Effects of Exercise and Sports Intervention and the Involvement Level on the Mineral Health of Different Bone Sites in the Leg, Hip, and Spine: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria for the Studies
2.3. Data Extraction
2.4. Assessment of Methodological Quality and Risk of Bias
2.5. Statistical Analysis
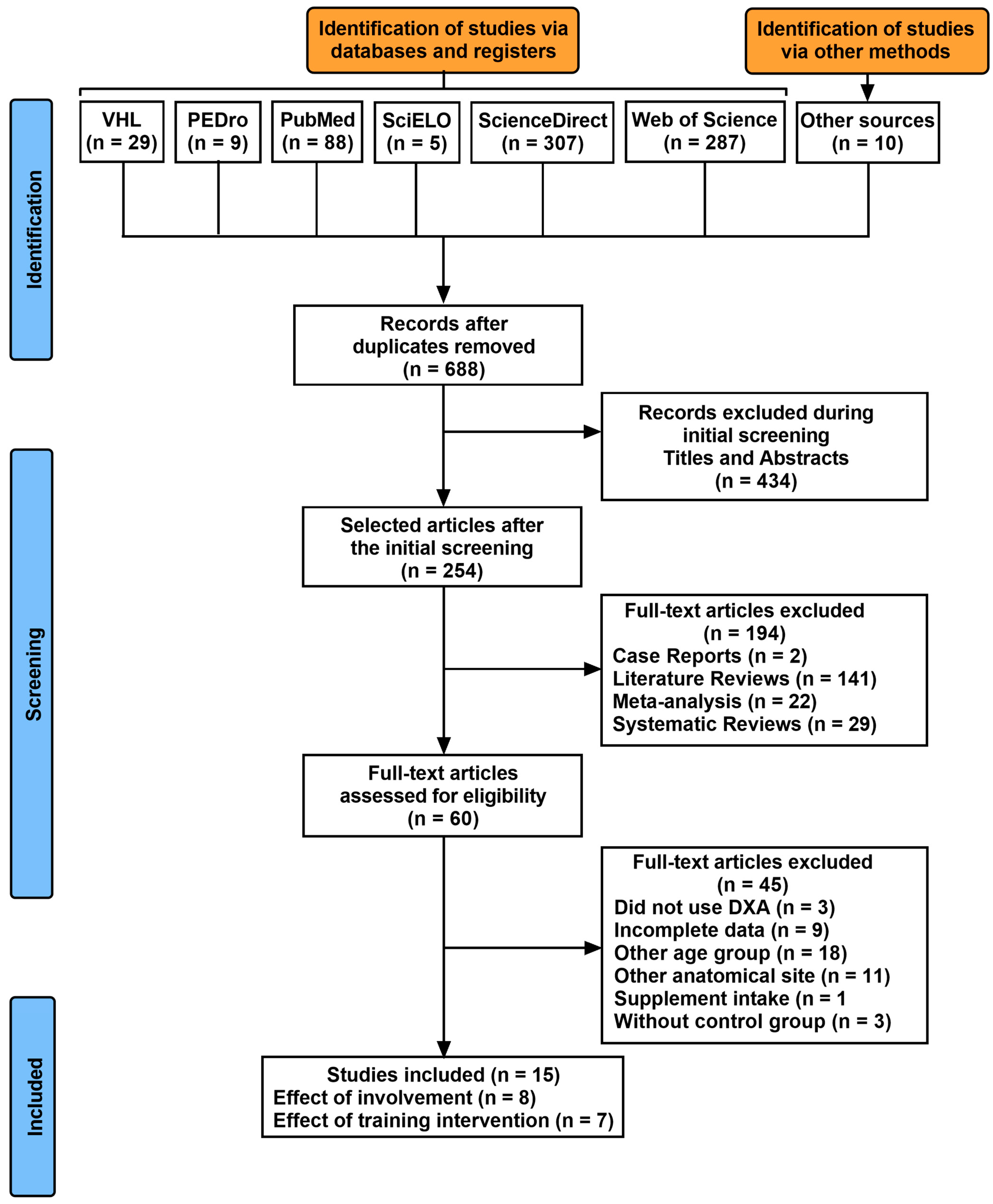
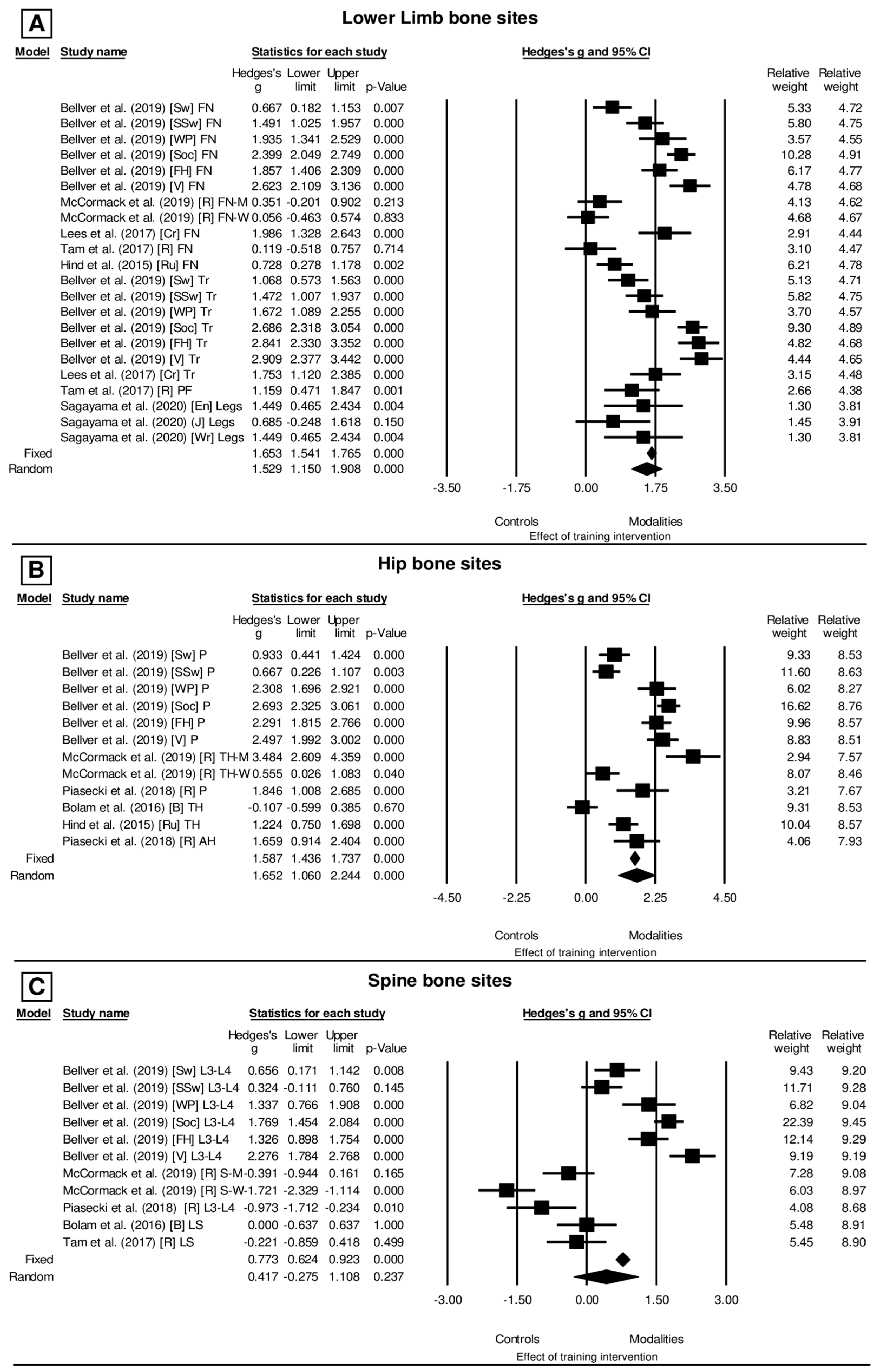
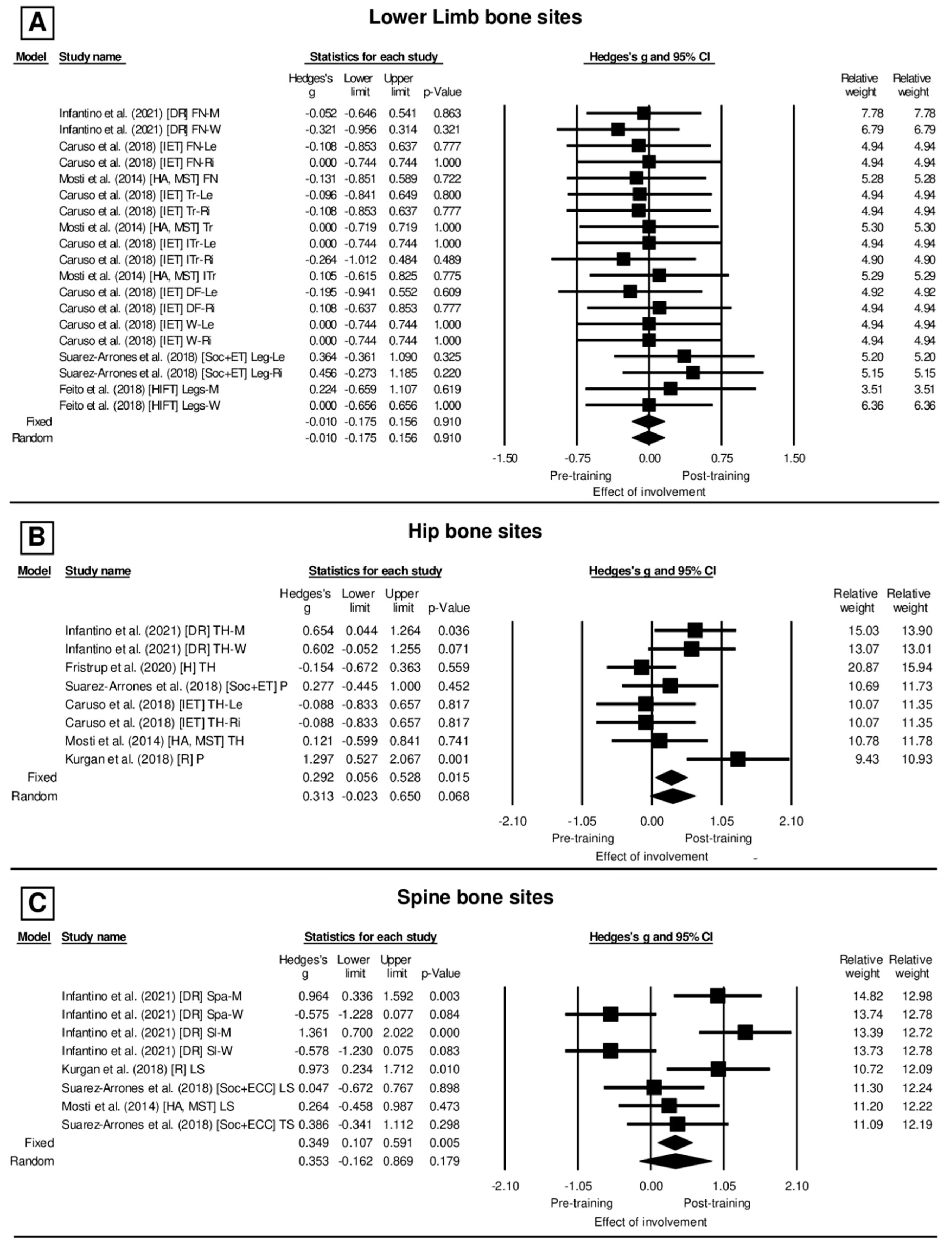
3. Results
| Study | Participants | Exercise/Sport (Bone Site) | BMD (g/cm2) | Methodological Quality | |
|---|---|---|---|---|---|
| Athlete | Control | Points | |||
| Classification | |||||
| Bellver et al. | 204 elite female athletes | Swimming (FN) | 0.994 ± 0.100 | 0.903 ± 0.140 | 4 |
| and 126 controls | Synchronized Swimming (FN) | 1.103 ± 0.090 | 0.903 ± 0.140 | Moderate | |
| (21.5 ± 4.6 years). | Water Polo (FN) | 1.172 ± 0.120 | 0.903 ± 0.140 | quality | |
| Soccer (FN) | 1.240 ± 0.140 | 0.903 ± 0.140 | |||
| Field Hockey (FN) | 1.155 ± 0.110 | 0.903 ± 0.140 | |||
| Volleyball (FN) | 1.272 ± 0.140 | 0.903 ± 0.140 | |||
| Swimming (Tr) | 0.811 ± 0.08 | 0.677 ± 0.13 | |||
| Synchronized Swimming (Tr) | 0.865 ± 0.11 | 0.677 ± 0.13 | |||
| Water Polo (Tr) | 0.889 ± 0.08 | 0.677 ± 0.13 | |||
| Soccer (Tr) | 1.039 ± 0.14 | 0.677 ± 0.13 | |||
| Field Hockey (Tr) | 1.030 ± 0.09 | 0.677 ± 0.13 | |||
| Volleyball (Tr) | 1.048 ± 0.11 | 0.677 ± 0.13 | |||
| Swimming (P) | 1.019 ± 0.110 | 0.924 ± 0.100 | |||
| Synchronized Swimming (P) | 0.991 ± 0.100 | 0.924 ± 0.100 | |||
| Water Polo (P) | 1.149 ± 0.060 | 0.924 ± 0.100 | |||
| Soccer (P) | 1.231 ± 0.130 | 0.924 ± 0.100 | |||
| Field Hockey (P) | 1.185 ± 0.160 | 0.924 ± 0.100 | |||
| Volleyball (P) | 1.184 ± 0.120 | 0.924 ± 0.100 | |||
| Swimming (L3–L4) | 1.161 ± 0.140 | 1.057 ± 0.160 | |||
| Synchronized Swimming (L3–L4) | 1.107 ± 0.110 | 1.057 ± 0.160 | |||
| Water Polo (L3–L4) | 1.265 ± 0.090 | 1.057 ± 0.160 | |||
| Soccer (L3–L4) | 1.341 ± 0.160 | 1.057 ± 0.160 | |||
| Field Hockey (L3–L4) | 1.258 ± 0.100 | 1.057 ± 0.160 | |||
| Volleyball (L3–L4) | 1.431 ± 0.180 | 1.057 ± 0.160 | |||
| Lees et al. | 26 elite male fast bowlers and 26 | Cricket Fast bowlers (FN) | 2.138 ± 0.185 | 1.715 ± 0.232 | 4 |
| normally active controls | Cricket Fast bowlers (Tr) | 1.811 ± 0.161 | 1.469 ± 0.219 | Moderate | |
| (24.3 ± 4.2 years) | quality | ||||
| Tam | 15 elite male Kenyan runners | Running (FN) | 0.945 ± 0.166 | 0.927 ± 0.135 | 4 |
| et al. | (24.4 ± 4.7 years) and 23 controls | Running (PF) | 1.265 ± 0.184 | 1.074 ± 0.145 | Moderate |
| (29.0 ± 4.1 years) | Running (LS) | 1.009 ± 0.166 | 1.040 ± 0.116 | quality | |
| Hind | 52 male rugby players | Rugby (FN) | 1.325 ± 0.200 | 1.178 ± 0.200 | 4 |
| et al. | (26.6 ± 4.4 years) and 32 | Rugby (TH) | 1.442 ± 0.200 | 1.195 ± 0.200 | Moderate |
| controls (25.0 ± 3.9 years). | quality | ||||
| Piasecki | 15 female long-distance runners | Running (P) | 1.140 ± 0.020 | 1.11 ± 0.01 | 5 |
| et al. | (eumenorrheic) and non-athletic | Running (L3–L4) | 1.16 0± 0.030 | 1.19 ± 0.03 | Good |
| controls (n = 15) (17 ± 42 years) | quality | ||||
