A Systematic Review of Areal Units and Adjacency Used in Bayesian Spatial and Spatio-Temporal Conditional Autoregressive Models in Health Research
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source and Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Risk of Bias Assessment
2.5. Data Synthesis and Analysis
3. Results
3.1. Characteristics of Included Studies
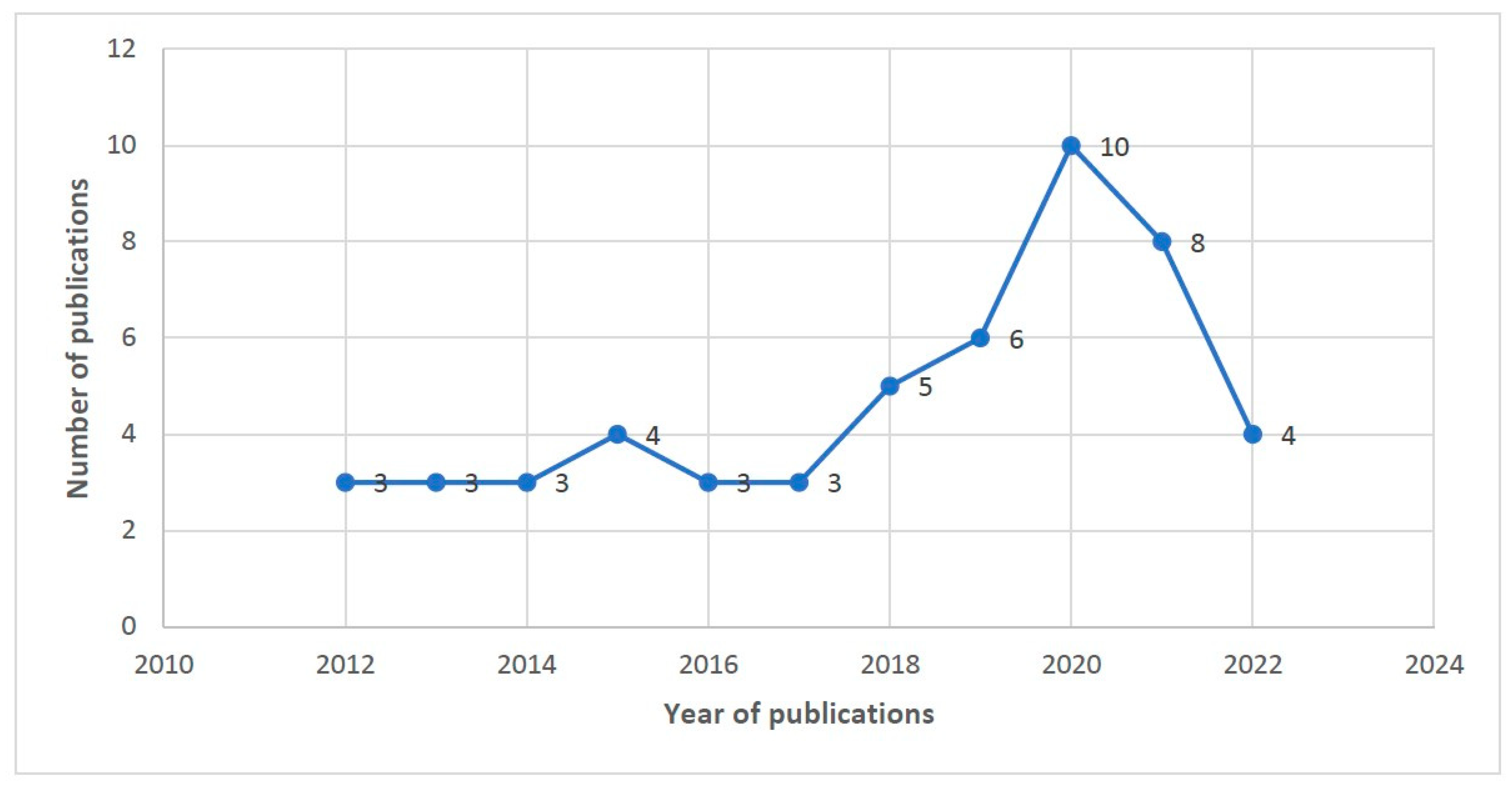
3.2. Publications by Year and Country of Study
3.3. Summary Statistics of Included Studies
3.4. Prior Distribution Selection, Sensitivity Analysis, and Adjacency Matrices
3.5. Modifiable Area Unit Problem (MAUP)
3.6. Key Considerations in Applying Bayesian Spatial and Spatio-Temporal Conditional Autoregressive Models and Methodological Gaps
3.7. Assessment of Quality
4. Discussion
4.1. Strengths and Limitations of the Review
4.2. Implications of the Study and Its Contributions
5. Conclusions
- ✓
- To report the rationale for the choice of areal units.
- ✓
- To perform sensitivity analyses on priors.
- ✓
- To evaluate the choice of neighbourhood adjacency.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krieger, N. Place, space, and health: GIS and epidemiology. Epidemiology 2003, 14, 384–385. [Google Scholar] [CrossRef]
- Meliker, J.R.; Sloan, C.D. Spatio-temporal epidemiology: Principles and opportunities. Spat. Spatio-Temporal Epidemiol. 2011, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Musa, G.J.; Chiang, P.-H.; Sylk, T.; Bavley, R.; Keating, W.; Lakew, B.; Tsou, H.-C.; Hoven, C.W. Use of GIS mapping as a public health Tool—From cholera to cancer. Health Serv. Insights 2013, 6, 111–116. [Google Scholar] [CrossRef]
- Lawson, A.B. Bayesian Disease Mapping: Hierarchical Modeling in Spatial Epidemiology; Chapman and Hall/CRC: Boca Raton, FL, USA, 2018. [Google Scholar]
- Beale, L.; Abellan, J.J.; Hodgson, S.; Jarup, L. Methodologic issues and approaches to spatial epidemiology. Environ. Health Perspect. 2008, 116, 1105–1110. [Google Scholar] [CrossRef]
- Wah, W.; Evans, M.; Ahern, S.; Earnest, A. A multi-level spatio-temporal analysis on prostate cancer outcomes. Cancer Epidemiol. 2021, 72, 101939. [Google Scholar] [CrossRef]
- Lope, V.; Pollán, M.; Pérez-Gómez, B.; Aragonés, N.; Ramis, R.; Gómez-Barroso, D.; López-Abente, G. Municipal mortality due to thyroid cancer in Spain. BMC Public Health 2006, 6, 302. [Google Scholar] [CrossRef] [PubMed]
- Besag, J.; York, J.; Mollié, A. Bayesian image restoration, with two applications in spatial statistics. Ann. Inst. Stat. Math. 1991, 43, 1–20. [Google Scholar] [CrossRef]
- Elliott, P.; Wartenberg, D. Spatial epidemiology: Current approaches and future challenges. Environ. Health Perspect. 2004, 112, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Ver Hoef, J.M.; Peterson, E.E.; Hooten, M.B.; Hanks, E.M.; Fortin, M.J. Spatial autoregressive models for statistical inference from ecological data. Ecol. Monogr. 2018, 88, 36–59. [Google Scholar] [CrossRef]
- Obaromi, D. Spatial modelling of some conditional autoregressive priors in a disease mapping model: The Bayesian approach. Biomed. J. Sci. Tech. Res. 2019, 14, 10680–10686. [Google Scholar] [CrossRef]
- Best, N.; Richardson, S.; Thomson, A. A comparison of Bayesian spatial models for disease mapping. Stat. Methods Med. Res. 2005, 14, 35–59. [Google Scholar] [CrossRef]
- Earnest, A.; Morgan, G.; Mengersen, K.; Ryan, L.; Summerhayes, R.; Beard, J. Evaluating the effect of neighbourhood weight matrices on smoothing properties of Conditional Autoregressive (CAR) models. Int. J. Health Geogr. 2007, 6, 54. [Google Scholar] [CrossRef]
- Lee, D. A comparison of conditional autoregressive models used in Bayesian disease mapping. Spat. Spatio-Temporal Epidemiol. 2011, 2, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Tuson, M.; Yap, M.; Kok, M.R.; Boruff, B.; Murray, K.; Vickery, A.; Turlach, B.A.; Whyatt, D. Overcoming inefficiencies arising due to the impact of the modifiable areal unit problem on single-aggregation disease maps. Int. J. Health Geogr. 2020, 19, 40. [Google Scholar] [CrossRef] [PubMed]
- Dark, S.J.; Bram, D. The modifiable areal unit problem (MAUP) in physical geography. Prog. Phys. Geogr. 2007, 31, 471–479. [Google Scholar] [CrossRef]
- Kok, M.R.; Tuson, M.; Yap, M.; Turlach, B.; Boruff, B.; Vickery, A.; Whyatt, D. Impact of the modifiable areal unit problem in assessing determinants of emergency department demand. Emerg. Med. Australas 2021, 33, 794–802. [Google Scholar] [CrossRef]
- Hanigan, I.C.; Cochrane, T.; Davey, R. Impact of scale of aggregation on associations of cardiovascular hospitalization and socio-economic disadvantage. PLoS ONE 2017, 12, e0188161. [Google Scholar] [CrossRef]
- Duncan, E.W.; White, N.M.; Mengersen, K. Spatial smoothing in Bayesian models: A comparison of weights matrix specifications and their impact on inference. Int. J. Health Geogr. 2017, 16, 47. [Google Scholar] [CrossRef]
- Louzada, F.; Nascimento, D.C.d.; Egbon, O.A. Spatial statistical models: An overview under the Bayesian approach. Axioms 2021, 10, 307. [Google Scholar] [CrossRef]
- van de Schoot, R.; Depaoli, S.; King, R.; Kramer, B.; Märtens, K.; Tadesse, M.G.; Vannucci, M.; Gelman, A.; Veen, D.; Willemsen, J. Bayesian statistics and modelling. Nat. Rev. Methods Prim. 2021, 1, 1. [Google Scholar] [CrossRef]
- Gelman, A.; Simpson, D.; Betancourt, M. The prior can often only be understood in the context of the likelihood. Entropy 2017, 19, 555. [Google Scholar] [CrossRef]
