0.20 | 0.01 | 0.03 | 0.02 | 11.73 | |
| T-2 | 8.37 | - | - | - | ND | - | - | - | |
| HT-2 | 2.05 | - | - | - | ND | - | - | - | |
| AFB1 | 0.82 | 0.30 | - | - | 0.003 | 0.01 | - | - | |
| AFB2 | 1.17 | - | - | - | <LOQ | - | - | - | |
| FB1 | 12.99 | - | 15.30 | 4.60 | 0.24 | 0.01 | - | - |
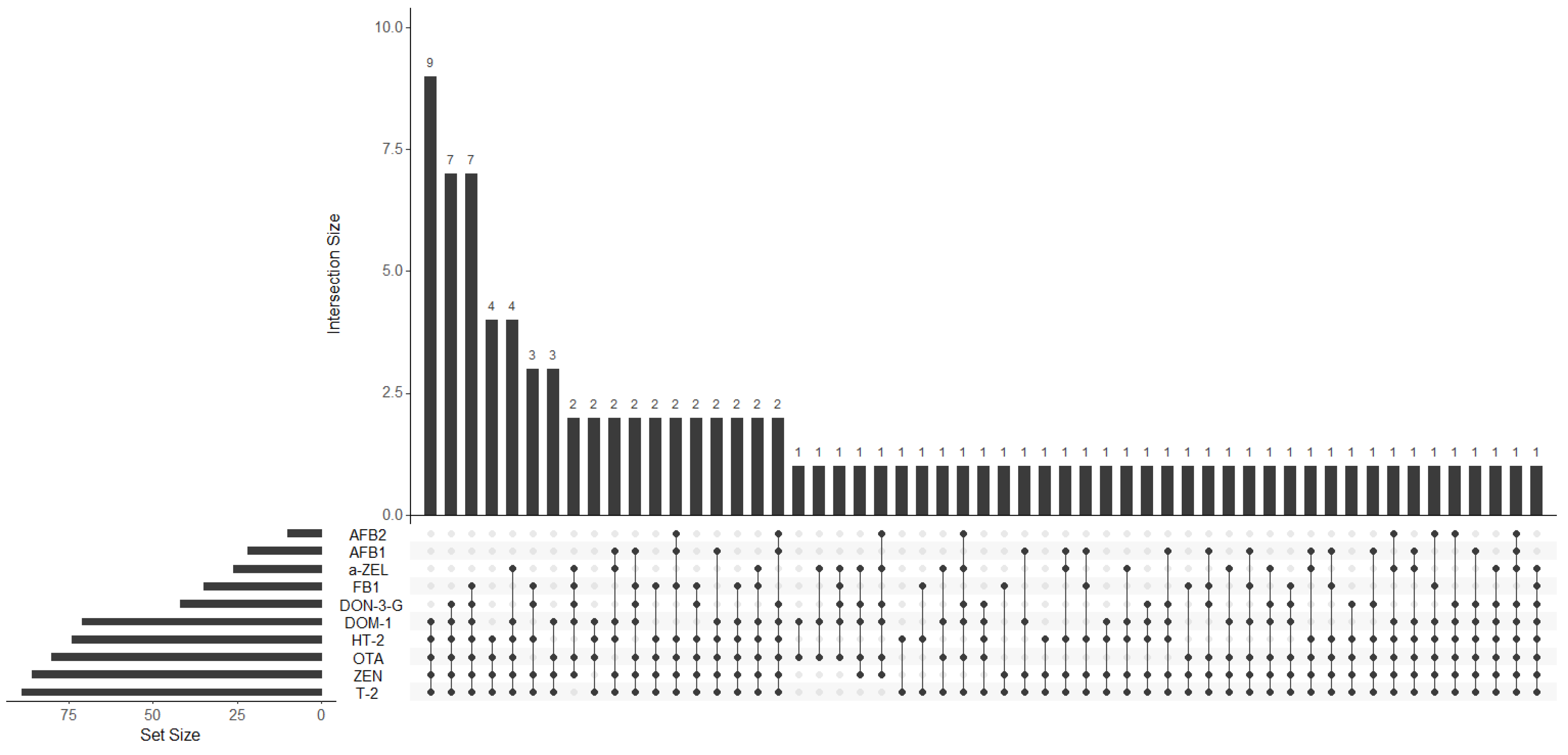
3.3.2. Co-Occurrence
3.3.3. Distribution of Mycotoxins and Their Metabolites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO (World Health Organization). Mycotoxins. Available online: https://www.who.int/news-room/fact-sheets/detail/mycotoxins (accessed on 30 January 2022).
