Psychological Interventions for Cannabis Use among Adolescents and Young Adults: A Systematic Review
Abstract
1. Introduction
- To systematically review all the existing psychological interventions that target CU specifically among youths;
- To describe the different criteria, methodologies, and frameworks used in these interventions;
- To assess the effectiveness of different techniques employed in interventions and guide future evaluations during this critical time of development.
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Searching Strategy
3. Results
3.1. Characteristics of Included Studies
3.2. Types of Interventions
3.2.1. In-Person Interventions
3.2.2. Interventions with Parents
3.2.3. Online Interventions
3.2.4. Control Groups
3.3. Participants
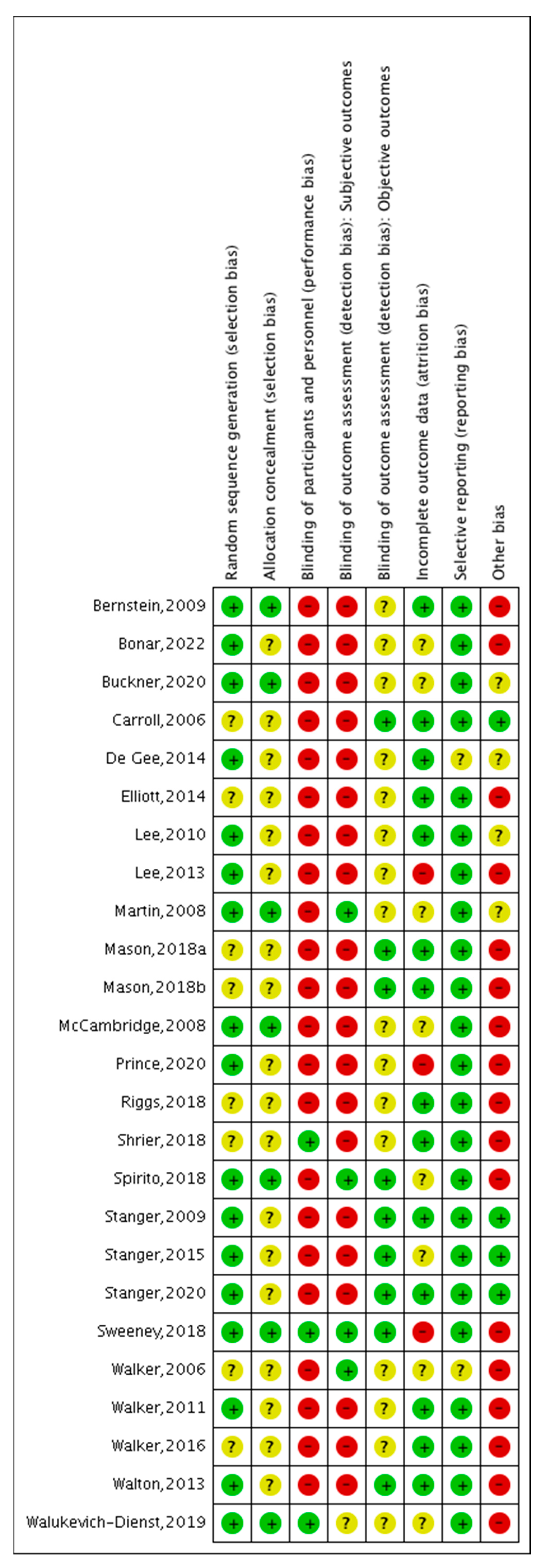
3.4. Risk of Bias
3.5. Effects of Interventions
3.5.1. In-Person Interventions
Interventions Based on MET
Interventions Based on MET and CBT
Interventions with Parents
Interventions Based on Working Memory
3.5.2. Online Interventions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Substance Abuse and Mental Health Services Administration. Key Substance Use and Mental Health Indicators in the United States: Results from the 2020 National Survey on Drug Use and Health; Substance Abuse and Mental Health Services Administration: Rockville, MD, USA, 2020; p. 156.