- Wah, W.; Ahern, S.; Earnest, A. A systematic review of Bayesian spatial–temporal models on cancer incidence and mortality. Int. J. Public Health 2020, 65, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Aswi, A.; Cramb, S.; Moraga, P.; Mengersen, K. Bayesian spatial and spatio-temporal approaches to modelling dengue fever: A systematic review. Epidemiol. Infect. 2019, 147, e33. [Google Scholar] [CrossRef]
- Byun, H.G.; Lee, N.; Hwang, S.-s. A systematic review of spatial and spatio-temporal analyses in public health research in Korea. J. Prev. Med. Public Health 2021, 54, 301. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef]
- Fone, D.; Hollinghurst, S.; Temple, M.; Round, A.; Lester, N.; Weightman, A.; Roberts, K.; Coyle, E.; Bevan, G.; Palmer, S. Systematic review of the use and value of computer simulation modelling in population health and health care delivery. J. Public Health 2003, 25, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.C.; Sumner, T.; Knight, G.M.; White, R.G. Systematic review of mathematical models exploring the epidemiological impact of future TB vaccines. Hum. Vaccines Immunother. 2016, 12, 2813–2832. [Google Scholar] [CrossRef] [PubMed]
- Danwang, C.; Khalil, É.; Achu, D.; Ateba, M.; Abomabo, M.; Souopgui, J.; De Keukeleire, M.; Robert, A. Fine scale analysis of malaria incidence in under-5: Hierarchical Bayesian spatio-temporal modelling of routinely collected malaria data between 2012–2018 in Cameroon. Sci. Rep. 2021, 11, 11408. [Google Scholar] [CrossRef]
- Donkor, E.; Kelly, M.; Eliason, C.; Amotoh, C.; Gray, D.J.; Clements, A.C.; Wangdi, K. A bayesian spatio-temporal analysis of malaria in the greater Accra region of Ghana from 2015 to 2019. Int. J. Environ. Res. Public Health 2021, 18, 6080. [Google Scholar] [CrossRef] [PubMed]
- Hanandita, W.; Tampubolon, G. Geography and social distribution of malaria in Indonesian Papua: A cross-sectional study. Int. J. Health Geogr. 2016, 15, 13. [Google Scholar] [CrossRef]
- Ibeji, J.U.; Mwambi, H.; Iddrisu, A.-K. Spatial variation and risk factors of malaria and anaemia among children aged 0 to 59 months: A cross-sectional study of 2010 and 2015 datasets. Sci. Rep. 2022, 12, 11498. [Google Scholar] [CrossRef]
- Kigozi, S.P.; Kigozi, R.N.; Sebuguzi, C.M.; Cano, J.; Rutazaana, D.; Opigo, J.; Bousema, T.; Yeka, A.; Gasasira, A.; Sartorius, B. Spatial-temporal patterns of malaria incidence in Uganda using HMIS data from 2015 to 2019. BMC Public Health 2020, 20, 1913. [Google Scholar] [CrossRef] [PubMed]
- Lubinda, J.; Bi, Y.; Hamainza, B.; Haque, U.; Moore, A.J. Modelling of malaria risk, rates, and trends: A spatiotemporal approach for identifying and targeting sub-national areas of high and low burden. PLoS Comput. Biol. 2021, 17, e1008669. [Google Scholar] [CrossRef] [PubMed]
- Okunlola, O.A.; Oyeyemi, O.T.; Lukman, A.F. Modeling the relationship between malaria prevalence and insecticide-treated bed net coverage in Nigeria using a Bayesian spatial generalized linear mixed model with a Leroux prior. Epidemiol. Health 2021, 43, e2021041. [Google Scholar] [CrossRef]
- Reid, H.L.; Haque, U.; Roy, S.; Islam, N.; Clements, A.C. Characterizing the spatial and temporal variation of malaria incidence in Bangladesh, 2007. Malar. J. 2012, 11, 170. [Google Scholar] [CrossRef]
- Wangdi, K.; Wetzler, E.; Cox, H.; Marchesini, P.; Villegas, L.; Canavati, S. Spatial patterns and climate drivers of malaria in three border areas of Brazil, Venezuela and Guyana, 2016–2018. Sci. Rep. 2022, 12, 10995. [Google Scholar] [CrossRef] [PubMed]
- Wangdi, K.; Xu, Z.; Suwannatrai, A.T.; Kurscheid, J.; Lal, A.; Namgay, R.; Glass, K.; Gray, D.J.; Clements, A.C. A spatio-temporal analysis to identify the drivers of malaria transmission in Bhutan. Sci. Rep. 2020, 10, 7060. [Google Scholar] [CrossRef]
- Akter, R.; Hu, W.; Gatton, M.; Bambrick, H.; Cheng, J.; Tong, S. Climate variability, socio-ecological factors and dengue transmission in tropical Queensland, Australia: A Bayesian spatial analysis. Environ. Res. 2021, 195, 110285. [Google Scholar] [CrossRef]
- Aswi, A.; Cramb, S.; Duncan, E.; Hu, W.; White, G.; Mengersen, K. Climate variability and dengue fever in Makassar, Indonesia: Bayesian spatio-temporal modelling. Spat. Spatio-Temporal Epidemiol. 2020, 33, 100335. [Google Scholar] [CrossRef]
- Hu, W.; Clements, A.; Williams, G.; Tong, S.; Mengersen, K. Spatial patterns and socioecological drivers of dengue fever transmission in Queensland, Australia. Environ. Health Perspect. 2012, 120, 260–266. [Google Scholar] [CrossRef]
- Tsheten, T.; Clements, A.C.; Gray, D.J.; Wangchuk, S.; Wangdi, K. Spatial and temporal patterns of dengue incidence in Bhutan: A Bayesian analysis. Emerg. Microbes Infect. 2020, 9, 1360–1371. [Google Scholar] [CrossRef] [PubMed]
- Wangdi, K.; Clements, A.C.; Du, T.; Nery, S.V. Spatial and temporal patterns of dengue infections in Timor-Leste, 2005–2013. Parasites Vectors 2018, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Aswi, A.; Cramb, S.; Duncan, E.; Mengersen, K. Evaluating the impact of a small number of areas on spatial estimation. Int. J. Health Geogr. 2020, 19, 39. [Google Scholar] [CrossRef] [PubMed]
- Gelaw, Y.A.; Magalhães, R.J.S.; Assefa, Y.; Williams, G. Spatial clustering and socio-demographic determinants of HIV infection in Ethiopia, 2015–2017. Int. J. Infect. Dis. 2019, 82, 33–39. [Google Scholar] [CrossRef]