- European Comission. Comission Regulation (EC) No 1881/2006 of 19 December 2006. Setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, L364, 5–24. [Google Scholar]
- U.S. DHHS. Chemical Contaminants, Metals, Natural Toxins & Pesticides > Guidance for Industry: Action Levels for Poisonous or Deleterious Substances in Human Food and Animal Feed; U.S. Food and Drug Administration: Washington, DC, USA, 2000. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-action-levels-poisonous-or-deleterious-substances-human-food-and-animal-feed (accessed on 4 February 2022).
- FAO. Food and Agriculture Organization of the United Nations Worldwide Regulations for Mycotoxins in Food and Feed in 2003; FAO Food and Nutrition Paper 81; FAO: Rome, Italy, 2004. [Google Scholar]
- Mahdjoubi, C.K.; Arroyo-Manzanares, N.; Hamini-Kadar, N.; García-Campaña, A.M.; Mebrouk, K.; Gámiz-Gracia, L. Multi-Mycotoxin Occurrence and Exposure Assessment Approach in Foodstuffs from Algeria. Toxins 2020, 12, 194. [Google Scholar] [CrossRef] [PubMed]
- Zebiri, S.; Mokrane, S.; Verheecke-Vaessen, C.; Choque, E.; Reghioui, H.; Sabaou, N.; Mathieu, F.; Riba, A. Occurrence of ochratoxin A in Algerian wheat and its milling derivatives. Toxin Rev. 2018, 38, 206–211. [Google Scholar] [CrossRef]
- Riba, A.; Matmoura, A.; Mokrane, S.; Mathieu, F.; Sabaou, N. Investigations on aflatoxigenic fungi and aflatoxins contamination in some nuts sampled in Algeria. Afr. J. Microbiol. Res. 2013, 7, 4974–4980. [Google Scholar] [CrossRef][Green Version]
- Tantaoui-Elaraki, A.; Riba, A.; Oueslati, S.; Zinedine, A. Toxigenic fungi and mycotoxin occurrence and prevention in food and feed in northern Africa—A review. World Mycotoxin J. 2018, 11, 385–400. [Google Scholar] [CrossRef]
- Vidal, A.; Mengelers, M.; Yang, S.; De Saeger, S.; De Boevre, M. Mycotoxin Biomarkers of Exposure: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1127–1155. [Google Scholar] [CrossRef]
- Pallarés, N.; Carballo, D.; Ferrer, E.; Rodríguez-Carrasco, Y.; Berrada, H. High-Throughput Determination of Major Mycotoxins with Human Health Concerns in Urine by LC-Q TOF MS and Its Application to an Exposure Study. Toxins 2022, 14, 42. [Google Scholar] [CrossRef]
- Ediage, E.N.; Di Mavungu, J.D.; Song, S.; Wu, A.; Van Peteghem, C.; De Saeger, S. A direct assessment of mycotoxin biomarkers in human urine samples by liquid chromatography tandem mass spectrometry. Anal. Chim. Acta 2012, 741, 58–69. [Google Scholar] [CrossRef]
- Heyndrickx, E.; Sioen, I.; Huybrechts, B.; Callebaut, A.; De Henauw, S.; De Saeger, S. Human biomonitoring of multiple mycotoxins in the Belgian population: Results of the BIOMYCO study. Environ. Int. 2015, 84, 82–89. [Google Scholar] [CrossRef]
- Martins, C.; Vidal, A.; De Boevre, M.; De Saeger, S.; Nunes, C.; Torres, D.; Goios, A.; Lopes, C.; Assunção, R.; Alvito, P. Exposure assessment of Portuguese population to multiple mycotoxins: The human biomonitoring approach. Int. J. Hyg. Environ. Health 2019, 222, 913–925. [Google Scholar] [CrossRef]