- American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013; ISBN 978-0-89042-555-8. [CrossRef]
- European Monitoring Centre for Drugs and Drug Addiction. European Drug Report: Trends and Developments; European Monitoring Centre for Drugs and Drug Addiction: Lisbon, Portugal, 2022; p. 60. [Google Scholar]
- Blest-Hopley, G.; Colizzi, M.; Giampietro, V.; Bhattacharyya, S. Is the Adolescent Brain at Greater Vulnerability to the Effects of Cannabis? A Narrative Review of the Evidence. Front. Psychiatry 2020, 11, 859. [Google Scholar] [CrossRef]
- Hasbi, A.; Madras, B.K.; George, S.R. Endocannabinoid System and Exogenous Cannabinoids in Depression and Anxiety: A Review. Brain Sci. 2023, 13, 325. [Google Scholar] [CrossRef] [PubMed]
- Hammond, C.; Allick, A.; Park, G.; Rizwan, B.; Kim, K.; Lebo, R.; Nanavati, J.; Parvaz, M.; Ivanov, I. A Meta-Analysis of fMRI Studies of Youth Cannabis Use: Alterations in Executive Control, Social Cognition/Emotion Processing, and Reward Processing in Cannabis Using Youth. Brain Sci. 2022, 12, 1281. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, G.; Atkin, T.; Zytynski, T.; Wang, S.; Askari, S.; Boruff, J.; Ware, M.; Marmorstein, N.; Cipriani, A.; Dendukuri, N.; et al. Association of Cannabis Use in Adolescence and Risk of Depression, Anxiety, and Suicidality in Young Adulthood: A Systematic Review and Meta-analysis. JAMA Psychiatry 2019, 76, 426. [Google Scholar] [CrossRef]
- Albaugh, M.D.; Ottino-Gonzalez, J.; Sidwell, A.; Lepage, C.; Juliano, A.; Owens, M.M.; Chaarani, B.; Spechler, P.; Fontaine, N.; Rioux, P.; et al. Association of Cannabis Use During Adolescence With Neurodevelopment. JAMA Psychiatry 2021, 78, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Owens, M.M.; Albaugh, M.D.; Allgaier, N.; Yuan, D.; Robert, G.; Cupertino, R.B.; Spechler, P.A.; Juliano, A.; Hahn, S.; Banaschewski, T.; et al. Bayesian causal network modeling suggests adolescent cannabis use accelerates prefrontal cortical thinning. Transl. Psychiatry 2022, 12, 188. [Google Scholar] [CrossRef]
- Hines, L.A.; Freeman, T.P.; Gage, S.H.; Zammit, S.; Hickman, M.; Cannon, M.; Munafo, M.; MacLeod, J.; Heron, J. Association of High-Potency Cannabis Use With Mental Health and Substance Use in Adolescence. JAMA Psychiatry 2020, 77, 1044. [Google Scholar] [CrossRef]
- Mericle, A.A.; Arria, A.M.; Meyers, K.; Cacciola, J.; Winters, K.C.; Kirby, K. National Trends in Adolescent Substance Use Disorders and Treatment Availability: 2003–2010. J. Child Adolesc. Subst. Abus. 2015, 24, 255–263. [Google Scholar] [CrossRef]
- Dennis, M.; Godley, S.H.; Diamond, G.; Tims, F.M.; Babor, T.; Donaldson, J.; Liddle, H.; Titus, J.C.; Kaminer, Y.; Webb, C.; et al. The Cannabis Youth Treatment (CYT) Study: Main findings from two randomized trials. J. Subst. Abuse Treat. 2004, 27, 197–213. [Google Scholar] [CrossRef]
- Halladay, J.; Scherer, J.; MacKillop, J.; Woock, R.; Petker, T.; Linton, V.; Munn, C. Brief interventions for cannabis use in emerging adults: A systematic review, meta-analysis, and evidence map. Drug Alcohol Depend. 2019, 204, 107565. [Google Scholar] [CrossRef]
- Li, L.Y.; Mann, R.E.; Wickens, C.M. Brief Interventions for Cannabis Problems in the Postsecondary Setting: A Systematic Review. Int. J. Ment. Health Addict. 2019, 17, 681–698. [Google Scholar] [CrossRef]