| Bolam | 30 male boxers (30.1 ± 6.4 years), | Boxing (TH) | 1.045 ± 0.134 | 1.059 ± 0.124 | 5 |
| et al. | and 32 non-boxing active | Boxing (LS) | 1.131 ± 0.128 | 1.131 ± 0.124 | Good |
| controls (30.7 ± 6.1 years) | quality | ||||
| McCormack | 60 runners (age): 27 male (age 19.7 | Male Runners (FN) | 0.934 ± 0.031 | 0.866 ± 0.280 | 4 |
| et al. | ± 1.2) and 33 female (age 20.3 ± | Female Runners (FN) | 0.921 ± 0.024 | 0.910 ± 0.300 | Moderate |
| 1.8); 47 Control: 23 male (age 20.0 | Male Runners (TH) | 1.062 ± 0.03 | 0.959 ± 0.028 | quality | |
| ± 0.8) and 24 female (age 19.8 ± 0.6) | Female Runners (TH) | 1.039 ± 0.024 | 1.024 ± 0.03 | ||
| Male Runners (S-M) | 0.912 ± 0.029 | 0.923 ± 0.026 | |||
| Female Runners (S-W) | 1.002 ± 0.023 | 1.046 ± 0.028 | |||
| Sagayama | 33 college athletes (aged 18 ± 22) | Wrestler (Legs) | 1.422 ± 0.09 | 1.279 ± 0.100 | 4 |
| et al. | years): 11 male wrestlers, 9 judo, | Judo (Legs) | 1.346 ± 0.086 | 1.279 ± 0.100 | Moderate |
| 13 endurance athletes, | Endurance Exercise (Legs) | 1.262 ± 0.097 | 1.279 ± 0.100 | quality | |
| and 8 control | |||||
| Study | Participants | Exercises/Sport (Bone Site) | BMD (g/cm2) | Methodological Quality | |
|---|---|---|---|---|---|
| Pre-Training | Post-Training | Points Classification | |||
| Caruso et al. | 13 healthy subjects, 2 men and 11 women (29.4 ± 12 years). Study Time: 30 training sessions in 70 ± 6.3 days (range 58–84 days. 30 healthy women (22 ± 2 years). Study Time: 12 weeks | Inertial Exercise Trainer (FN-Le) Inertial Exercise Trainer (FN-Ri) Inertial Exercise Trainer (Tr-Le) Inertial Exercise Trainer (Tr-Ri) Inertial Exercise Trainer (ITr-Le) Inertial Exercise Trainer (ITr-Ri) Inertial Exercise Trainer (DF-Le) Inertial Exercise Trainer (DF-Ri) Inertial Exercise Trainer (W-Le) Inertial Exercise Trainer (W-Ri) Inertial Exercise Trainer (TH-Le) Inertial Exercise Trainer (TH-Ri) | 0.990 ± 0.180 0.990 ± 0.110 0.810 ± 0.110 0.800 ± 0.090 1.270 ± 0.180 1.240 ± 0.110 1.190 ± 0.110 1.180 ± 0.180 1.000 ± 0.300 1.000 ± 0.300 1.070 ± 0.110 1.060 ± 0.110 | 1.010 ± 0.180 0.990 ± 0.180 0.820 ± 0.090 0.810 ± 0.090 1.270 ± 0.180 1.270 ± 0.110 1.220 ± 0.180 1.160 ± 0.180 1.000 ± 0.200 1.000 ± 0.300 1.080 ± 0.110 1.070 ± 0.110 | 5 Good quality |
| Mosti et al. | 14 healthy male professional soccer players (17.5 ± 0.8 years). Study Time: 27 weeks | HA strength training (FN) HA, maximal strength training (Tr) HA maximal strength training (ITr) HA, maximal strength training (TH) HA maximal strength training (LS) | 0.851 ± 0.086 0.747 ± 0.054 1.149 ± 0.103 0.977 ± 0.081 1.023 ± 0.073 | 0.863 ± 0.092 0.747 ± 0.059 1.138 ± 0.100 0.967 ± 0.079 1.002 ± 0.081 | 4 Moderate quality |
| Suarez-Arrones et al. | EOT in elite soccer players (Leg-Le) EOT in elite soccer players (Leg-Ri) EOT in elite soccer players (P) EOT in elite soccer players (TS) | 1.410 ± 0.080 1.400 ± 0.090 1.600 ± 0.210 1.230 ± 0.220 | 1.380 ± 0.080 1.360 ± 0.080 1.540 ± 0.210 1.220 ± 0.190 | 5 Good quality | |