- Kandhasamy, C.; Ghosh, K. Relative risk for HIV in India–An estimate using conditional auto-regressive models with Bayesian approach. Spat. Spatio-Temporal Epidemiol. 2017, 20, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Okango, E.; Mwambi, H.; Ngesa, O. Spatial modeling of HIV and HSV-2 among women in Kenya with spatially varying coefficients. BMC Public Health 2016, 16, 355. [Google Scholar] [CrossRef] [PubMed]
- Okango, E.; Mwambi, H.; Ngesa, O.; Achia, T. Semi-parametric spatial joint modeling of HIV and HSV-2 among women in Kenya. PLoS ONE 2015, 10, e0135212. [Google Scholar] [CrossRef] [PubMed]
- Alene, K.A.; Xu, Z.; Bai, L.; Yi, H.; Tan, Y.; Gray, D.J.; Viney, K.; Clements, A.C. Spatiotemporal Patterns of Tuberculosis in Hunan Province, China. Int. J. Environ. Res. Public Health 2021, 18, 6778. [Google Scholar] [CrossRef]
- Amsalu, E.; Liu, M.; Li, Q.; Wang, X.; Tao, L.; Liu, X.; Luo, Y.; Yang, X.; Zhang, Y.; Li, W. Spatial-temporal analysis of tuberculosis in the geriatric population of China: An analysis based on the Bayesian conditional autoregressive model. Arch. Gerontol. Geriatr. 2019, 83, 328–337. [Google Scholar] [CrossRef]
- Roza, D.L.d.; Caccia-Bava, M.d.C.G.G.; Martinez, E.Z. Spatio-temporal patterns of tuberculosis incidence in Ribeirão Preto, State of São Paulo, southeast Brazil, and their relationship with social vulnerability: A Bayesian analysis. Rev. Soc. Bras. Med. Trop. 2012, 45, 607–615. [Google Scholar] [CrossRef]
- Aragonés, N.; Goicoa, T.; Pollán, M.; Militino, A.F.; Pérez-Gómez, B.; López-Abente, G.; Ugarte, M.D. Spatio-temporal trends in gastric cancer mortality in Spain: 1975–2008. Cancer Epidemiol. 2013, 37, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.; White, N.; Mengersen, K.; Rolfe, M.; Morgan, G.G. Joint modelling of potentially avoidable hospitalisation for five diseases accounting for spatiotemporal effects: A case study in New South Wales, Australia. PLoS ONE 2017, 12, e0183653. [Google Scholar] [CrossRef]
- Cramb, S.M.; Baade, P.D.; White, N.M.; Ryan, L.M.; Mengersen, K.L. Inferring lung cancer risk factor patterns through joint Bayesian spatio-temporal analysis. Cancer Epidemiol. 2015, 39, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Darikwa, T.B.; Manda, S.O. Spatial Co-Clustering of Cardiovascular Diseases and Select Risk Factors among Adults in South Africa. Int. J. Envion. Res. Public Health 2020, 17, 3583. [Google Scholar] [CrossRef] [PubMed]
- Jürgens, V.; Ess, S.; Phuleria, H.C.; Früh, M.; Schwenkglenks, M.; Frick, H.; Cerny, T.; Vounatsou, P. Bayesian spatio-temporal modelling of tobacco-related cancer mortality in Switzerland. Geospat. Health 2013, 7, 219–236. [Google Scholar] [CrossRef]
- Lal, A.; Swaminathan, A.; Holani, T. Spatial clusters of Clostridium difficile infection and an association with neighbourhood socio-economic disadvantage in the Australian Capital Territory, 2004–2014. Infect. Dis. Health 2020, 25, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Ogunsakin, R.E.; Ginindza, T.G. Bayesian Spatial Modeling of Diabetes and Hypertension: Results from the South Africa General Household Survey. Int. J. Environ. Res. Public Health 2022, 19, 8886. [Google Scholar] [CrossRef] [PubMed]
- Raei, M.; Schmid, V.J.; Mahaki, B. Bivariate spatiotemporal disease mapping of cancer of the breast and cervix uteri among Iranian women. Geospat. Health 2018, 13, 645. [Google Scholar] [CrossRef]
- Saijo, Y.; Yoshioka, E.; Kawanishi, Y.; Nakagi, Y.; Hanley, S.J.; Yoshida, T. Relationships between road-distance to primary care facilities and ischemic heart disease and stroke mortality in Hokkaido, Japan: A Bayesian hierarchical approach to ecological count data. J. Gen. Fam. Med. 2018, 19, 4–8. [Google Scholar] [CrossRef]
- Sharafi, Z.; Asmarian, N.; Hoorang, S.; Mousavi, A. Bayesian spatio-temporal analysis of stomach cancer incidence in Iran, 2003–2010. Stoch. Environ. Res. Risk Assess. 2018, 32, 2943–2950. [Google Scholar] [CrossRef]
- Li, M.; Baffour, B.; Richardson, A. Bayesian spatial modelling of early childhood development in Australian regions. Int. J. Health Geogr. 2020, 19, 43. [Google Scholar] [CrossRef]
- Lome-Hurtado, A.; Lartigue-Mendoza, J.; Trujillo, J.C. Modelling local patterns of child mortality risk: A Bayesian Spatio-temporal analysis. BMC Public Health 2021, 21, 29. [Google Scholar] [CrossRef]
- Lome-Hurtado, A.; Li, G.; Touza-Montero, J.; White, P.C. Patterns of low birth weight in greater Mexico City: A Bayesian spatio-temporal analysis. Appl. Geogr. 2021, 134, 102521. [Google Scholar] [CrossRef]
- Xu, Z.; Hu, W.; Tong, S. The geographical co-distribution and socio-ecological drivers of childhood pneumonia and diarrhoea in Queensland, Australia. Epidemiol. Infect. 2015, 143, 1096–1104. [Google Scholar] [CrossRef]
- Adeyemi, R.A.; Zewotir, T.; Ramroop, S. Joint spatial mapping of childhood anemia and malnutrition in sub-Saharan Africa: A cross-sectional study of small-scale geographical disparities. Afr. Health Sci. 2019, 19, 2692–2712. [Google Scholar] [CrossRef]
- Ngwira, A. Shared geographic spatial risk of childhood undernutrition in Malawi: An application of joint spatial component model. Public Health Pract. 2022, 3, 100224. [Google Scholar] [CrossRef] [PubMed]
