An Exploratory Study Investigating the Prevalence of Gastrointestinal Symptoms in Collegiate Division I American Football Athletes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Questionnaire
2.3. Data Analysis
3. Results
3.1. Characteristics
3.2. General GI Issues, Food Omissions, Supplements, and Medication
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilson, P.B.; Fearn, R.; Pugh, J. Occurrence and Impacts of Gastrointestinal Symptoms in Team-Sport Athletes: A Preliminary Survey. Clin. J. Sports Med. 2023, 33, 239–245. [Google Scholar] [CrossRef]
- Costa, R.J.S.; Snipe, R.M.J.; Kitic, C.M.; Gibson, P.R. Systematic Review: Exercise-Induced Gastrointestinal Syndrome—Implications for Health and Intestinal Disease. Aliment. Pharmacol. Ther. 2017, 46, 246–265. [Google Scholar] [CrossRef]
- Clark, A.; Mach, N. Exercise-Induced Stress Behavior, Gut-Microbiota-Brain Axis and Diet: A Systematic Review for Athletes. J. Int. Soc. Sports Nutr. 2016, 13, 43. [Google Scholar] [CrossRef] [PubMed]
- Lamprecht, M.; Bogner, S.; Schippinger, G.; Steinbauer, K.; Fankhauser, F.; Hallstroem, S.; Schuetz, B.; Greilberger, J.F. Probiotic Supplementation Affects Markers of Intestinal Barrier, Oxidation, and Inflammation in Trained Men; a Randomized, Double-Blinded, Placebo-Controlled Trial. J. Int. Soc. Sports Nutr. 2012, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Hoogervorst, D.; van der Burg, N.; Versteegen, J.J.; Lambrechtse, K.J.; Redegeld, M.I.; Cornelissen, L.A.J.; Wardenaar, F.C. Gastrointestinal Complaints and Correlations with Self-Reported Macronutrient Intake in Independent Groups of (Ultra)Marathon Runners Competing at Different Distances. Sports 2019, 7, 140. [Google Scholar] [CrossRef]
- Ter Steege, R.W.F.; Van Der Palen, J.; Kolkman, J.J. Prevalence of Gastrointestinal Complaints in Runners Competing in a Long-Distance Run: An Internet-Based Observational Study in 1281 Subjects. Scand. J. Gastroenterol. 2008, 43, 1477–1482. [Google Scholar] [CrossRef]
- Costa, R.J.S.; Miall, A.; Khoo, A.; Rauch, C.; Snipe, R.; Camões-Costa, V.; Gibson, P. Gut-Training: The Impact of Two Weeks Repetitive Gut-Challenge during Exercise on Gastrointestinal Status, Glucose Availability, Fuel Kinetics, and Running Performance. Appl. Physiol. Nutr. Metab. 2017, 42, 547–557. [Google Scholar] [CrossRef]
- Pugh, J.N.; Fearn, R.; Morton, J.P.; Close, G.L. Gastrointestinal Symptoms in Elite Athletes: Time to Recognise the Problem? Br. J. Sports Med. 2018, 52, 487–488. [Google Scholar] [CrossRef]
- Harper, D.J.; Carling, C.; Kiely, J. High-Intensity Acceleration and Deceleration Demands in Elite Team Sports Competitive Match Play: A Systematic Review and Meta-Analysis of Observational Studies. Sports Med. 2019, 49, 1923–1947. [Google Scholar] [CrossRef]
- Grillner, S.; Nilsson, J.; Thorstensson, A. Intra-Abdominal Pressure Changes during Natural Movements in Man. Acta Physiol. Scand. 1978, 103, 275–283. [Google Scholar] [CrossRef]
- Guyer, H.; Georgescu, M.; Hondula, D.M.; Wardenaar, F.; Vanos, J. Identifying the Need for Locally-Observed Wet Bulb Globe Temperature across Outdoor Athletic Venues for Current and Future Climates in a Desert Environment. Environ. Res. Lett. 2021, 16, 124042. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Lai, J.; Zhu, A.; Zhang, X.; Zheng, Z.; Zhu, H.; Shi, Y.; Wang, L.; Chen, Z. The Effects of a Passive Exoskeleton on Human Thermal Responses in Temperate and Cold Environments. Int. J. Environ. Res. Public Health 2021, 18, 3889. [Google Scholar] [CrossRef]
- NCAA Demographics Database—NCAA.Org. Available online: https://www.ncaa.org/sports/2018/12/13/ncaa-demographics-database.aspx (accessed on 23 October 2022).