| Feito et al. Kurgan et al. Fristrup et al. | 26 recreationally active adult men (n = 9, 34.2 ± 9.1 years) and women (n = 17, 36.4 ± 7.9 years). Study Time: 16 weeks 15 elite women heavyweight rowers (27.0 ± 0.8 years). Study Time: 42 weeks 28 Recreationally Handball training 14 men and 14 women age (24.1 ± 2.6 years). Study Time: 12 weeks | High Intensity functional training: Multimodal exercises (Leg-W) High Intensity functional training: Multimodal exercises (Leg-M) Heavy-weight row (P) Heavy-weight row (LS) Recreationally Handball training (TH) | 1.250 ± 0.090 1.530 ± 0.090 1.260 ± 0.030 1.230 ± 0.030 1.099 ± 0.115 | 1.250 ± 0.090 1.510 ± 0.080 1.220 ± 0.030 1.200 ± 0.030 1.117 ± 0.115 | 4 Moderate quality 5 Good quality 7 Excellent quality |
| Infantino et al. | 21 male and 18 distance females Runners (19.5 ± 0.8 years). Study Time: 12 months | DR (TH-M) DR (TH-F) DR (FN-M) DR (FN-F) DR (Sl-M) DR (Sl-F) DR (Spa-M) DR (Spa-F) | 1.065 ± 0.037 1.057 ± 0.033 0.944 ± 0.039 0.930 ± 0.035 0.783 ± 0.025 0.736 ± 0.022 0.956 ± 0.029 0.970 ± 0.026 | 1.089± 0.035 1.037 ± 0.032 0.942 ± 0.036 0.919 ± 0.032 0.817 ± 0.024 0.723 ± 0.022 0.984 ± 0.028 0.955 ± 0.025 | 4 Moderate quality |
4. Discussion
4.1. Evidence of BMD Alteration According to Exercise and Sport Involvement
4.2. Evidence of BMD Alteration after Different Interventions Planned with Exercise and Sport
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vicente-Rodríguez, G. How Does Exercise Affect Bone Development during Growth? Sports Med. 2006, 36, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Min, S.K.; Oh, T.; Kim, S.H.; Cho, J.; Chung, H.Y.; Park, D.H.; Kim, C.S. Position Statement: Exercise Guidelines to Increase Peak Bone Mass in Adolescents. J. Bone Metab. 2019, 26, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Robling, A.G.; Castillo, A.B.; Turner, C.H. Biomechanical and Molecular Regulation of Bone Remodeling. Annu. Rev. Biomed. Eng. 2006, 8, 455–498. [Google Scholar] [CrossRef] [PubMed]
- Troy, K.L.; Mancuso, M.E.; Butler, T.A.; Johnson, J.E. Exercise Early and Often: Effects of Physical Activity and Exercise on Women’s Bone Health. Int. J. Environ. Res. Public Health 2018, 15, 878. [Google Scholar] [CrossRef]
- Bellver, M.; Del Rio, L.; Jovell, E.; Drobnic, F.; Trilla, A. Bone Mineral Density and Bone Mineral Content among Female Elite Athletes. Bone 2019, 127, 393–400. [Google Scholar] [CrossRef]
- Vlachopoulos, D.; Barker, A.R.; Ubago-Guisado, E.; Fatouros, I.G.; Knapp, K.M.; Williams, C.A.; Gracia-Marco, L. Longitudinal Adaptations of Bone Mass, Geometry, and Metabolism in Adolescent Male Athletes: The PRO-BONE Study. J. Bone Miner. Res. 2017, 32, 2269–2277. [Google Scholar] [CrossRef]
- Magkos, F.; Kavouras, S.A.; Yannakoulia, M.; Karipidou, S.M.; Sidossi, S. The Bone Response to Non-Weight-Bearing Exercise Is Sport-, Site-, and Sex Specific. Clin. J. Sport Med. 2007, 17, 123–128. [Google Scholar] [CrossRef]
- Villareal, D.T.; Aguirre, L.; Gurney, A.B.; Waters, D.L.; Sinacore, D.R.; Colombo, E.; Armamento-Villareal, R.; Qualls, C. Aerobic or Resistance Exercise, or Both, in Dieting Obese Older Adults. N. Eng. J. Med. 2017, 376, 1943–1955. [Google Scholar] [CrossRef]