- Odhiambo, J.N.; Sartorius, B. Mapping of anaemia prevalence among pregnant women in Kenya (2016–2019). BMC Pregnancy Childbirth 2020, 20, 711. [Google Scholar] [CrossRef] [PubMed]
- Chou, M.P.; Clements, A.C.A.; Thomson, R.M. A spatial epidemiological analysis of nontuberculous mycobacterial infections in Queensland, Australia. BMC Infect. Dis. 2014, 14, 279. [Google Scholar] [CrossRef]
- Law, J. Exploring the specifications of spatial adjacencies and weights in Bayesian spatial modeling with intrinsic conditional autoregressive priors in a small-area study of fall injuries. AIMS Public Health 2016, 3, 65. [Google Scholar] [CrossRef]
- Ntirampeba, D.; Neema, I.; Kazembe, L. Modelling spatio-temporal patterns of disease for spatially misaligned data: An application on measles incidence data in Namibia from 2005–2014. PLoS ONE 2018, 13, e0201700. [Google Scholar] [CrossRef]
- Qi, X.; Hu, W.; Mengersen, K.; Tong, S. Socio-environmental drivers and suicide in Australia: Bayesian spatial analysis. BMC Public Health 2014, 14, 681. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.S.; Hossain, S.S.; Sheela, F.F. Spatial smoothing of low birth weight rate in Bangladesh using Bayesian hierarchical model. J. Appl. Stat. 2019, 46, 1870–1885. [Google Scholar] [CrossRef]
- Blain, A.; Thomas, M.; Shirley, M.; Simmister, C.; Elemraid, M.; Gorton, R.; Pearce, M.; Clark, J.; Rushton, S.; Spencer, D. Spatial variation in the risk of hospitalization with childhood pneumonia and empyema in the North of England. Epidemiol. Infect. 2014, 142, 388–398. [Google Scholar] [CrossRef]
- Desjardins, M.R.; Eastin, M.D.; Paul, R.; Casas, I.; Delmelle, E.M. Space–Time Conditional Autoregressive Modeling to Estimate Neighborhood-Level Risks for Dengue Fever in Cali, Colombia. Am. J. Trop. Med. Hyg. 2020, 103, 2040. [Google Scholar] [CrossRef]
- Dhewantara, P.W.; Marina, R.; Puspita, T.; Ariati, Y.; Purwanto, E.; Hananto, M.; Hu, W.; Magalhaes, R.J.S. Spatial and temporal variation of dengue incidence in the island of Bali, Indonesia: An ecological study. Travel Med. Infect. Dis. 2019, 32, 101437. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.X. Bayesian joint modeling of correlated counts data with application to adverse birth outcomes. J. Appl. Stat. 2015, 42, 1206–1222. [Google Scholar] [CrossRef]
- Huang, X.; Lambert, S.; Lau, C.; Magalhaes, R.S.; Marquess, J.; Rajmokan, M.; Milinovich, G.; Hu, W. Assessing the social and environmental determinants of pertussis epidemics in Queensland, Australia: A Bayesian spatio-temporal analysis. Epidemiol. Infect. 2017, 145, 1221–1230. [Google Scholar] [CrossRef]
- Thiam, S.; Cissé, G.; Stensgaard, A.-S.; Niang-Diène, A.; Utzinger, J.; Vounatsou, P. Bayesian conditional autoregressive models to assess spatial patterns of diarrhoea risk among children under the age of 5 years in Mbour, Senegal. Geospat. Health 2019, 14, 823. [Google Scholar] [CrossRef]
- Wangdi, K.; Clements, A.C. Spatial and temporal patterns of diarrhoea in Bhutan 2003–2013. BMC Infect. Dis. 2017, 17, 507. [Google Scholar] [CrossRef]
- Bhutta, Z.A.; Sommerfeld, J.; Lassi, Z.S.; Salam, R.A.; Das, J.K. Global burden, distribution, and interventions for infectious diseases of poverty. Infect. Dis. Poverty 2014, 3, 21. [Google Scholar] [CrossRef]
- Baker, R.E.; Mahmud, A.S.; Miller, I.F.; Rajeev, M.; Rasambainarivo, F.; Rice, B.L.; Takahashi, S.; Tatem, A.J.; Wagner, C.E.; Wang, L.-F. Infectious disease in an era of global change. Nat. Rev. Microbiol. 2022, 20, 193–205. [Google Scholar] [CrossRef]
- Desjardins, M.; Whiteman, A.; Casas, I.; Delmelle, E. Space-time clusters and co-occurrence of chikungunya and dengue fever in Colombia from 2015 to 2016. Acta Trop. 2018, 185, 77–85. [Google Scholar] [CrossRef]
- Kang, J.; Zhang, N.; Shi, R. A Bayesian nonparametric model for spatially distributed multivariate binary data with application to a multidrug-resistant tuberculosis (MDR-TB) study. Biometrics 2014, 70, 981–992. [Google Scholar] [CrossRef]
- Kinyoki, D.K.; Manda, S.O.; Moloney, G.M.; Odundo, E.O.; Berkley, J.A.; Noor, A.M.; Kandala, N.B. Modelling the ecological comorbidity of acute respiratory infection, diarrhoea and stunting among children under the age of 5 years in Somalia. Int. Stat. Rev. 2017, 85, 164–176. [Google Scholar] [CrossRef]
- Price, S.; Center, G.; Shipping, F. “Everything Is Related to Everything Else, but Near Things Are More Related than Distant Things”.~Waldo Tobler, American-Swiss Geographer (1970) First Law of Geography; Mini Museum: Blenheim, VA, USA, 2023. [Google Scholar]
- Rutstein, S.O.; Rojas, G. Guide to DHS Statistics; ORC Macro: Calverton, MD, USA, 2006; Volume 38, p. 78. [Google Scholar]
- Mahaki, B.; Mehrabi, Y.; Kavousi, A.; Schmid, V.J. Joint spatio-temporal shared component model with an application in Iran Cancer Data. Asian Pac. J. Cancer Prev. APJCP 2018, 19, 1553. [Google Scholar] [PubMed]
- De Smedt, T.; Simons, K.; Van Nieuwenhuyse, A.; Molenberghs, G. Comparing MCMC and INLA for disease mapping with Bayesian hierarchical models. Arch. Public Health 2015, 73, O2. [Google Scholar] [CrossRef]
- Cramb, S.; Duncan, E.; Baade, P.; Mengersen, K. Investigation of Bayesian Spatial Models; Cancer Council Queensland and Queensland University of Technology (QUT): Brisbane, QLD, Australia, 2018. [Google Scholar]
- Bohr, A.; Memarzadeh, K. The rise of artificial intelligence in healthcare applications. In Artificial Intelligence in Healthcare; Elsevier: Amsterdam, The Netherlands, 2020; pp. 25–60. [Google Scholar]