- Beneria, A.; Santesteban-Echarri, O.; Daigre, C.; Tremain, H.; Ramos-Quiroga, J.A.; McGorry, P.D.; Alvarez-Jimenez, M. Online interventions for cannabis use among adolescents and young adults: Systematic review and meta-analysis. Early Interv. Psychiatry 2022, 16, 821–844. [Google Scholar] [CrossRef]
- Olmos, A.; Tirado-Muñoz, J.; Farré, M.; Torrens, M. The efficacy of computerized interventions to reduce cannabis use: A systematic review and meta-analysis. Addict. Behav. 2018, 79, 52–60. [Google Scholar] [CrossRef]
- Boumparis, N.; Loheide-Niesmann, L.; Blankers, M.; Ebert, D.D.; Korf, D.; Schaub, M.P.; Spijkerman, R.; Tait, R.J.; Riper, H. Short- and long-term effects of digital prevention and treatment interventions for cannabis use reduction: A systematic review and meta-analysis. Drug Alcohol Depend. 2019, 200, 82–94. [Google Scholar] [CrossRef]
- Cochrane Handbook for Systematic Reviews of Interventions. Available online: https://training.cochrane.org/handbook/current (accessed on 22 March 2023).
- Martin, G.; Copeland, J. The adolescent cannabis check-up: Randomized trial of a brief intervention for young cannabis users. J. Subst. Abuse Treat. 2008, 34, 407–414. [Google Scholar] [CrossRef]
- de Gee, E.A.; Verdurmen, J.E.E.; Bransen, E.; de Jonge, J.M.; Schippers, G.M. A randomized controlled trial of a brief motivational enhancement for non-treatment-seeking adolescent cannabis users. J. Subst. Abuse Treat. 2014, 47, 181–188. [Google Scholar] [CrossRef]
- McCambridge, J.; Slym, R.L.; Strang, J. Randomized controlled trial of motivational interviewing compared with drug information and advice for early intervention among young cannabis users. Addiction 2008, 103, 1809–1818. [Google Scholar] [CrossRef] [PubMed]
- Carroll, K.M.; Easton, C.J.; Nich, C.; Hunkele, K.A.; Neavins, T.M.; Sinha, R.; Ford, H.L.; Vitolo, S.A.; Doebrick, C.A.; Rounsaville, B.J. The use of contingency management and motivational/skills-building therapy to treat young adults with marijuana dependence. J. Consult. Clin. Psychol. 2006, 74, 955–966. [Google Scholar] [CrossRef] [PubMed]
- Stanger, C.; Budney, A.J.; Kamon, J.L.; Thostensen, J. A randomized trial of contingency management for adolescent marijuana abuse and dependence. Drug Alcohol Depend. 2009, 105, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Stanger, C.; Ryan, S.R.; Scherer, E.A.; Norton, G.E.; Budney, A.J. Clinic- and Home-Based Contingency Management Plus Parent Training for Adolescent Cannabis Use Disorders. J. Am. Acad. Child Adolesc. Psychiatry 2015, 54, 445–453.e2. [Google Scholar] [CrossRef]
- Stanger, C.; Scherer, E.A.; Vo, H.T.; Babbin, S.F.; Knapp, A.A.; McKay, J.R.; Budney, A.J. Working memory training and high magnitude incentives for youth cannabis use: A SMART pilot trial. Psychol. Addict. Behav. 2020, 34, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Spirito, A.; Hernandez, L.; Cancilliere, M.K.; Graves, H.R.; Rodriguez, A.M.; Operario, D.; Jones, R.; Barnett, N.P. Parent and Adolescent Motivational Enhancement Intervention for Substance-Using, Truant Adolescents: A Pilot Randomized Trial. J. Clin. Child Adolesc. Psychol. 2018, 47, S467–S479. [Google Scholar] [CrossRef] [PubMed]