- Guadalupe-Grau, A.; Fuentes, T.; Guerra, B.; Calbet, J.A. Exercise and Bone Mass in Adults. Sports Med. 2009, 39, 439–468. [Google Scholar] [CrossRef]
- Piasecki, J.; Ireland, A.; Piasecki, M.; Cameron, J.; McPhee, J.S.; Degens, H. The Strength of Weight-bearing Bones is Similar in Amenorrheic and Eumenorrheic Elite Long-Distance Runners. Scand. J. Med. Sci. Sports 2018, 28, 1559–1568. [Google Scholar] [CrossRef]
- Lane, N.E. Epidemiology, Etiology, and Diagnosis of Osteoporosis. Am. J. Obstet. Gynecol. 2006, 194, S3–S11. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.M.; Gordon, C.M.; Janz, K.F.; Kalkwarf, H.J.; Lappe, J.M.; Lewis, R.; O’Karma, M.; Wallace, T.C.; Zemel, B.S. The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: A systematic review and implementation recommendations. Osteoporos. Int. 2016, 27, 1281–1386. [Google Scholar] [CrossRef] [PubMed]
- Hinton, P.S.; Nigh, P.; Thyfault, J. Effectiveness of Resistance Training or Jumping-exercise to Increase Bone Mineral Density in Men with Low Bone Mass: A 12-month Randomized, Clinical Trial. Bone 2015, 79, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Winters-Stone, K.M.; Snow, C.M. Site-Specific Response of Bone to Exercise in Premenopausal Women. Bone 2006, 39, 1203–1209. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, B.R.; Pimenta, L.D.; Massini, D.A.; Dos Santos, D.; Siqueira, L.; Simionato, A.R.; Dos Santos, L.G.A.; Neiva, C.M.; Pessôa Filho, D.M. Muscle Strength and Regional Lean Body Mass Influence on Mineral Bone Health in Young Male Adults. PLoS ONE 2018, 13, E0191769. [Google Scholar] [CrossRef]
- Makovey, J.; Naganathan, V.; Sambrook, P. Gender Differences in Relationships between Body Composition Components, their Distribution and Bone Mineral Density: A Cross-Sectional Opposite Sex Twin Study. Osteoporos. Int. 2005, 16, 1495–1505. [Google Scholar] [CrossRef][Green Version]
- Taaffe, D.R.; Cauley, J.A.; Danielson, M.; Nevitt, M.C.; Lang, T.F.; Bauer, D.C.; Harris, T.B. Race and Sex Effects on the Association between Muscle Strength, Soft Tissue, and Bone Mineral Density in Healthy Elders: The Health, Aging, and Body Composition Study. J. Bone Miner. Res. 2001, 16, 1343–1352. [Google Scholar] [CrossRef]
- Edwards, M.H.; Ward, K.A.; Ntani, G.; Parsons, C.; Thompson, J.; Sayer, A.A.; Dennison, E.M.; Cooper, C. Lean Mass and Fat Mass have Differing Associations with Bone Microarchitecture Assessed by High Resolution Peripheral Quantitative Computed Tomography in Men and Women from the Hertfordshire Cohort Study. Bone 2015, 81, 145–151. [Google Scholar] [CrossRef][Green Version]
- Sharir, A.; Stern, T.; Rot, C.; Shahar, R.; Zelzer, E. Muscle Force Regulates Bone Shaping for Optimal Load-Bearing Capacity during Embryogenesis. Development 2011, 138, 3247–3259. [Google Scholar] [CrossRef]
- Hamrick, M.W.; Mcneil, P.L.; Patterson, S. L Role of Muscle-Derived Growth Factors in Bone Formation. J. Musculoskelet. Neuronal Interact. 2010, 10, 64. [Google Scholar]