- Jansson-Knodell, C.L.; Krajicek, E.J.; Savaiano, D.A.; Shin, A.S. Lactose Intolerance: A Concise Review to Skim the Surface. Mayo Clin. Proc. 2020, 95, 1499–1505. [Google Scholar] [CrossRef]
- Wright, H.; Collins, M.; Schwellnus, M.P. Gastrointestinal (GIT) Symptoms in Athletes: A Review of Risk Factors Associated with the Development of GIT Symptoms during Exercise. Int. SportMed J. 2009, 10, 116–123. [Google Scholar]
- Rauch, C.E.; Mika, A.S.; McCubbin, A.J.; Huschtscha, Z.; Costa, R.J.S. Effect of Prebiotics, Probiotics, and Synbiotics on Gastrointestinal Outcomes in Healthy Adults and Active Adults at Rest and in Response to Exercise—A Systematic Literature Review. Front. Nutr. 2022, 9, 2981. [Google Scholar] [CrossRef] [PubMed]
- Vento, K.A.; Wardenaar, F.C. Third-Party Testing Nutritional Supplement Knowledge, Attitudes, and Use among an NCAA I Collegiate Student-Athlete Population. Front. Sports Act Living 2020, 2, 115. [Google Scholar] [CrossRef] [PubMed]
- Walzem, R.L.; German, B.; Walzem, R.L.; Dillard, C.J.; German, J.B. Whey Components: Millennia of Evolution Create Functionalities for Mammalian Nutrition: What We Know and What We May Be Overlooking. Crit. Rev. Food Sci. Nutr. 2002, 42, 353–375. [Google Scholar] [CrossRef] [PubMed]
- Ostojic, S.M.; Ahmetovic, Z. Gastrointestinal Distress after Creatine Supplementation in Athletes: Are Side Effects Dose Dependent? Res. Sports Med. 2008, 16, 15–22. [Google Scholar] [CrossRef]
- Tricker, R. Painkilling Drugs in Collegiate Athletics: Knowledge, Attitudes, and Use of Student Athletes. J. Drug Educ. 2000, 30, 313–324. [Google Scholar] [CrossRef]
- Emerson, D.M.; Davis, J.M.; Chen, S.C.L.; Torres-McGehee, T.M.; Pfeifer, C.E.; Emerson, C.C.; Bivona, J.D.; Stone, J.V. A 24 Hour Naproxen Dose on Gastrointestinal Distress and Performance during Cycling in the Heat. Sports Med. Health Sci. 2020, 2, 19–24. [Google Scholar] [CrossRef]
- Allen, A. The Structure and Function of Gastrointestinal Mucus. Attach. Org. Gut Mucosa 2018, 2, 3–11. [Google Scholar] [CrossRef]
- Whittle, B.J.R. Gastrointestinal Effects of Nonsteroidal Anti-Inflammatory Drugs. Fundam. Clin. Pharmacol. 2003, 17, 301–313. [Google Scholar] [CrossRef]