- Shrier, L.A.; Burke, P.J.; Kells, M.; Scherer, E.A.; Sarda, V.; Jonestrask, C.; Xuan, Z.; Harris, S.K. Pilot randomized trial of MOMENT, a motivational counseling-plus-ecological momentary intervention to reduce marijuana use in youth. mHealth 2018, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.D.; Roffman, R.A.; Stephens, R.S.; Wakana, K.; Berghuis, J. Motivational enhancement therapy for adolescent marijuana users: A preliminary randomized controlled trial. J. Consult. Clin. Psychol. 2006, 74, 628–632. [Google Scholar] [CrossRef]
- Walker, D.D.; Stephens, R.; Roffman, R.; DeMarce, J.; Lozano, B.; Towe, S.; Berg, B. Randomized controlled trial of motivational enhancement therapy with nontreatment-seeking adolescent cannabis users: A further test of the teen marijuana check-up. Psychol. Addict. Behav. 2011, 25, 474–484. [Google Scholar] [CrossRef]
- Walker, D.D.; Stephens, R.S.; Blevins, C.E.; Banes, K.E.; Matthews, L.; Roffman, R.A. Augmenting brief interventions for adolescent marijuana users: The impact of motivational check-ins. J. Consult. Clin. Psychol. 2016, 84, 983–992. [Google Scholar] [CrossRef]
- Lee, C.M.; Kilmer, J.R.; Neighbors, C.; Atkins, D.C.; Zheng, C.; Walker, D.D.; Larimer, M.E. Indicated prevention for college student marijuana use: A randomized controlled trial. J. Consult. Clin. Psychol. 2013, 81, 702–709. [Google Scholar] [CrossRef]
- Bernstein, E.; Edwards, E.; Dorfman, D.; Heeren, T.; Bliss, C.; Bernstein, J. Screening and Brief Intervention to Reduce Marijuana Use Among Youth and Young Adults in a Pediatric Emergency Department. Acad. Emerg. Med. 2009, 16, 1174–1185. [Google Scholar] [CrossRef]
- Prince, M.A.; Collins, R.L.; Wilson, S.D.; Vincent, P.C. A preliminary test of a brief intervention to lessen young adults’ cannabis use: Episode-level smartphone data highlights the role of protective behavioral strategies and exercise. Exp. Clin. Psychopharmacol. 2020, 28, 150–156. [Google Scholar] [CrossRef]
- Walton, M.A.; Bohnert, K.; Resko, S.; Barry, K.L.; Chermack, S.T.; Zucker, R.A.; Zimmerman, M.A.; Booth, B.M.; Blow, F.C. Computer and therapist based brief interventions among cannabis-using adolescents presenting to primary care: One year outcomes. Drug Alcohol Depend. 2013, 132, 646–653. [Google Scholar] [CrossRef]
- Buckner, J.D.; Zvolensky, M.J.; Lewis, E.M. On-line personalized feedback intervention for negative affect and cannabis: A pilot randomized controlled trial. Exp. Clin. Psychopharmacol. 2020, 28, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Elliott, J.C.; Carey, K.B.; Vanable, P.A. A preliminary evaluation of a web-based intervention for college marijuana use. Psychol. Addict. Behav. 2014, 28, 288–293. [Google Scholar] [CrossRef]
- Lee, C.M.; Neighbors, C.; Kilmer, J.R.; Larimer, M.E. A brief, web-based personalized feedback selective intervention for college student marijuana use: A randomized clinical trial. Psychol. Addict. Behav. 2010, 24, 265–273. [Google Scholar] [CrossRef]
- Riggs, N.R.; Conner, B.T.; Parnes, J.E.; Prince, M.A.; Shillington, A.M.; George, M.W. Marijuana eCHECKUPTO GO: Effects of a personalized feedback plus protective behavioral strategies intervention for heavy marijuana-using college students. Drug Alcohol Depend. 2018, 190, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Walukevich-Dienst, K.; Neighbors, C.; Buckner, J.D. Online personalized feedback intervention for cannabis-using college students reduces cannabis-related problems among women. Addict. Behav. 2019, 98, 106040. [Google Scholar] [CrossRef]