- Kaji, H. Interaction between Muscle and Bone. J. Bone Metab. 2014, 21, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Hind, K.; Truscott, J.G.; Evans, J.A. Low Lumbar Spine Bone Mineral Density in Both Male and Female Endurance Runners. Bone 2006, 39, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 372, n71. [Google Scholar] [CrossRef]
- Guimarães, B.R.; Pimenta, L.D.; Massini, D.A.; Santos, D.; Siqueira, L.O.C.; Simionato, A.R.; dos Santos, L.G.A.; Neiva, C.M.; Pessoa Filho, D.M. Muscular Strength and Regional Lean Mass Influence Bone Mineral Health among Young Females. Rev. Bras. Med. Esporte 2018, 24, 186–191. [Google Scholar] [CrossRef]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro Scale for Rating Quality of Randomized Controlled Trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef]
- Kümmel, J.; Kramer, A.; Giboin, L.S.; Gruber, M. Specificity of Balance Training in Healthy Individuals: A Systematic Review and Meta-Analysis. Sports Med. 2016, 46, 1261–1271. [Google Scholar] [CrossRef]
- Grgic, J.; Lazinica, B.; Mikulic, P.; Krieger, J.W.; Schoenfeld, B.J. The Effects of Short Versus Long Inter-Set Rest Intervals in Resistance Training on Measures of Muscle Hypertrophy: A Systematic Review. Eur. J. Sport Sci. 2017, 17, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, J.A. Qualitative descriptors of strength of association and effect size. J. Soc. Serv. Res. 1996, 21, 37–59. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying Heterogeneity in a Meta-Analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Su, L.; Fu, J.; Sun, S.; Zhao, G.; Cheng, W.; Dou, C.; Quan, M. Effects of HIIT and MICT on Cardiovascular Risk Factors in Adults with Overweight and/or Obesity: A Meta-Analysis. PLoS ONE 2019, 14, e0210644. [Google Scholar] [CrossRef]
- Grgic, J.; Diaz-Lara, F.J.; Coso, J.D.; Duncan, M.J.; Tallis, J.; Pickering, C.; Schoenfeld, B.J.; Mikulic, P. The Effects of Caffeine Ingestion on Measures of Rowing Performance: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 434. [Google Scholar] [CrossRef] [PubMed]
- Olmedillas, H.; González-Agüero, A.; Moreno, L.A.; Casajus, J.A.; Vicente-Rodríguez, G. Cycling and Bone Health: A Systematic Review. BMC Med. 2012, 10, 168. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Bruton, A.; Gónzalez-Agüero, A.; Gómez-Cabello, A.; Casajús, J.A.; Vicente-Rodríguez, G. Is Bone Tissue Really Affected by Swimming? A Systematic Review. PLoS ONE 2013, 8, e70119. [Google Scholar] [CrossRef] [PubMed]
- Fehling, P.C.; Alekel, L.; Clasey, J.; Rector, A.; Stillman, R.J. A Comparison of Bone Mineral Densities among Female Athletes in Impact Loading and Active Loading Sports. Bone 1995, 17, 205–210. [Google Scholar] [CrossRef]
- Mudd, L.M.; Fornetti, W.; Pivarnik, J.M. Bone Mineral Density in Collegiate Female Athletes: Comparisons among Sports. J. Athl. Train. 2007, 42, 403–408. [Google Scholar]
- Tenforde, A.S.; Fredericson, M. Influence of Sports Participation on Bone Health in the Young Athlete: A Review of the Literature. PM&R 2011, 3, 861–867. [Google Scholar] [CrossRef]
- Akgül, S.; Kanbur, N.; Cinemre, Ş.A.; Karabulut, E.; Derman, O. The Effect of Swimming and Type of Stroke on Bone Metabolism in Competitive Adolescent Swimmers: A Pilot Study. Turk. J. Med. Sci. 2015, 45, 827–832. [Google Scholar] [CrossRef]