- Smith, K.A.; Pugh, J.N.; Duca, F.A.; Close, G.L.; Ormsbee, M.J. Gastrointestinal Pathophysiology during Endurance Exercise: Endocrine, Microbiome, and Nutritional Influences. Eur. J. Appl. Physiol. 2021, 121, 2657–2674. [Google Scholar] [CrossRef]
- Gaskell, S.K.; Burgell, R.; Wiklendt, L.; Dinning, P.; Costa, R.J.S. Does Exertional Heat Stress Impact Gastrointestinal Function and Symptoms? J. Sci. Med. Sport 2022, 25, 960–967. [Google Scholar] [CrossRef]
- Emerenziani, S.; Guarino, M.P.L.; Asensio, L.M.T.; Altomare, A.; Ribolsi, M.; Balestrieri, P.; Cicala, M. Role of Overweight and Obesity in Gastrointestinal Disease. Nutrients 2020, 12, 111. [Google Scholar] [CrossRef] [PubMed]
- Lilly, K.F. Athletes, NSAID, Coxibs, and the Gastrointestinal Tract. Curr. Sports Med. Rep. 2010, 9, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Papantoniou, K.; Michailides, C.; Bali, M.; Papantoniou, P.; Thomopoulos, K. Gastrointestinal Bleeding in Athletes. Ann. Gastroenterol. 2023, 36, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.B. ‘I Think I’m Gonna Hurl’: A Narrative Review of the Causes of Nausea and Vomiting in Sport. Sports 2019, 7, 162. [Google Scholar] [CrossRef] [PubMed]
- Ljótsson, B.; Jones, M.; Talley, N.J.; Kjellström, L.; Agréus, L.; Andreasson, A. Discriminant and Convergent Validity of the GSRS-IBS Symptom Severity Measure for Irritable Bowel Syndrome: A Population Study. United Eur. Gastroenterol. J. 2020, 8, 284–292. [Google Scholar] [CrossRef]
- Svedlund, J.; Sjödin, I.; Dotevall, G. GSRS—A Clinical Rating Scale for Gastrointestinal Symptoms in Patients with Irritable Bowel Syndrome and Peptic Ulcer Disease. Dig. Dis. Sci. 1988, 33, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Dimenäs, E.; Carlsson, G.; Glise, H.; Israelsson, B.; Wiklund, I. Relevance of Norm Values as Part of the Documentation of Quality of Life Instruments for Use in Upper Gastrointestinal Disease. Scand. J. Gastroenterol. Suppl. 1996, 221, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Peters, H. Gastrointestinal Symptoms in Long-Distance Runners, Cyclists, and Triathletes: Prevalence, Medication, and Etiology. Am. J. Gastroenterol. 1999, 94, 1570–1581. [Google Scholar] [CrossRef]