- Koo, K.; Papa, N.; Evans, M.; Jefford, M.; IJzerman, M.; White, V.; Evans, S.M.; Ristevski, E.; Emery, J.; Millar, J. Mapping disadvantage: Identifying inequities in functional outcomes for prostate cancer survivors based on geography. BMC Cancer 2022, 22, 283. [Google Scholar] [CrossRef] [PubMed]
- Deeth, L.E.; Deardon, R.; Gillis, D.J. Model choice using the Deviance Information Criterion for latent conditional individual-level models of infectious disease spread. Epidemiol. Methods 2015, 4, 47–68. [Google Scholar] [CrossRef]
- Tessema, Z.T.; Tesema, G.A.; Ahern, S.; Earnest, A. Bayesian spatio-temporal modelling of child anemia in Ethiopia using conditional autoregressive model. Sci. Rep. 2022, 12, 20297. [Google Scholar] [CrossRef]
- Kamary, K.; Robert, C.P. Reflecting about selecting noninformative priors. arXiv 2014, arXiv:1402.6257. [Google Scholar]
- Berger, J.O.; Insua, D.R.; Ruggeri, F. Bayesian robustness. In Robust Bayesian Analysis; Springer: New York, NY, USA, 2000; pp. 1–32. [Google Scholar]
- Depaoli, S.; Winter, S.D.; Visser, M. The importance of prior sensitivity analysis in Bayesian statistics: Demonstrations using an interactive Shiny App. Front. Psychol. 2020, 11, 608045. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | Frequency | Percentage (%) |
|---|---|---|
| Study category (n = 52) | ||
| Infectious diseases | 26 | 50.0 |
| Chronic diseases | 10 | 19.2 |
| Maternal and child health outcome | 8 | 15.3 |
| Nutrition | 3 | 5.7 |
| Others * | 5 | 9.6 |
| Data source | ||
| Survey | 24 | 46.1 |
| Hospital records/Administrative | 20 | 38.4 |
| Registry | 7 | 13.4 |
| Others ** | 1 | 0.03 |
| Study design(n = 52) | ||
| Ecological | 42 | 80.7 |
| Cross-sectional | 10 | 19.2 |
| Spatial unit (n = 52) | ||
| District | 9 | 17.3 |
| County | 7 | 13.1 |
| Region | 6 | 11.5 |
| Province | 6 | 11.5 |
| Postcode | 4 | 7.6 |
| SLAs | 3 | 5.7 |
| LGA | 2 | 3.8 |
| Municipalities | 2 | 3.8 |
| Others *** | 13 | 25.0 |
| Temporal unit | ||
| Year | 42 | 80.7 |
| Month | 9 | 17.3 |
| Week | 1 | 0 |
| Estimation technique (n = 52) | ||
| MCMC | 46 | 88.4 |
| INLA | 6 | 11.5 |
| Software used for analysis | ||
| WinBuGs | 31 | 59.6 |
| R | 11 | 21.1 |
| ArcGIS | 8 | 15.3 |
| Geoda | 2 | 0.03 |
| Types of distribution for outcome variable | ||
| Poisson | 30 | 57.7 |
| Negative Binomial/ZIP | 6 | 11.6 |
| Binomial/Bernoulli | 16 | 30.7 |
| Model comparison | ||
| DIC | 50 | 96.2 |
| WAIC | 2 | 3.8 |
| Effect measures reported | ||
| RR | 31 | 59.6 |
| Mean | 12 | 23.0 |
| OR | 9 | 17.4 |
| Types of spatial unit | ||
| Area | 42 | 80.7 |
| Point | 10 | 19.3 |
| Spatial structure | ||
| ICAR/CAR/MCAR | 39 | 75.0 |
| GMRF | 3 | 5.7 |
| Not reported | 10 | 19.2 |
| Spatial modes used | ||
| Bayesian spatial Poisson regression | 30 | 57.7 |
| Negative Binomial model | 6 | 11.5 |
| Binomial/Bernoulli model | 16 | 30.7 |
| Covariates | ||
| Demographic | 21 | 41.1 |
| Socio-economic | 19 | 37.2 |
| Climatology/Environmental | 16 | 30.7 |
| Clinical | 3 | 5.7 |
| Others **** | 7 | 13.4 |
| Model diagnostic reported | ||
| Yes | 24 | 46.1 |
| No | 28 | 53.8 |
| Map reported | ||
| Yes | 44 | 84.6 |
| No | 8 | 15.3 |
| Script provided | ||
| Yes | 5 | 9.6 |
| No | 47 | 90.4 |
| Burn-in, thinning, and iteration reported | ||
| Yes | 28 | 53.8 |
| No | 24 | 46.2 |
| Reasons of uses of specified prior distribution provided | ||
| Yes | 7 | 13.4 |
| No | 45 | 85.6 |
| Reasons for use of specified adjacency matrix provided | ||
| Yes | 2 | 3.8 |
| No | 50 | 96.2 |
| Id | Prior Distribution | Sensitivity Analysis for Hyperpriors | Adjacency | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intercept | Regression Coefficient | Spatially Structured Random Effect (Precision) | Spatially Unstructured Random Effect (Precision) | Yes | No | Queen Contiguity | Distance-Based Matrix | Rook Contiguity | Not Reported | |
| 1 | Diffuse | Highly dispersed normal prior distributions | Non-informative gamma | Non-informative gamma | ✓ | ✓ | ||||
| 2 | Flat | Normal | Non-informative gamma (0.001, 0.001) | Non-informative gamma (0.001, 0.001) | ✓ | ✓ | ||||
| 3 | Flat | Normal | Gamma | Beta | ✓ | ✓ | ||||
| 4 | Dflat | norm (0,0.000001 ) | non-informative gamma (0.001, 0.001) | Non-informative gamma (0.001, 0.001) | ✓ | ✓ | ||||
| 5 | Flat | Normal | Non-informative gamma (0.05, 0.0005) | Non-informative gamma (0.05, 0.0005) | ✓ | ✓ | ||||
| 6 | NA | Normal | Non-informative gamma | Non-informative gamma | ✓ | ✓ | ||||
| 7 | Uniform | Normal | Inverse gamma (1, 0.01) | Inverse gamma (1, 0.01) | ✓ | ✓ | ||||
| 8 | Uniform | Gaussian | Inverse gamma (1, 0.01) | Inverse gamma (1, 0.01) | ✓ | ✓ | ||||
| 9 | Normal | Normal | Inverse gamma (1, 0.01) | Inverse gamma (1, 0.01) | ✓ | ✓ | ||||
| 10 | NA | NA | NA | NA | ✓ | ✓ | ||||
| 11 | Flat | Normal with mean = 0 and 1/variance = 1 × 10−4 | Inverse gamma (0.5, 0.005) | Inverse gamma (0.5, 0.005) | ✓ | ✓ | ||||
| 12 | Normal | Normal | Inverse gamma (0.5, 0.0005) | Inverse gamma (0.5, 0.0005) | ✓ | ✓ | ||||
| 13 | NA | NA | NA | NA | ✓ | ✓ | ||||
| 14 | NA | NA | NA | NA | ✓ | ✓ | ||||
| 15 | Uninformative | Normal (0, 1000) | Weakly informative hyperpriors uniform (0, 0.001) | Weakly informative hyperpriors uniform (0, 0.001) | ✓ | ✓ | ||||
| 16 | Flat | Normal (0, 0.0001) | Noninformative gamma (0.1, 0.1) | Noninformative gamma (0.1, 0.1) | ✓ | ✓ | ||||