- Mason, M.J.; Zaharakis, N.M.; Moore, M.; Brown, A.; Garcia, C.; Seibers, A.; Stephens, C. Who responds best to text-delivered cannabis use disorder treatment? A randomized clinical trial with young adults. Psychol. Addict. Behav. 2018, 32, 699–709. [Google Scholar] [CrossRef]
- Mason, M.J.; Zaharakis, N.M.; Russell, M.; Childress, V. A pilot trial of text-delivered peer network counseling to treat young adults with cannabis use disorder. J. Subst. Abuse Treat. 2018, 89, 1–10. [Google Scholar] [CrossRef]
- Bonar, E.E.; Goldstick, J.E.; Chapman, L.; Bauermeister, J.A.; Young, S.D.; McAfee, J.; Walton, M.A. A social media intervention for cannabis use among emerging adults: Randomized controlled trial. Drug Alcohol Depend. 2022, 232, 109345. [Google Scholar] [CrossRef]
- Sweeney, M.M.; Rass, O.; DiClemente, C.; Schacht, R.L.; Vo, H.T.; Fishman, M.J.; Leoutsakos, J.-M.S.; Mintzer, M.Z.; Johnson, M.W. Working Memory Training for Adolescents With Cannabis Use Disorders: A Randomized Controlled Trial. J. Child Adolesc. Subst. Abuse 2018, 27, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Gates, P.J.; Sabioni, P.; Copeland, J.; Le Foll, B.; Gowing, L. Psychosocial interventions for cannabis use disorder. Cochrane Database Syst. Rev. 2016, 5, CD005336. [Google Scholar] [CrossRef]
- Steele, D.W.; Becker, S.J.; Danko, K.J.; Balk, E.M.; Saldanha, I.J.; Adam, G.P.; Bagley, S.M.; Friedman, C.; Spirito, A.; Scott, K.; et al. Interventions for Substance Use Disorders in Adolescents: A Systematic Review; Agency for Healthcare Research and Quality (AHRQ): Rockville, MD, USA, 2020. [CrossRef]
- Manchanda, T.; Stein, A.; Fazel, M. Investigating the Role of Friendship Interventions on the Mental Health Outcomes of Adolescents: A Scoping Review of Range and a Systematic Review of Effectiveness. Int. J. Environ. Res. Public. Health 2023, 20, 2160. [Google Scholar] [CrossRef] [PubMed]

| n | Mean Age (SD) | Gender (Female %) | Follow-Up | Length of Treatment | Mode | Treatment Group | Control Group | |
|---|---|---|---|---|---|---|---|---|
| Martin & Copeland (2008) [19] | 40 | 16.5 (1.3) | 33 | 3 months post-intervention | 2 sessions | In person | MET and CBT | Delayed treatment control |
| De Gee et al. (2014) [20] | 119 | 18.1 (1.8) | 26 | 3 months post-intervention | 2 sessions (60–90 min) | In person | MI | 1 information session (56 min) |
| McCambridge et al. (2008) [21] | 326 | 17.95 (NR) | 31 | 3 and 6 months post-intervention | 1 session | In person | MI | Drug information and giving advice |
| Carroll et al. (2006) [22] | 136 | 21 (2.1) | 11 | 3 and 6 months post-intervention | 8 sessions | In person | MET, CBT, and CM |
|
| Stanger et al. (2009) [23] | 69 | 16 (NR) | 17 | 3, 6, and 9 months post-treatment | 14 sessions (40 min): 14 sessions with the adolescent and 14 sessions with the parents | In person | MET, CBT, CM (abstinence), and family management | MET, CBT, CM (attendance), and parent psychoeducation |
| Stanger et al. (2015) [24] | 153 | 15.8 (1.3) | 11 | 3, 6, 9, and 12 months post-intervention | 14 sessions (40 min): 14 sessions with the adolescent and 14 sessions with the parents | In person, computerized | MET, CBT, CM, and parent training |
|