- Jeukendrup, A.E.; Vet-Joop, K.; Sturk, A.; Stegen, J.H.; Senden, J.; Saris, W.H.; Wagenmakers, A.J. Relationship between Gastro-Intestinal Complaints and Endotoxaemia, Cytokine Release and the Acute-Phase Reaction during and after a Long-Distance Triathlon in Highly Trained Men. Clin. Sci. 2000, 98, 47–55. [Google Scholar] [CrossRef]
- van Wijck, K.; Lenaerts, K.; van Loon, L.J.C.; Peters, W.H.M.; Buurman, W.A.; Dejong, C.H.C. Exercise-Induced Splanchnic Hypoperfusion Results in Gut Dysfunction in Healthy Men. PLoS ONE 2011, 6, e22366. [Google Scholar] [CrossRef] [PubMed]
- Hespel, P.; Maughan, R.J.; Greenhaff, P.L. Dietary Supplements for Football. J. Sports Sci. 2006, 24, 749–761. [Google Scholar] [CrossRef]
- Wardenaar, F.C.; Ceelen, I.J.M.; Van Dijk, J.W.; Hangelbroek, R.W.J.; Van Roy, L.; Van Der Pouw, B.; De Vries, J.H.M.; Mensink, M.; Witkamp, R.F. Nutritional Supplement Use by Dutch Elite and Sub-Elite Athletes: Does Receiving Dietary Counselling Make a Difference? Int. J. Sport Nutr. Exerc. Metab. 2017, 27, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Close, G.L.; Kasper, A.M.; Walsh, N.P.; Maughan, R.J. “Food First but Not Always Food Only”: Recommendations for Using Dietary Supplements in Sport. Int. J. Sport Nutr. Exerc. Metab. 2022, 32, 371–386. [Google Scholar] [CrossRef]
- Camilleri, M. Diagnosis and Treatment of Irritable Bowel Syndrome: A Review. JAMA 2021, 325, 865–877. [Google Scholar] [CrossRef]
- de Oliveira, E.P.; Burini, R.C. Carbohydrate-Dependent, Exercise-Induced Gastrointestinal Distress. Nutrients 2014, 6, 4191–4199. [Google Scholar] [CrossRef]
- Shepherd, S.J. Short-Chain Carbohydrates and Functional Gastrointestinal Disorders Low FODMAP Diet and Colonic Volume View Project Developing a Dietary Management Algorithm for People with IBD View Project. Am. J. Gastroenterol. 2013, 108, 707–717. [Google Scholar] [CrossRef]
- Lis, D.M. Exit Gluten-Free and Enter Low FODMAPs: A Novel Dietary Strategy to Reduce Gastrointestinal Symptoms in Athletes. Sports Med. 2019, 49, 87–97. [Google Scholar] [CrossRef] [PubMed]