- Song, S.; Ediage, E.N.; Wu, A.; De Saeger, S. Development and application of salting-out assisted liquid/liquid extraction for multi-mycotoxin biomarkers analysis in pig urine with high performance liquid chromatography/tandem mass spectrometry. J. Chromatogr. A 2013, 1292, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Dasí-Navarro, N.; Lozano, M.; Llop, S.; Esplugues, A.; Cimbalo, A.; Font, G.; Manyes, L.; Mañes, J.; Vila-Donat, P. Development and Validation of LC-Q-TOF-MS Methodology to Determine Mycotoxin Biomarkers in Human Urine. Toxins 2022, 14, 651. [Google Scholar] [CrossRef] [PubMed]
- Escrivá, L.; Manyes, L.; Font, G.; Berrada, H. Mycotoxin Analysis of Human Urine by LC-MS/MS: A Comparative Extraction Study. Toxins 2017, 9, 330. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Wang, L.; Wang, J.; Tan, Y.; Yu, D.; Chang, X.; Fan, Y.; Zhao, D.; Wang, C.; De Boevre, M.; et al. A QuEChERS-Based Liquid Chromatography-Tandem Mass Spectrometry Method for the Simultaneous Determination of Nine Zearalenone-Like Mycotoxins in Pigs. Toxins 2018, 10, 129. [Google Scholar] [CrossRef]
- Rausch, A.K.; Brockmeyer, R.; Schwerdtle, T. Development and Validation of a QuEChERS-Based Liquid Chromatography Tandem Mass Spectrometry Multi-Method for the Determination of 38 Native and Modified Mycotoxins in Cereals. J. Agric. Food Chem. 2020, 68, 4657–4669. [Google Scholar] [CrossRef]
- Sedova, I.; Kiseleva, M.; Tutelyan, V. Mycotoxins in Tea: Occurrence, Methods of Determination and Risk Evaluation. Toxins 2018, 10, 444. [Google Scholar] [CrossRef]
- Caldeirão, L.; Sousa, J.; Nunes, L.; Godoy, H.T.; Fernandes, J.O.; Cunha, S.C. Herbs and herbal infusions: Determination of natural contaminants (mycotoxins and trace elements) and evaluation of their exposure. Food Res. Int. 2021, 144, 110322. [Google Scholar] [CrossRef]
- Cunha, S.C.; Sá, S.; Fernandes, J.O. Multiple mycotoxin analysis in nut products: Occurrence and risk characterization. Food Chem. Toxicol. 2018, 114, 260–269. [Google Scholar] [CrossRef]
- Cao, X.; Li, X.; Li, J.; Niu, Y.; Shi, L.; Fang, Z.; Zhang, T.; Ding, H. Quantitative determination of carcinogenic mycotoxins in human and animal biological matrices and animal-derived foods using multi-mycotoxin and analyte-specific high performance liquid chromatography-tandem mass spectrometric methods. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2018, 1073, 191–200. [Google Scholar] [CrossRef]
- Warth, B.; Petchkongkaew, A.; Sulyok, M.; Krska, R. Utilising an LC-MS/MS-based multi-biomarker approach to assess mycotoxin exposure in the Bangkok metropolitan area and surrounding provinces. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2014, 31, 2040–2046. [Google Scholar] [CrossRef]
- Foerster, C.; Ríos-Gajardo, G.; Gómez, P.; Muñoz, K.; Cortés, S.; Maldonado, C.; Ferreccio, C. Assessment of Mycotoxin Exposure in a Rural County of Chile by Urinary Biomarker Determination. Toxins 2021, 13, 439. [Google Scholar] [CrossRef]
- Martins, C.; Vidal, A.; De Boevre, M.; De Saeger, S.; Nunes, C.; Torres, D.; Goios, A.; Lopes, C.; Alvito, P.; Assunção, R. Burden of disease associated with dietary exposure to carcinogenic aflatoxins in Portugal using human biomonitoring approach. Food Res. Int. 2020, 134, 109210. [Google Scholar] [CrossRef] [PubMed]
- Huybrechts, B.; Martins, J.C.; Debongnie, P.; Uhlig, S.; Callebaut, A. Fast and sensitive LC-MS/MS method measuring human mycotoxin exposure using biomarkers in urine. Arch. Toxicol. 2015, 89, 1993–2005. [Google Scholar] [CrossRef] [PubMed]