- Massini, D.A.; de Souza Martins, N.D.; de Oliveira, T.P.; Macedo, A.G.; Castro, E.A.; Almeida, T.A.; Santos, F.J.; Espada, M.C.; Pessôa Filho, D.M. The effect of the exercise environment and the level of involvement on bone mineral health. J. Bone Miner. Metab. 2023, 41, 113–123. [Google Scholar] [CrossRef]
- Lees, M.J.; Beggs, C.B.; Barlow, M.J.; Rutherford, Z.H.; Bansil, K.; Gannon, L.; Hind, K. Bone Density and Cross-Sectional Geometry of the Proximal Femur Are Bilaterally Elevated in Elite Cricket Fast Bowlers. J. Clin. Densitom. 2018, 21, 399–405. [Google Scholar] [CrossRef]
- Ubago-Guisado, E.; Sánchez Sánchez, J.; Vila Maldonado, S.; Gallardo, L. Effects of Zumba® and Aquagym on Bone Mass in Inactive Middle-Aged Women. Medicina 2019, 55, 23. [Google Scholar] [CrossRef]
- Bolam, K.A.; Skinner, T.L.; Jenkins, D.G.; Galvão, D.A.; Taaffe, D.R. The Osteogenic Effect of Impact-Loading and Resistance Exercise on Bone Mineral Density in Middle-Aged and Older Men: A Pilot Study. Gerontology 2015, 62, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Sagayama, H.; Kondo, E.; Tanabe, Y.; Ohnishi, T.; Yamada, Y.; Takahashi, H. Bone mineral density in male weight-classified athletes is higher than that in male endurance-athletes and non-athletes. Clin. Nutr. 2020, 36, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Schoenau, E. From mechanostat theory to development of the functional muscle-bone-unit. J. Musculoskelet. Neuronal Interact. 2005, 5, 232–238. [Google Scholar]
- Tam, N.; Santos-Concejero, J.; Tucker, R.; Lamberts, R.P.; Micklesfield, L.K. Bone Health in Elite Kenyan Runners. J. Sports Sci. 2018, 36, 456–461. [Google Scholar] [CrossRef]
- McCormack, W.P.; Shoepe, T.C.; LaBrie, J.; Almstedt, H.C. Bone mineral density, energy availability, and die-tary restraint in collegiate cross-country runners and non-running controls. Eur. J. Appl. Physiol. 2019, 119, 1747–1756. [Google Scholar] [CrossRef] [PubMed]
- Milgrom, C.; Finestone, A.; Simkin, A.; Ekenman, I.; Mendelson, S.; Millgram, M.; Nyska, M.; Larsson, E.; Burr, D. In-vivo Strain Measurements to Evaluate the Strengthening Potential of Exercises on the Tibial Bone. J. Bone Jt. Surg. Br. 2000, 82, 591–594. [Google Scholar] [CrossRef]
- Dengel, D.R.; Keller, K.A.; Stanforth, P.R.; Oliver, J.M.; Carbuhn, A.; Bosch, T.A. Body Composition and Bone Mineral Density of Division 1 Collegiate Track and Field Athletes, a Consortium of College Athlete Research (C-CAR) Study. J. Clin. Densitom. 2020, 23, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Heikura, I.A.; Uusitalo, A.L.T.; Stellingwerff, T.; Bergland, D.; Mero, A.A.; Burke, L.M. Low Energy Availability Is Difficult to Assess but Outcomes Have Large Impact on Bone Injury Rates in Elite Distance Athletes. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Duplanty, A.A.; Levitt, D.E.; Hill, D.W.; McFarlin, B.K.; DiMarco, N.M.; Vingren, J.L. Resistance Training Is Associated with Higher Bone Mineral Density among Young Adult Male Distance Runners Independent of Physiological Factors. J. Strength Cond. Res. 2018, 32, 1594–1600. [Google Scholar] [CrossRef]