- de Roest, R.H.; Dobbs, B.R.; Chapman, B.A.; Batman, B.; O’Brien, L.A.; Leeper, J.A.; Hebblethwaite, C.R.; Gearry, R.B. The Low FODMAP Diet Improves Gastrointestinal Symptoms in Patients with Irritable Bowel Syndrome: A Prospective Study. Int. J. Clin. Pract. 2013, 67, 895–903. [Google Scholar] [CrossRef]
- van Tilburg, M.A.; Squires, M.M.; Blois-Martin, N.; Zucker, N.; Bulik, C.; Chitkara, D. Diet and Eating Associated Symptoms in Adolescents with IBS. Gastroenterology 2012, 5 (Suppl. S1), S381. [Google Scholar] [CrossRef]
- van Tilburg, M.A.L.; Felix, C.T. Diet and Functional Abdominal Pain in Children and Adolescents. J. Pediatr. Gastroenterol. Nutr. 2013, 57, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Biesiekierski, J.R.; Newnham, E.D.; Irving, P.M.; Barrett, J.S.; Haines, M.; Doecke, J.D.; Shepherd, S.J.; Muir, J.G.; Gibson, P.R. Gluten Causes Gastrointestinal Symptoms in Subjects Without Celiac Disease: A Double-Blind Randomized Placebo-Controlled Trial. Am. J. Gasteroenterol. 2011, 106, 508–514. [Google Scholar] [CrossRef]
- Shaw, A.D.; Davies, G.J. Lactose Intolerance: Problems in Diagnosis and Treatment. J. Clin. Gastroenterol. 1999, 28, 208–216. [Google Scholar] [CrossRef]
- Gujral, N.; Freeman, H.J.; Thomson, A.B.R. Celiac Disease: Prevalence, Diagnosis, Pathogenesis and Treatment. World J. Gastroenterol. 2012, 18, 6036. [Google Scholar] [CrossRef]
- Agrawal, A.; Whorwell, P.J. Abdominal Bloating and Distension in Functional Gastrointestinal Disorders—Epidemiology and Exploration of Possible Mechanisms. Aliment. Pharmacol. Ther. 2008, 27, 2–10. [Google Scholar] [CrossRef]
- Francis, C.Y.; Whorwell, P.J. Bran and Irritable Bowel Syndrome: Time for Reappraisal. Lancet 1994, 344, 39–40. [Google Scholar] [CrossRef]
- Grabitske, H.A.; Slavin, J.L. Effects of Low-Digestible Carbohydrates Gastrointestinal Effects of Low-Digestible Carbohydrates. Crit. Rev. Food Sci. Nutr. Gastrointest. 2009, 49, 327–360. [Google Scholar] [CrossRef]