| Stanger et al. (2020) [25] | 59 | 16.4 (1.8) | 71 | Post-intervention | 25 sessions | In person | Phase 1: CM and WMT Phase 2: standard CM and WMT or enhanced CM + WMT | Phase 1: CM Phase 2: Standard CM, or enhanced CM |
| Spirito et al. (2018) [26] | 69 | 15.8 (NR) | 39 | 3 and 6 months post-baseline | 1 session (90 min): 1 session with the adolescent and 1 session with the parents | In person | MET and family checkup | 1 session of psychoeducation (60 min): 1 session with the adolescent and 1 session with the parents separately |
| Shrier et al. (2018) [27] | 70 | 20.7 6 (NR) | 61 | 3 month post-intervention | 2 sessions | In person | MET, mobile self-monitoring, and feedback messages |
|
| Walker et al. (2006) [28] | 97 | 15.75 (1.32) | 52 | 3 months post-intervention | 2 sessions | In person | MET | Delayed treatment control |
| Walker et al. (2011) [29] | 310 | 15.97 (1.24) | 39 | 3 and 12 months post- baseline | 2 sessions (45–50 min) | In person | MET and optional sessions of CBT |
|
| Walker et al. (2016) [30] | 252 | 15.84 (0.96) | 32 | 6, 9, 12, and 15 months post-baseline | 5 sessions | In person | MET and optional sessions of CBT | 2 sessions of MET and optional sessions of individual CBT |
| Lee et al. (2013) [31] | 212 | 20.0 (1.6) | 45 | 3 and 6 months post-baseline | 1 session (60 min) | In person | MI | Assessment only |
| Bernstein et al. (2009) [32] | 210 | NR (14–21) | 66 | 3 and 12 months post-baseline | 1 session (20–30 min) | In person | MI |
|
| Prince et al. (2020) [33] | 37 | 20.36 (1.71) | 35 | 1, 3, and 6 months post-intervention | 4 sessions | In person | MET, CBT, and exercise condition | MET and CBT |
| Walton et al. (2013) [34] | 328 | 16.3 (1.6) | 67 | 1, 3, and 12 months post-intervention | 1 session | Online In person | MI delivered by computer or therapist | Brochure |
| Buckner et al. (2020) [35] | 63 | 19.1 (NR) | 83 | 2 weeks post-baseline | 1 session | Online | PFI for negative affect and cannabis based on MI | Assessment only |
| Elliott et al. (2014) [36] | 317 | 19.34 (1.22) | 52 | 1 month post-intervention | 1 session (45 min) | Online | Educational program and PFI | Assessment only |
| Lee et al. (2010) [37] | 341 | 18.03 (0.31) | 55 | 3 and 6 months post-baseline | 1 session | Online | PFI | Assessment only |
| Riggs et al. (2018) [38] | 298 | 19.97 (2.0) | 49 | 6 weeks post-intervention | 1 session | Online | PFI based on MI | Healthy stress management |
| Walukevich-Dienst et al. (2019) [39] | 227 | 19.83 (1.43) | 77 | 1 month post-baseline | 1 session | Online | PFI based on MI | Personalized normative feedback |
| Mason, Zaharakis, Moore et al. (2018a) [40] | 101 | 20.33 (1.76) | 43 | 1, 2, and 3 months post-baseline | 30 days | Text | Peer network counseling based on MI | Assessment only |
| Mason, Zaharakis, Russell et al. (2018b) [41] | 30 | 20.75 (NR) | 50 | 1, 2, and 3 months post-baseline | 30 days | Text | Peer network counseling text based on MI | Delayed treatment control |
|
Bonar et al. (2022) [42] | 149 | 21.0 (2.2) | 56 | 3 and 6 months post-baseline | 8 sessions | Online e-coach | MI and CBT | Manuel-based content unrelated to substance use and mental health |
| Sweeney et al. (2018) [43] | 87 | 16.2 (1.6) | 16 | 3 and 6 months post-intervention | 25 sessions (30 min) | In person | Cognitive training involved an adaptive procedure | Cognitive training involved a static procedure |