- Kurgan, N.; Logan-Sprenger, H.; Falk, B.; Klentrou, P. Bone and Inflammatory Responses to Training in Female Rowers over an Olympic Year. Med. Sci. Sports Exerc. 2018, 50, 1810–1817. [Google Scholar] [CrossRef]
- Infantino, N.A.; McCormack, W.P.; Almstedt, H.C. Bone mineral density and hip structure changes over one-year in collegiate distance runners and non-athlete controls. Bone Rep. 2021, 14, 101056. [Google Scholar] [CrossRef]
- Suarez-Arrones, L.; Saez de Villarreal, E.; Núñez, F.J.; Di Salvo, V.; Petri, C.; Buccolini, A.; Maldonado, R.A.; Torreno, N.; Mendez-Villanueva, A. In-season Eccentric-overload Training in Elite Soccer Players: Effects on Body Composition, Strength and Sprint Performance. PLoS ONE 2018, 13, e0205332. [Google Scholar] [CrossRef] [PubMed]
- Arija-Blázquez, A.; Ceruelo-Abajo, S.; Díaz-Merino, M.S.; Godino-Durán, J.A.; Martínez-Dhier, L.; Martin, J.L.; Florensa-Vila, J. Effects of Electromyostimulation on Muscle and Bone in Men with Acute Traumatic Spinal Cord Injury: A randomized Clinical Trial. J. Spinal Cord Med. 2014, 37, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Vainionpää, A.; Korpelainen, R.; Sievänen, H.; Vihriälä, E.; Leppäluoto, J.; Jämsä, T. Effect of Impact Exercise and its Intensity on Bone Geometry at Weight-bearing Tibia and Femur. Bone 2007, 40, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, L.D.; Massini, D.A.; Santos, D.D.; Vasconcelos, C.M.T.; Simionato, A.R.; Gomes, L.A.T.; Guimarães, B.R.; Neiva, C.M.; Pessôa Filho, D.M. Bone Health, Muscle Strength and Lean Mass: Relationships and Exercise Recommendations. Rev. Bras. Med. Esporte 2019, 25, 245–251. [Google Scholar] [CrossRef]
- Caruso, J.F.; Voor, M.; Jaggers, J. Musculoskeletal Outcomes from Chronic High-Speed, High-Impulse Resistance Exercise. Int. J. Sports Med. 2018, 39, 791–801. [Google Scholar] [CrossRef]
- Mosti, M.P.; Kaehler, N.; Stunes, A.K.; Hoff, J.; Syversen, U. Maximal Strength Training in Postmenopausal Women with Osteoporosis or Osteopenia. J. Strength Cond. Res. 2013, 27, 2879–2886. [Google Scholar] [CrossRef]
- Feito, Y.; Hoffstetter, W.; Serafini, P.; Mangine, G. Changes in Body Composition, Bone Metabolism, Strength, and Skill-specific Performance Resulting from 16-weeks of HIFT. PLoS ONE 2018, 13, e0198324. [Google Scholar] [CrossRef]
- Massini, D.A.; Nedog, F.H.; de Oliveira, T.P.; Almeida, T.A.F.; Santana, C.A.A.; Neiva, C.M.; Macedo, A.G.; Castro, E.A.; Espada, M.C.; Santos, F.J.; et al. The Effect of Resistance Training on Bone Mineral Density in Older Adults: A Systematic Review and Meta-Analysis. Healthcare 2022, 10, 1129. [Google Scholar] [CrossRef]
- Fristrup, B.; Krustrup, P.; Andersen, J.L.; Hornstrup, T.; Løwenstein, F.T.; Larsen, M.A.; Helge, J.W.; Póvoas, S.C.A.; Aagaard, P. Effects of small-sided recreational team handball training on mechanical muscle function, body composition and bone miner-alization in untrained young adults-A randomized controlled trial. PLoS ONE 2020, 15, e0241359. [Google Scholar] [CrossRef]