- Manichanh, C.; Eck, A.; Varela, E.; Roca, J.; Clemente, J.C.; González, A.; Knights, D.; Knight, R.; Estrella, S.; Hernandez, C.; et al. Anal Gas Evacuation and Colonic Microbiota in Patients with Flatulence: Effect of Diet. Gut 2014, 63, 401–408. [Google Scholar] [CrossRef]
- Sepple, C.P.; Read, N.W. Gastrointestinal Correlates of the Development of Hunger in Man. Appetite 1989, 13, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Nunez-Salces, M.; Li, H.; Feinle-Bisset, C.; Young, R.L.; Page, A.J. The Regulation of Gastric Ghrelin Secretion. Acta Physiol. 2021, 231, e13588. [Google Scholar] [CrossRef] [PubMed]
- Jakubowicz, D.; Froy, O.; Wainstein, J.; Boaz, M. Meal Timing and Composition Influence Ghrelin Levels, Appetite Scores and Weight Loss Maintenance in Overweight and Obese Adults. Steroids 2012, 77, 323–331. [Google Scholar] [CrossRef]
- Bo, S.; Broglio, F.; Settanni, F.; Parasiliti Caprino, M.; Ianniello, A.; Mengozzi, G.; de Francesco, A.; Fadda, M.; Fedele, D.; Guggino, A.; et al. Effects of Meal Timing on Changes in Circulating Epinephrine, Norepinephrine, and Acylated Ghrelin Concentrations: A Pilot Study. Nutr. Diabetes 2017, 7, 12. [Google Scholar] [CrossRef]
- Kinirons, M.; Ellis, H. French’s Index of Differential Diagnosis, 15th ed.; CRC Press: Florida, FL, USA, 2010; Volume 69. [Google Scholar]
- Lohiniemi, S.; Maki, M.; Kaukinen, K.; Laippala, P.; Collin, P. Gastrointestinal Symptoms Rating Scale in Coeliac Disease Patients on Wheat Starch-Based Gluten-Free Diets. Scand. J. Gastroenterol. 2009, 35, 947–949. [Google Scholar] [CrossRef]
| Number or Percentage | |
|---|---|
| Age (years) | 20.7 ± 1.7 |
| Race | |
| Afro-American or black | 52% (n = 23) |
| White | 34% (n = 15) |
| Other | 14% (n = 6) |
| Playing position | |
| Size | |
| Defensive Back | 14% (n = 6) |
| Defensive-Line | 11% (n = 5) |
| Line Backer | 16% (n = 7) |
| Offensive-Line | 20% (n = 9) |
| Skill | |
| Kicker/Punter | 7% (n = 3) |
| Quarter Back | 5% (n = 2) |
| Running Back | 9% (n = 4) |
| Tight End | 2% (n = 1) |
| Wide Receiver | 16% (n = 7) |
| Use of medication | |
| Use of non-steroidal anti-inflammatory drugs | 18% (n = 8) |
| Use of antibiotics in the last 12 months | 46% (n = 20) |