| 17 | Flat | Normal | Noninformative gamma (0.01, 0.01) | Noninformative gamma (0.01, 0.01) | ✓ | |||||
| 18 | Normal (0, 100) | Normal | Gamma (0.01, 0.01) | Gamma (0.01, 0.01) | ✓ | ✓ | ||||
| 19 | Flat with bounds −1 and +1 | Normal (0, 0.000001) | Non-informative gamma (0.001, 0.001) | Non-informative gamma (0.001, 0.001) | ✓ | ✓ | ||||
| 20 | Uninformed | Uninformed | Uninformed | Uninformed | ✓ | ✓ | ||||
| 21 | Diffuse | Normal (0, 0.0001) | Gamma (0.001, 0.001) | Gamma (0.001, 0.001) | ✓ | ✓ | ||||
| 22 | NA | NA | Gamma | Gamma | ✓ | |||||
| 23 | NA | NA | Gamma (0.01, 0.01) | Gamma (0.01, 0.01) | ✓ | ✓ | ||||
| 23 | NA | NA | Inverse gamma | Inverse gamma | ✓ | ✓ | ||||
| 24 | Flat | Uniform | Inverse gamma | Inverse gamma | ✓ | ✓ | ||||
| 25 | NA | Normal (0, 1000) | Uniform prior on (0, 1000) | Uniform prior on (0, 1000) | ✓ | ✓ | ||||
| 26 | NA | Informative Gaussian | Informative Gaussian | Informative Gaussian | ✓ | ✓ | ||||
| 27 | NA | NA | Gamma | Gamma | ✓ | ✓ | ||||
| 28 | Dflat() | Normal | Inverse gamma (0.5, 0.0005) | Inverse gamma (0.5, 0.0005) | ✓ | ✓ | ||||
| 29 | NA | NA | Normal | Inverse gamma | ✓ | ✓ | ||||
| 30 | NA | Normal | Inverse gamma | Inverse gamma | ✓ | |||||
| 31 | NA | NA | Non-informative gamma | Non-informative gamma | ✓ | ✓ | ||||
| 32 | Improper uniform | Vague normal prior | Improper uniform | Normal distribution | ✓ | ✓ | ||||
| 33 | NA | Normal | Gamma | Gamma | ✓ | ✓ | ||||
| 34 | Flat | Weakly informative | Inverse gamma (0.0001, 0.0001) | Inverse gamma (0.0001, 0.0001) | ✓ | ✓ | ||||
| 35 | Flat | Normal | Gamma (0.5, 0.0005) | Gamma (0.5, 0.0005) | ✓ | ✓ | ||||
| 36 | NA | Normal | Gamma | Gamma | ✓ | ✓ | ||||
| 37 | Normal | Normal | Inverse gamma | Inverse gamma | ✓ | ✓ | ||||
| 38 | Normal | Normal | Inverse gamma | Inverse gamma | ✓ | ✓ | ||||
| 39 | NA | Normal | Uniform prior (0, 0.001) | Uniform (0, 0.001) | ✓ | ✓ | ||||
| 40 | Dflat() | Normal | Gamma (0.5, 0.0005) | Gamma (0.01, 0.01) | ✓ | ✓ | ||||
| 41 | Flat | Normal | Weekly informative gamma | Weekly informative gamma | ✓ | ✓ | ||||
| 42 | Non-informative | Normal | Gamma (0.01, 0.01) | Gamma (0.01, 0.01) | ✓ | ✓ | ||||
| 43 | Non-informative | Normal | Gamma (0.5, 0.0005) | Gamma (0.5, 0.0005) | ✓ | ✓ | ||||
| 44 | NA | NA | Vague prior distribution gamma (0.5, 0.0005) | Vague prior distribution Gamma (0.5, 0.0005) | ✓ | ✓ | ||||
| 45 | Uniform | Normal | Gamma (0.5, 0.0005) | Gamm (0.5, 0.005) | ✓ | ✓ | ||||
| 46 | Flat | Non-informative normal | Non-informative gamma (0.5, 0.5) | Non-informative gamma (0.5, 0.5) | ✓ | ✓ | ||||
| 47 | Flat | Normal | Non-informative gamma (0.001, 0.001) | Non-informative gamma (0.001, 0.001) | ✓ | ✓ | ||||
| 48 | Flat | Normal | Non-informative gamma (0.01, 0.01) | Non-informative gamma (0.01, 0.01) | ✓ | ✓ | ||||
| 49 | Flat | Normal | Non-informative gamma (0.001, 0.001) | Non-informative gamma (0.001, 0.001) | ✓ | ✓ | ||||
| 50 | Flat | Normal | Non-informative gamma (0.01, 0.01) | Non-informative gamma (0.01, 0.01) | ✓ | ✓ | ||||
| 51 | Flat | Normal | Non-informative gamma (0.01, 0.01) | Non-informative gamma (0.01, 0.01) | ✓ | ✓ | ||||
| 52 | NA | NA | NA | NA | ✓ | ✓ | ||||
| Total | 8 | 44 | 41 | 2 | 1 | 8 | ||||
| % | 15.3% | 88.4% | 75.0% | 3.8% | 1.9% | 15.3% | ||||
| Article | Country of Study | Spatial Scale Used for This Study | Available Spatial Scale in the Country | Reasons for Using Spatial Scale |
|---|---|---|---|---|
| Adeyemi et al., 2019 [67] | Burkina Faso and Mozambique | Region for Burkina Faso Province for Mozambique | Region, province, and department (Burkina Faso) and province, district, and pesto (Mozambique) | Not mentioned |
| Akter et al., 2021 [40] | Australia | Statistical Local Area (SLA) | Postcode, SA1, SA2, SA3, SA4, and LGA | Not mentioned |
| Alam et al., 2019 [74] | Bangladesh | District | Division, district, thanas, and union | Not mentioned |
| Alene et al., 2021 [50] | China | Province | Province(level 1), prefecture(level 2) | Not mentioned |
| Amsalu et al., 2019 [51] | China | Province | Province(level 1), prefecture(level 2) | Not mentioned |
| Aragonés et al., 2013 [53] | Spain | Province | Autonomous community(level 1), province (level 2), and municipality(level 3) | Not mentioned |
| Aswi et al., 2020 [41] | Indonesia | Province | Province, district, sub-district, and village | Not mentioned |
| Aswi et al., 2020 [45] | Indonesia | Province | Province, district, sub-district, and village | Not mentioned |
| Baker et al., 2017 [54] | Australia | SLA | Postcode, SA1, SA2, SA3, SA4, and LGA | Not mentioned |
| Blain et al., 2013 [75] | England | County | ||
| Chou et al., 2014 [70] | Australia | Postcode | SA1, SA2, SA3, SA4, SLA, and LGA | Postcodes are widely used by researchers in Australia because they are readily available in datasets; others not found |
| Cramb et al., 2015 [55] | Australia | SLAs | Postcode, SA1, SA2, SA3, SA4, and LGA | To overcome limitations of changing geographical boundaries |
| Danwang et al., 2021 [30] | Cameroon | Region | Region(level 1), department(level 2), and arrondissement(level 3) | Not mentioned |
| Darikwa et al., 2020 [56] | South Africa | District | Province, district, and municipality | Not mentioned |
| Desjardins et al., 2020 [76] | Colombia | Municipality | Department, municipality | Not mentioned |
| Dhewantara et al., 2019 [77] | Indonesia | County | Province, district, sub-district, and village | Not mentioned |