- Vidal, A.; Claeys, L.; Mengelers, M.; Vanhoorne, V.; Vervaet, C.; Huybrechts, B.; De Saeger, S.; De Boevre, M. Humans significantly metabolize and excrete the mycotoxin deoxynivalenol and its modified form deoxynivalenol-3-glucoside within 24 hours. Sci. Rep. 2018, 8, 5255. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.L.; Walsh, J.P.; Renaud, J.B.; McMillan, A.; Rulisa, S.; Miller, J.D.; Reid, G.; Sumarah, M.W. Improved methods for biomarker analysis of the big five mycotoxins enables reliable exposure characterization in a population of childbearing age women in Rwanda. Food Chem. Toxicol. 2021, 147, 111854. [Google Scholar] [CrossRef] [PubMed]
- Carballo, D.; Pallarés, N.; Ferrer, E.; Barba, F.J.; Berrada, H. Assessment of Human Exposure to Deoxynivalenol, Ochratoxin A, Zearalenone and Their Metabolites Biomarker in Urine Samples Using LC-ESI-qTOF. Toxins 2021, 13, 530. [Google Scholar] [CrossRef]
- Ezekiel, C.N.; Warth, B.; Ogara, I.M.; Abia, W.A.; Ezekiel, V.C.; Atehnkeng, J.; Sulyok, M.; Turner, P.C.; Tayo, G.O.; Krska, R.; et al. Mycotoxin exposure in rural residents in northern Nigeria: A pilot study using multi-urinary biomarkers. Environ. Int. 2014, 66, 138–145. [Google Scholar] [CrossRef]
- Gerding, J.; Ali, N.; Schwartzbord, J.; Cramer, B.; Brown, D.L.; Degen, G.H.; Humpf, H.U. A comparative study of the human urinary mycotoxin excretion patterns in Bangladesh, Germany, and Haiti using a rapid and sensitive LC-MS/MS approach. Mycotoxin Res. 2015, 31, 127–136. [Google Scholar] [CrossRef]
- Shephard, G.S.; Burger, H.M.; Gambacorta, L.; Gong, Y.Y.; Krska, R.; Rheeder, J.P.; Solfrizzo, M.; Srey, C.; Sulyok, M.; Visconti, A.; et al. Multiple mycotoxin exposure determined by urinary biomarkers in rural subsistence farmers in the former Transkei, South Africa. Food Chem. Toxicol. 2013, 62, 217–225. [Google Scholar] [CrossRef]
- Abia, W.A.; Warth, B.; Sulyok, M.; Krska, R.; Tchana, A.; Njobeh, P.B.; Turner, P.C.; Kouanfack, C.; Eyongetah, M.; Dutton, M.; et al. Bio-monitoring of mycotoxin exposure in Cameroon using a urinary multi-biomarker approach. Food Chem. Toxicol. 2013, 62, 927–934. [Google Scholar] [CrossRef]
- Kouadio, J.H.; Lattanzio, V.M.; Ouattara, D.; Kouakou, B.; Visconti, A. Assessment of Mycotoxin Exposure in Côte d’ivoire (Ivory Coast) Through Multi-Biomarker Analysis and Possible Correlation with Food Consumption Patterns. Toxicol. Int. 2014, 21, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Blesa, J.; Moltó, J.C.; El Akhdari, S.; Mañes, J.; Zinedine, A. Simultaneous determination of Fusarium mycotoxins in wheat grain from Morocco by liquid chromatography coupled to triple quadrupole mass spectrometry. Food Control 2014, 46, 1–5. [Google Scholar] [CrossRef]
- Rodríguez-Carrasco, Y.; Ruiz, M.J.; Font, G.; Berrada, H. Exposure estimates to Fusarium mycotoxins through cereals intake. Chemosphere 2013, 93, 2297–2303. [Google Scholar] [CrossRef]
- Juan, C.; Ritieni, A.; Mañes, J. Occurrence of Fusarium mycotoxins in Italian cereal and cereal products from organic farming. Food Chem. 2013, 141, 1747–1755. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J. Mycotoxins: Of molds and maladies. Environ. Health Perspect. 2000, 108, A20–A23. [Google Scholar] [CrossRef] [PubMed]