- Izard, R.M.; Fraser, W.D.; Negus, C.; Sale, C.; Greeves, J.P. Increased Density and Periosteal Expansion of the Tibia in Young Adult Men Following Short-Term Arduous Training. Bone 2016, 88, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Ravnholt, T.; Tybirk, J.; Jørgensen, N.R.; Bangsbo, J. High-intensity intermittent “5-10-15” running reduces body fat, and increases lean body mass, bone mineral density, and performance in untrained subjects. Eur. J. Appl. Physiol. 2018, 118, 1221–1230. [Google Scholar] [CrossRef]
- Battafarano, G.; Rossi, M.; Marampon, F.; Minisola, S.; Del Fattore, A. Bone Control of Muscle Function. Int. J. Mol. Sci. 2020, 21, 1178. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.X.; Yang, T. Roles of Leptin in Bone Metabolism and Bone Diseases. J. Bone Miner. Metab. 2015, 33, 474–485. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.T.; Morandé, G.; García-Centenera, J.A.; Hervás, F.; Pozo, J.; Argente, J. The Effects of Estrogen Administration on Bone Mineral Density in Adolescents with Anorexia Nervosa. Eur. J. Endocrinol. 2002, 146, 45–50. [Google Scholar] [CrossRef]
- Maïmoun, L.; Lumbroso, S.; Manetta, J.; Paris, F.; Leroux, J.L.; Sultan, C. Testosterone is Significantly Reduced in Endurance Athletes without Impact on Bone Mineral Density. Horm. Res. 2003, 59, 285–292. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, T.P.; Espada, M.C.; Massini, D.A.; Robalo, R.A.M.; Almeida, T.A.F.; Hernández-Beltrán, V.; Gamonales, J.M.; Castro, E.A.; Pessôa Filho, D.M. Effects of Exercise and Sports Intervention and the Involvement Level on the Mineral Health of Different Bone Sites in the Leg, Hip, and Spine: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2023, 20, 6537. https://doi.org/10.3390/ijerph20156537
Oliveira TP, Espada MC, Massini DA, Robalo RAM, Almeida TAF, Hernández-Beltrán V, Gamonales JM, Castro EA, Pessôa Filho DM. Effects of Exercise and Sports Intervention and the Involvement Level on the Mineral Health of Different Bone Sites in the Leg, Hip, and Spine: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2023; 20(15):6537. https://doi.org/10.3390/ijerph20156537
Chicago/Turabian StyleOliveira, Thiago P., Mário C. Espada, Danilo A. Massini, Ricardo A. M. Robalo, Tiago A. F. Almeida, Víctor Hernández-Beltrán, José M. Gamonales, Eliane A. Castro, and Dalton M. Pessôa Filho. 2023. "Effects of Exercise and Sports Intervention and the Involvement Level on the Mineral Health of Different Bone Sites in the Leg, Hip, and Spine: A Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 20, no. 15: 6537. https://doi.org/10.3390/ijerph20156537
APA StyleOliveira, T. P., Espada, M. C., Massini, D. A., Robalo, R. A. M., Almeida, T. A. F., Hernández-Beltrán, V., Gamonales, J. M., Castro, E. A., & Pessôa Filho, D. M. (2023). Effects of Exercise and Sports Intervention and the Involvement Level on the Mineral Health of Different Bone Sites in the Leg, Hip, and Spine: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 20(15), 6537. https://doi.org/10.3390/ijerph20156537