| Donkor et al., 2021 [31] | Ghana | Region | Region, district, and municipality | Not mentioned |
| Feng et al., 2015 [78] | Canada | County | Province, census division, and census sub-division | Not mentioned |
| Gelaw et al., 2019 [46] | Ethiopia | District | Region, zone, district, and kebele | Not mentioned |
| Hanandita et al., 2016 [32] | Indonesia | County | Province, district, sub-district, and village | Not mentioned |
| Hu et al., 2012 [42] | Australia | LGA | Postcode, SA1, SA2, SA3, SA4, and SLA | Not mentioned |
| Huang et al., 2017 [79] | Australia | Postcode | SA1, SA2, SA3, SA4, LGA, and SLA | Since postcode is the most readily available spatial unit in the dataset and other spatial units were not available |
| Ibeji et al., 2022 [33] | Nigeria | State | State(level 1), local government area (level 2), and district(level 3) | Not mentioned |
| Jurgens et al., 2013 [57] | Switzerland | Cantons | Cantons, district, and postcode | Not mentioned |
| Kandhasamy et al., 2017 [47] | India | State | State, union territories, and district | Not mentioned |
| Kigozi et al., 2020 [34] | Uganda | County | Region, district, county, and sub-country | Not mentioned |
| Lal et al., 2020 [58] | Australia | Postcode | SA1, SA2, SA3, SA4, LGA, and SLA | Not mentioned |
| Law, 2016 [71] | Canada | Census division | Province, census division, and census sub-division | Not mentioned |
| Li et al., 2020 [63] | Australia | SA3 | Postcode, SA1, SA2, SA4, LGA, and SLA | Since the objective of the study was mapping at regional-level estimate |
| Lubinda et al., 2021 [35] | Zambia | Province | Province and district | Not mentioned |
| Lome-Hurtado et al., 2021 [64] | Mexico | Municipalities | Estado and municipality | Not mentioned |
| Lome-Hurtado et al., 2021 [65] | Mexico | Municipalities | Estado and municipality | Not mentioned |
| Ngwira, 2022 [68] | Malawi | District | Region, district, and traditional authority area | Not mentioned |
| Ntirampeb et al., 2018 [72] | Namibia | Region | Region and constituency | Not mentioned |
| Odhiambo et al., 2020 [69] | Kenya | Sub-county | County, sub-county | Not mentioned |
| Ogunsakin et al., 2022 [59] | South Africa | Region | Province, district, and municipality | Not mentioned |
| Okango et al., 2015 [48] | Kenya | County | County, sub-county | Not mentioned |
| Okango et al., 2016 [49] | Kenya | County | County, sub-county | Not mentioned |
| Okunlola et al., 2021 [36] | Nigeria | Enumeration Area (Cluster) | State(level 1), local government area (level 2), and district(level-3) | Not mentioned |
| Qi et al., 2014 [73] | Australia | LGA | Postcode, SA1, SA2, SA3, SA4, and SLA | Each LGA contains one or more SLAs and LGA boundaries are more stable compared with SLA boundaries |
| Raei et al.,2018 [60] | Iran | Province | Province and district | Not mentioned |
| Reid et al., 2012 [37] | Bangladesh | District | Division, district, thanas, and union | Not mentioned |
| Roza et al., 2012 [52] | Brazil | County | Region, federal unit, and municipality | Not mentioned |
| Saijo et al., 2018 [61] | Japan | Prefecture | Region, prefecture, and municipality | Not mentioned |
| Sharafi et al., 2018 [62] | Iran | District | Province and district | Not mentioned |
| Thiam et al., 2019 [80] | Senegal | Region | Region, department, and arrondissement | Not mentioned |
| Tsheten el al, 2020 [43] | Bhutan | Sub-district | District, block | Not mentioned |
| Wangdi et al., 2017 [81] | Bhutan | District | District, block | Not mentioned |
| Wangdi et al., 2018 [44] | Timor-Leste | District | Department and municipality | Not mentioned |
| Wangdi et al., 2022 [38] | Brazil | Region | Region, federal unit, and municipality | Not mentioned |
| Wangdi et al., 2020 [39] | Bhutan | District | District, block | Not mentioned |
| Xu et al., 2015 [66] | Australia | Postcode | SA1, SA2, SA3, SA4, LGA, and SLA | Since the only available spatial unit in the dataset is postcode |
| Item | Number | % | Reference |
|---|---|---|---|
| I Reasons of use of specified prior distribution and sensitivity analysis | |||
| ✓ Non-informative prior distribution was used to minimise the risk of substantive influence on the estimates produced | 8 | 15.3 | [40,41,50,53,54,62,70,77] |
| ✓ The values of the hyperpriors distribution are selected in a way so that our Bayesian inferences are robust and not sensitive to these choices’ different priors | |||
| ✓ Sensitivity analysis using assigned parameters of variance component done to yield a robust finding of the posterior estimate | |||
| ✓ Weakly specified hyperpriors used following a normal and inverse gamma distribution, respectively, for structured and unstructured random effect and our final choice made based on goodness of fit, computation time and plausibility of estimates | |||
| ✓ Sensitivity analysis of different assigned priors were done to see the effect of different priors on posterior estimation using DIC and models with the smallest DIC were reported and discussed. | |||
| ✓ Non-informative priors were used to minimize the risk of major influence of estimates. | |||