| Mycotoxins | Calibration Range (μg/L) | Correlation Coefficient (r) | LOQ (μg/L) | LOD (μg/L) | Repeatability (RSD %) | |
|---|---|---|---|---|---|---|
| First Level | Second Level | |||||
| DON | 6.75–225 | 0.997 | 6 | 2.05 | 3.1 * | 1.1 * |
| DON-3-G | 6.75–225 | 0.981 | 6 | 2.05 | 1.1 * | 0.2 * |
| DOM-1 | 6.75–225 | 0.997 | 6 | 2.05 | 6.4 * | 0.9 * |
| ZEN | 6.75–225 | 0.997 | 6 | 2.05 | 7.5 * | 26.8 * |
| α-ZEL | 0.25–5 | 0.978 | 0.2 | 0.08 | 6.2 ** | 0.8 ** |
| OTA | 0.25–5 | 0.999 | 0.2 | 0.08 | 22.3 ** | 3.1 ** |
| T-2 | 0.25–5 | 0.997 | 0.2 | 0.08 | 1.9 ** | 2.8 ** |
| HT-2 | 0.25–5 | 0.907 | 0.2 | 0.08 | 17.9 ** | 14.1 ** |
| AFB1 | 0.25–5 | 0.997 | 0.2 | 0.08 | 43.0 ** | 33.3 ** |
| AFB2 | 0.25–5 | 0.995 | 0.2 | 0.08 | 6.0 ** | 6.8 ** |
| FB1 | 0.25–5 | 0.962 | 0.2 | 0.08 | 0.01 ** | 0.02 ** |
| Mycotoxin | Frequency (%) | Average ± RSD (µg/L) | Min (µg/L) | Max (µg/L) |
|---|---|---|---|---|
| DON | 0 | ND | ND | ND |
| DON-3-G | 44 (45.8) | 13.28 ± 6.5 | 6.8 | 37.80 |
| DOM-1 | 73 (76.0) | 47.97 ± 18.8 | 6.9 | 189.1 |
| ZEN | 86 (89.6) | 28.87 ± 20.9 | 7.6 | 126.8 |
| α-ZEL | 23 (24.9) | 0.43 ± 0.2 | 0.3 | 1.0 |
| OTA | 83 (86.4) | 0.82 ± 0.5 | 0.3 | 3.5 |
| T-2 | 89 (92.7) | 8.37 ± 7.6 | 0.3 | 36.3 |
| HT-2 | 74 (77.1) | 2.05 ± 1.9 | 0.3 | 11.0 |
| AFB1 | 18 (18.8) | 0.82 ± 0.9 | 0.3 | 4.7 |
| AFB2 | 10 (10.4) | 1.17 ± 1.5 | 0.3 | 5.8 |
| FB1 | 35 (36.5) | 12.99 ± 17.1 | 0.5 | 96.2 |
| Rural (13) | Urban (82) | p-Value | ||||
|---|---|---|---|---|---|---|
| Positive Samples (%) | Average (µg/L) | Positive Samples (%) | Average (µg/L) | Frequency a | Concentration b | |
| DON-3-G | 7 (53.8) | 11.71 | 36 (43.9) | 13.66 | 0.559 | 0.834 |
| DOM-1 | 13 (100) | 88.58 | 59 (71.9) | 39.31 | 0.034 | <0.01 |
| ZEN | 8 (61.5) | 20.32 | 77 (93.9) | 29.97 | 0.004 | 0.155 |
| α-ZEL | 10 (76.9) | 0.38 | 13 (15.85) | 0.48 | <0.01 | 0.148 |
| OTA | 13 (100) | 0.92 | 69 (84.1) | 0.82 | 0.203 | 0.793 |
| T-2 | 9 (69.2) | 1.37 | 81 (98.8) | 9.01 | <0.01 | <0.01 |
| HT-2 | 5 (38.5) | 2.06 | 67 (81.7) | 2.08 | 0.01 | 0.848 |
| AFB1 | 0 | <LOQ | 18 (22.0) | 0.82 | 0.12 | - |
| AFB2 | 2 (15.4) | 0.85 | 8 (9.8) | 1.25 | 0.623 | 0.533 |
| FB1 | 3 (23.1) | 1.37 | 33 (40.2) | 13.67 | 0.123 | 0.067 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendes, M.I.; Cunha, S.C.; Rebai, I.; Fernandes, J.O. Algerian Workers’ Exposure to Mycotoxins—A Biomonitoring Study. Int. J. Environ. Res. Public Health 2023, 20, 6566. https://doi.org/10.3390/ijerph20166566
Mendes MI, Cunha SC, Rebai I, Fernandes JO. Algerian Workers’ Exposure to Mycotoxins—A Biomonitoring Study. International Journal of Environmental Research and Public Health. 2023; 20(16):6566. https://doi.org/10.3390/ijerph20166566
Chicago/Turabian StyleMendes, Marta I., Sara C. Cunha, Iméne Rebai, and José O. Fernandes. 2023. "Algerian Workers’ Exposure to Mycotoxins—A Biomonitoring Study" International Journal of Environmental Research and Public Health 20, no. 16: 6566. https://doi.org/10.3390/ijerph20166566
APA StyleMendes, M. I., Cunha, S. C., Rebai, I., & Fernandes, J. O. (2023). Algerian Workers’ Exposure to Mycotoxins—A Biomonitoring Study. International Journal of Environmental Research and Public Health, 20(16), 6566. https://doi.org/10.3390/ijerph20166566