| Bowel movement and complaints | |
| Passing bowel movement at least once a day | 82% (n = 36) |
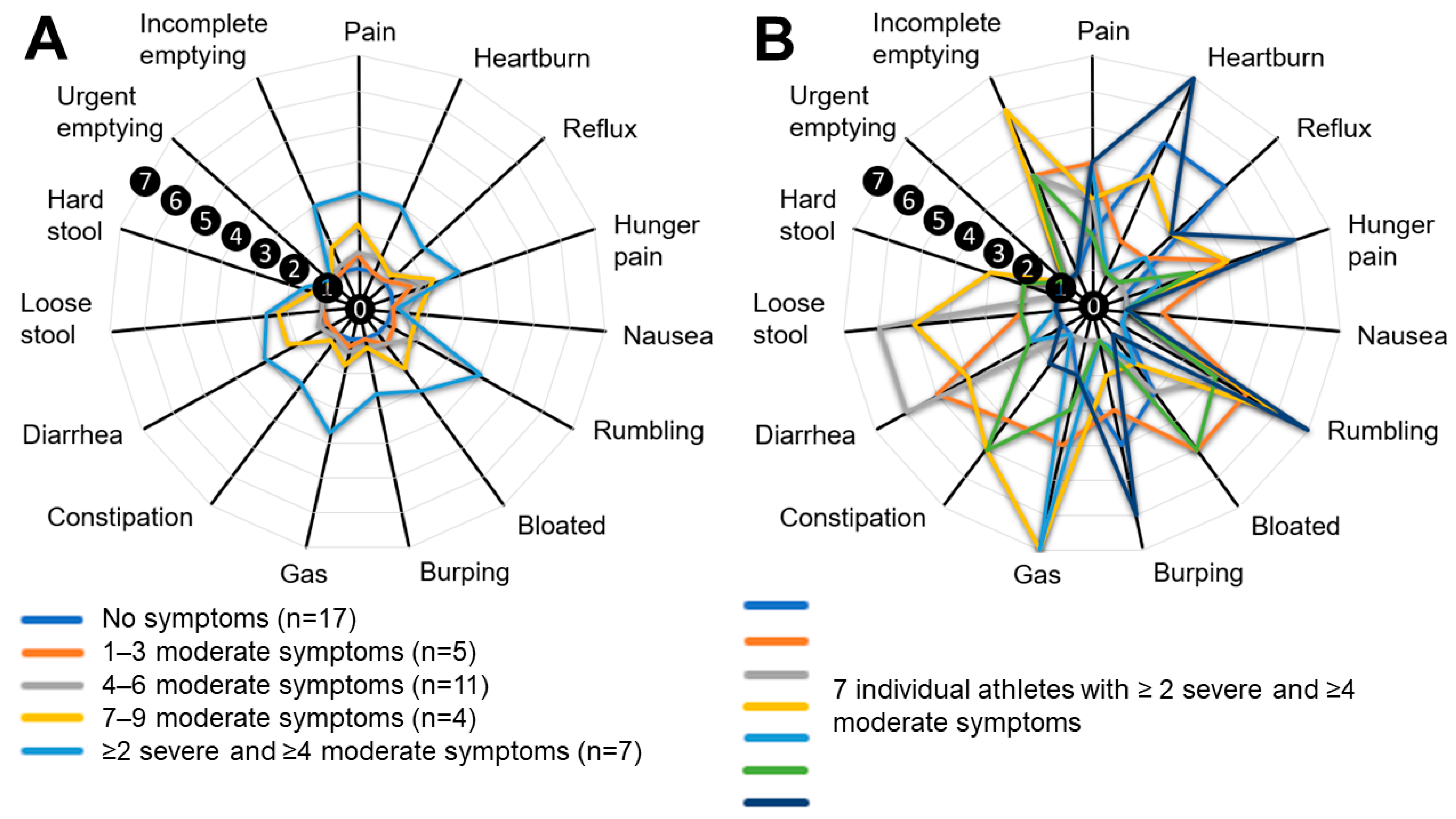
| Experience of gastrointestinal issues during exercise | 52% (n = 23) |
| Diagnosed with food intolerance of allergy | 11% (n = 5) |
| Tried to eliminate any dietary components | 11% (n = 5) |
| Self-reported use of frequently reported dietary supplements | |
| Protein and amino acids | 41% (n = 18) |
| Creatine | 34% (n = 15) |
| Probiotics | 27% (n = 12) |
| Caffeine | 11% (n = 5) |
| Sensations during the Last Week | All (n = 44) | Using Protein Supplements (n = 18) | Not Using Protein Supplements (n = 26) | p-Value and (η2). |
|---|---|---|---|---|
| Total Score | 21.8 ± 10.4 17.0 (15.0–23.5) | 27.1 ± 12.8 22.0 (17.0–31.8) | 18.1 ± 6.26 15.0 (15.0–19.3) | 0.001 * (0.24) |
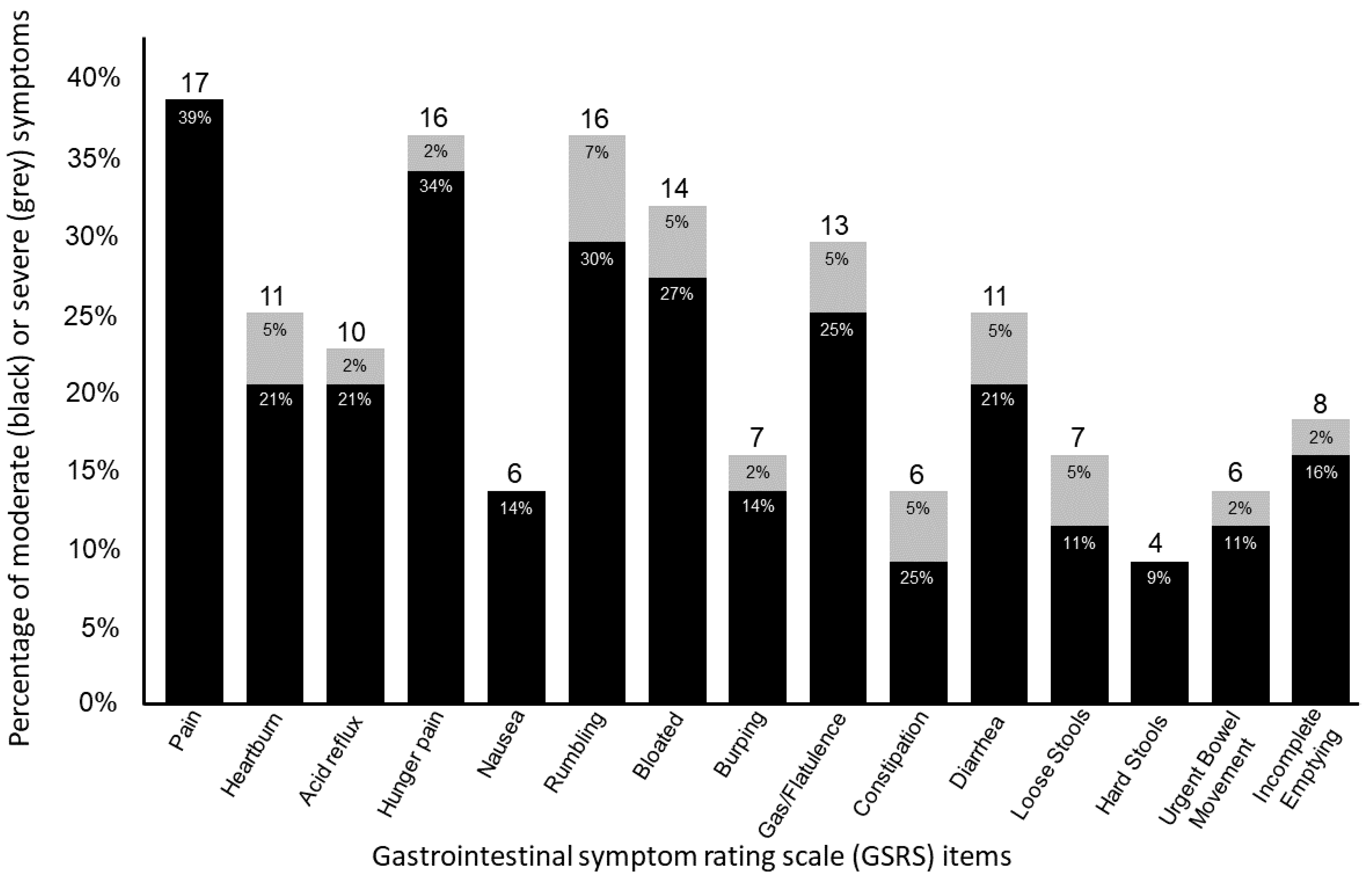
| Pain or discomfort in upper abdomen/stomach pit | 1.61 ± 0.92 1.00 (1.00–2.00) | 1.94 ± 0.94 2.00 (1.00–3.00) | 1.39 ± 0.85 1.00 (1.00–1.25) | 0.015 * (0.14) |
| Heartburn | 1.48 ± 1.17 1.00 (1.00–1.75) | 1.61 ± 1.15 1.00 (1.00–2.00) | 1.39 ± 1.20 1.00 (1.00–1.00) | 0.283 (0.03) |
| Acid reflux | 1.34 ± 0.78 0.00 (1.00–1.00) | 1.61 ± 1.04 1.00 (1.00–2.00) | 1.15 ± 0.46 1.00 (1.00–1.00) | 0.037 * (0.10) |
| Hunger pains | 1.73 ± 1.19 1.00 (1.00–2.00) | 1.89 ± 1.18 1.00 (1.00–3.00) | 1.61 ± 1.20 1.00 (1.00–2.00) | 0.324 (0.02) |
| Nausea | 1.18 ± 0.50 0.00 (1.00–1.00) | 1.28 ± 0.57 1.00 (1.00–1.25) | 1.12 ± 0.43 1.00 (1.00–1.00) | 0.186 (0.04) |
| Rumbling | 1.80 ± 1.42 1.00 (1.00–2.00) | 2.27 ± 1.56 2.00 (1.00–3.25) | 1.46 ± 1.24 1.00 (1.00–1.25) | 0.021 * (0.12) |
| Stomach feeling bloated | 1.57 ± 1.04 1.00 (1.00–2.00) | 2.11 ± 1.41 1.50 (1.00–3.00) | 1.19 ± 0.40 1.00 (1.00–1.00) | 0.020 * (0.15) |
| Burping | 1.34 ± 0.96 0.00 (1.00–1.00) | 1.50 ± 0.92 1.00 (1.00–2.00) | 1.23 ± 0.99 1.00 (1.00–1.00) | 0.085 (0.07) |
| Passing gas or flatus | 1.61 ± 1.37 1.00 (1.00–2.00) | 2.06 ± 1.51 2.00 (1.00–2.25) | 1.31 ± 1.19 1.00 (1.00–1.00) | 0.002 * (0.22) |
| Constipation | 1.32 ± 0.96 0.00 (1.00–1.00) | 1.67 ± 1.41 1.00 (1.00–1.25) | 1.08 ± 0.27 1.00 (1.00–1.00) | 0.139 (0.05) |