| ✓ Sensitivity analyses for investigating the choice of priors were carried out. The hyperprior distribution of the variance components is set to be vague to obtain most of the information from the data. The prior for the precision of the random effects (σ2) is often specified as a gamma distribution with scale and shape parameters both equal to 0.001. To investigate the influence of the hyperprior specifications gamma priors like gamma (0.001,0.001) and gamma (0.05,0.0005) and Uni (0,100) were done. The resulting posterior inference remains robust is selected. | |||
| ✓ For the structured and unstructured random effect precision gamma (0.5,0.0005) was specified and hyperprior is vague allows the model to get the most information from the data. Gamma (0.001,0.001) was tested for precision and the estimate remains robust and selected. | |||
| ✓ Articles did not report the reason for using specified prior distribution and not done sensitivity analysis. | 44 | 84.6 | |
| II Reasons of use of specified adjacency matrices | |||
| ✓ More complex specifications of the adjacency matrix, for instance using a distance-based measure introduce model complexity, unrealistic spatial dependence, and do not necessarily lead to better inference | 2 | 3.8 | [62,64] |
| ✓ Using Queens method of adjacency for smoothing relative risk have a little impact on modelling results and it’s not common to face irregular shape. | |||
| ✓ Articles the did not mentioned reasons for use of specified adjacency matrices in their model. | 50 | 96.1 | |
| III Reasons of use of specified spatial scale (modifiable area unit problem) | |||
| ✓ Each LGA contains one or more SLAs and LGA boundaries are more stable compared with SLA boundaries. | 6 | 11.5 | [54,62,69,70,72,78] |
| ✓ Since the only available spatial unit in the dataset is postcode due to that postcode used as a spatial scale for this study. | |||
| ✓ Since the objective of the study was mapping at regional level estimate we used large spatial scale due to this LGA used as spatial scale for this study | |||
| ✓ Since postcode is the readily available spatial unit in the dataset due to that postcode used as a spatial scale for this study. | |||
| ✓ To overcoming limitations of changing geographical boundaries for this study LGA used as spatial scale. | |||
| ✓ Since postcode are readily available in the dataset postcode used as a spatial scale for this study. | |||
| ✓ Articles do not reason out of use of specified spatial scale (modifiable area unit problem) | 46 | 88.4 | |
| IV Methodological gaps | |||
| ✓ The result of this study is analysed at the district level, which is the level at which primary health is provided in South Africa. Aggregation of the results has the effect of introducing ecological fallacy and large geographical units of analysis may mask some information of interest. Results and efficiency may be improved by having smaller units of analysis | 8 | 15.3 | [55] |
| ✓ We used covariates at the municipality level, but this potentially masks important variations within municipalities, and to obtain more reliable results on the role of covariates as individual risk factors for overall LBW risk, it would be better to analyse birth records at the small-scale level and individual level (MAUP) | [64] | ||
| ✓ Having daily or weekly data would have been preferable to examine climatic influences of dengue case as this study used monthly cases of dengue | [40] | ||
| ✓ The impact of using different smoothing priors has not been done in this study and needs to be further investigated. | [39] | ||
| ✓ Further studies are required to quantify the of extent the spatial differences in risk represent differences in health behaviour or in disease risks | [74] | ||
| ✓ For Further study, precise estimate at local level is recommended for internal policy making and implementation | [73] | ||
| ✓ First, while the level of analysis was by province/region (large geographic area), it might be desirable to examine even smaller geographical units, such as districts and cities. | [50] | ||
| ✓ Explores approaches that allow the data to inform on the appropriate spatial weights to be specified is recommended. | [70] | ||
| ✓ Articles did not report the methodological gaps | 44 | 44.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tessema, Z.T.; Tesema, G.A.; Ahern, S.; Earnest, A. A Systematic Review of Areal Units and Adjacency Used in Bayesian Spatial and Spatio-Temporal Conditional Autoregressive Models in Health Research. Int. J. Environ. Res. Public Health 2023, 20, 6277. https://doi.org/10.3390/ijerph20136277
Tessema ZT, Tesema GA, Ahern S, Earnest A. A Systematic Review of Areal Units and Adjacency Used in Bayesian Spatial and Spatio-Temporal Conditional Autoregressive Models in Health Research. International Journal of Environmental Research and Public Health. 2023; 20(13):6277. https://doi.org/10.3390/ijerph20136277
Chicago/Turabian StyleTessema, Zemenu Tadesse, Getayeneh Antehunegn Tesema, Susannah Ahern, and Arul Earnest. 2023. "A Systematic Review of Areal Units and Adjacency Used in Bayesian Spatial and Spatio-Temporal Conditional Autoregressive Models in Health Research" International Journal of Environmental Research and Public Health 20, no. 13: 6277. https://doi.org/10.3390/ijerph20136277
APA StyleTessema, Z. T., Tesema, G. A., Ahern, S., & Earnest, A. (2023). A Systematic Review of Areal Units and Adjacency Used in Bayesian Spatial and Spatio-Temporal Conditional Autoregressive Models in Health Research. International Journal of Environmental Research and Public Health, 20(13), 6277. https://doi.org/10.3390/ijerph20136277