| Inclusion | Exclusion | Measures | Key Findings (Last Follow-Up) | Dropouts (%) | |
|---|---|---|---|---|---|
| Martin & Copeland (2008) [19] |
|
| CU frequency and quantity: TLFB (90 days): Number of CUD symptoms: GAIN | Significant for frequency (p = 0.032) and quantity (p = 0.021) of CU, and number of CUD symptoms (p = 0.04) | 20 |
| De Gee et al. (2014) [20] |
|
| CU frequency and quantity: CUPIT CUD Severity: CUPIT, SDS Psychosocial functioning: YSR | Not significant | 17.65 |
| McCambridge et al. (2008) [21] |
| NR | CU frequency and quantity: Self-reporting Severity of CUD: SDS CU consequences: CPQ Alcohol use: AUDIT Nicotine use: Fagerstrom test | Not significant | 19.02 |
| Carroll et al. (2006) [22] |
|
| CU frequency: TLFB, urine and breath analyses Psychosocial functioning: ASI | Significant for CU: MET and CBT vs. DC (p = 0.02) Significant for treatment retention for CM vs. no CM (d = 0.42, 95% CI = 0.05, 0.84) and MET, CBT, and CM (d = 0.47, 95% CI = 0.12, 0.81) | 20.59 |
| Stanger et al. (2009) [23] |
|
| CU: VSDI, TLFB, urine drug screen Psychopathology: VSDI, CBCL/YSR Parenting measures: APQ | Not significant | 40.58 |
| Stanger et al. (2015) [24] |
|
| CU: VSDI, TLFB, urine drug screen Psychopathology: CBCL Parenting measures: APQ | Not significant | 33.33 |
| Stanger et al. (2020) [25] |
|
| CU frequency: TLFB (90 days) Visual spatial working memory: computerized task urine drug screen | Not significant | 57 |
| Spirito et al. (2018) [26] |
| Psychiatric or development disorders preventing participation | CU frequency and quantity: TLFB (90 days) Frequency and quantity of alcohol use: ADQ Other substance use: urine drug screen Parent-teen interaction: FAsTask | Significant for CU frequency (d = 0.49, 95% CI = 0.13, 0.84), parental monitoring (d = 0.58, 95% CI = −1.09, −0.05), parent (d = 0.42, 95% CI = −0.94, 0.11) and adolescent problem solving (d = −0.66, 95% CI = −1.17, −0.11) | 13.04 |
| Shrier et al. (2018) [27] |
|
| CU frequency: TLFB (30 days), momentary reports Consequences of cannabis: POSIT CU craving: momentary reports | Significant for momentary cannabis desire (MOMENT vs. MET only; p = 0.006) and for momentary CU after a trigger (MOMENT vs. No-message; p = 0.02) | 37.14 |
| Walker et al. (2006) [28] |
| Thought disorder | Frequency of cannabis and other substances: GAIN (60 days) Number of CUD symptoms: GAIN | Not significant | 5 |
| Walker et al. (2011) [29] |
| Thought disorder | Frequency of cannabis and other substances: GAIN-I (60 days) Number of CUD symptoms: GAIN-I CU consequences: MPI Other treatment: GAIN-I | Not significant | 9 |
| Walker et al. (2016) [30] |
| Severe medical or psychiatric condition | Frequency of cannabis and other substances: GAIN-I (60 days) Number of CUD symptoms: GAIN-I CU consequences: MPI | Not significant | 9.13 |
| Lee et al. (2013) [31] |
|
| CU frequency: TLFB (30 days) CU quantity: DDQ (60 days) CU consequences: RMPI | Not significant | 17.45 |
| Bernstein et al. (2009) [32] |
|
| CU frequency: TLFB (30 days) CU consequences: AIC | Significant for CU frequency among participants who reported CU in the last 30 days (p < 0.027) | 29.04 |
| Prince et al. (2020) [33] |
|