| Diarrhea | 1.50 ± 1.11 1.00 (1.00–1.75) | 2.11 ± 1.53 1.50 (1.00–3.00) | 1.08 ± 0.27 1.00 (1.00–1.00) | 0.001 * (0.25) |
| Loose stools | 1.36 ± 1.06 0.00 (1.00–1.00) | 1.83 ± 1.54 1.00 (1.00–2.00) | 1.04 ± 0.196 1.00 (1.00–1.00) | 0.008 * (0.16) |
| Hard stools | 1.11 ± 0.39 0.00 (1.00–1.00) | 1.28 ± 1.58 1.00 (1.00–1.25) | 1.00 ± 0.00 1.00 (1.00–1.00) | 0.013 * (0.14) |
| Urgent need to have a bowel movement | 1.41 ± 1.19 0.00 (1.00–1.00) | 2.00 ± 1.72 1.00 (1.00–3.25) | 1.00 ± 0.00 1.00 (1.00–1.00) | 0.002 * (0.23) |
| Not completely emptying bowels | 1.43 ± 1.09 0.00 (1.00–1.00) | 1.94 ± 1.55 1.00 (1.00–3.25) | 1.08 ± 0.27 1.00 (1.00–1.00) | 0.021 * (0.12) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wardenaar, F.C.; Schott, K.D.; Mohr, A.E.; Ortega-Santos, C.P.; Connolly, J.E. An Exploratory Study Investigating the Prevalence of Gastrointestinal Symptoms in Collegiate Division I American Football Athletes. Int. J. Environ. Res. Public Health 2023, 20, 6453. https://doi.org/10.3390/ijerph20156453
Wardenaar FC, Schott KD, Mohr AE, Ortega-Santos CP, Connolly JE. An Exploratory Study Investigating the Prevalence of Gastrointestinal Symptoms in Collegiate Division I American Football Athletes. International Journal of Environmental Research and Public Health. 2023; 20(15):6453. https://doi.org/10.3390/ijerph20156453
Chicago/Turabian StyleWardenaar, Floris C., Kinta D. Schott, Alex E. Mohr, Carmen P. Ortega-Santos, and John E. Connolly. 2023. "An Exploratory Study Investigating the Prevalence of Gastrointestinal Symptoms in Collegiate Division I American Football Athletes" International Journal of Environmental Research and Public Health 20, no. 15: 6453. https://doi.org/10.3390/ijerph20156453
APA StyleWardenaar, F. C., Schott, K. D., Mohr, A. E., Ortega-Santos, C. P., & Connolly, J. E. (2023). An Exploratory Study Investigating the Prevalence of Gastrointestinal Symptoms in Collegiate Division I American Football Athletes. International Journal of Environmental Research and Public Health, 20(15), 6453. https://doi.org/10.3390/ijerph20156453