| CU frequency and quantity: EMA Protective behavioral strategies: EMA Alcohol use: DDQ | Significant for CU | NR |
| Walton et al. (2013) [34] |
| NR | CU frequency and other substances: add health items (3 months) CU consequences: RAPI, SDS Alcohol use: AUDIT | Not significant | 16.2 |
| Buckner et al. (2020) [35] |
| NR | CU frequency and quantity: TLFB (2 weeks) Social anxiety: SIAS-S Positive and negative affect: PANSA | Significant for frequency of CU only for moderate (p = 0.035) or high levels of social anxiety (p = 0.008) | 38 |
| Elliott et al. (2014) [36] |
| NR | CU frequency: self-reporting (past month) Number of CUD symptoms: AUDADIS-IV CU Consequences: RMPI | Not significant | 1.58 |
| Lee et al. (2010) [37] |
| NR | CU: GAIN-I CU consequences: RMPI | Not significant | 5.57 |
| Riggs et al. (2018) [38] |
| NR | CU frequency: self-reporting CU consequences: self-reporting Strategies: PBSM | Significant for hours (p < 0.05), days (p < 0.01), and periods of CU (p < 0.05) per week and weeks per month (p < 0.01) | 23.59 |
| Walukevich-Dienst et al. (2019) [39] |
| <18 years old | CU frequency: MUF CU consequences: MPS | Significant for CU consequences only for women (p < 0.01) | 22.03 |
| Mason, Zaharakis, Moore et al. (2018b) [40] |
| Treatment for substance use disorder in the last 3 months | CU frequency: CUDIT-R, ASSIST (30 days), urine drug screen CU consequences: YBSR, MPI Peer network health: YASNA | Significant for frequency of heavy CU (p = 0.005) and interpersonal problems (p = 0.011) | 4.95 |
| Mason, Zaharakis, Russell et al. (2018a) [41] |
| Treatment for substance use disorder in the last 90 days | CU frequency and quantity: TLFB (30 days), urine drug screen CU consequences: MPI Craving: EMA Peer network health: YASNA | Significant for CU consequences (p = 0.04), and urine drug screen (p = 0.03) | 13.3 |
| Bonar et al. (2022) [42] |
| NR | CU frequency and alcohol use: TLFB (30 days) | Significant only for frequency of vaped cannabis (p = 0.02) | 10.74 |
| Sweeney et al. (2018) [43] |
|
| Frequency and quantity of CU, alcohol, and tobacco: TLFB (30 days) Psychosocial functioning: GAIN-I, DERS Therapeutic alliance: WAI-SR urine drug screen | Not significant | 57.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bou Nassif, Y.; Rahioui, H.; Varescon, I. Psychological Interventions for Cannabis Use among Adolescents and Young Adults: A Systematic Review. Int. J. Environ. Res. Public Health 2023, 20, 6346. https://doi.org/10.3390/ijerph20146346
Bou Nassif Y, Rahioui H, Varescon I. Psychological Interventions for Cannabis Use among Adolescents and Young Adults: A Systematic Review. International Journal of Environmental Research and Public Health. 2023; 20(14):6346. https://doi.org/10.3390/ijerph20146346
Chicago/Turabian StyleBou Nassif, Yara, Hassan Rahioui, and Isabelle Varescon. 2023. "Psychological Interventions for Cannabis Use among Adolescents and Young Adults: A Systematic Review" International Journal of Environmental Research and Public Health 20, no. 14: 6346. https://doi.org/10.3390/ijerph20146346
APA StyleBou Nassif, Y., Rahioui, H., & Varescon, I. (2023). Psychological Interventions for Cannabis Use among Adolescents and Young Adults: A Systematic Review. International Journal of Environmental Research and Public Health, 20(14), 6346. https://doi.org/10.3390/ijerph20146346






