Changes in Salivary Oxytocin Level of Term Pregnant Women after Aromatherapy Footbath for Spontaneous Labor Onset: A Non-Randomized Experimental Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
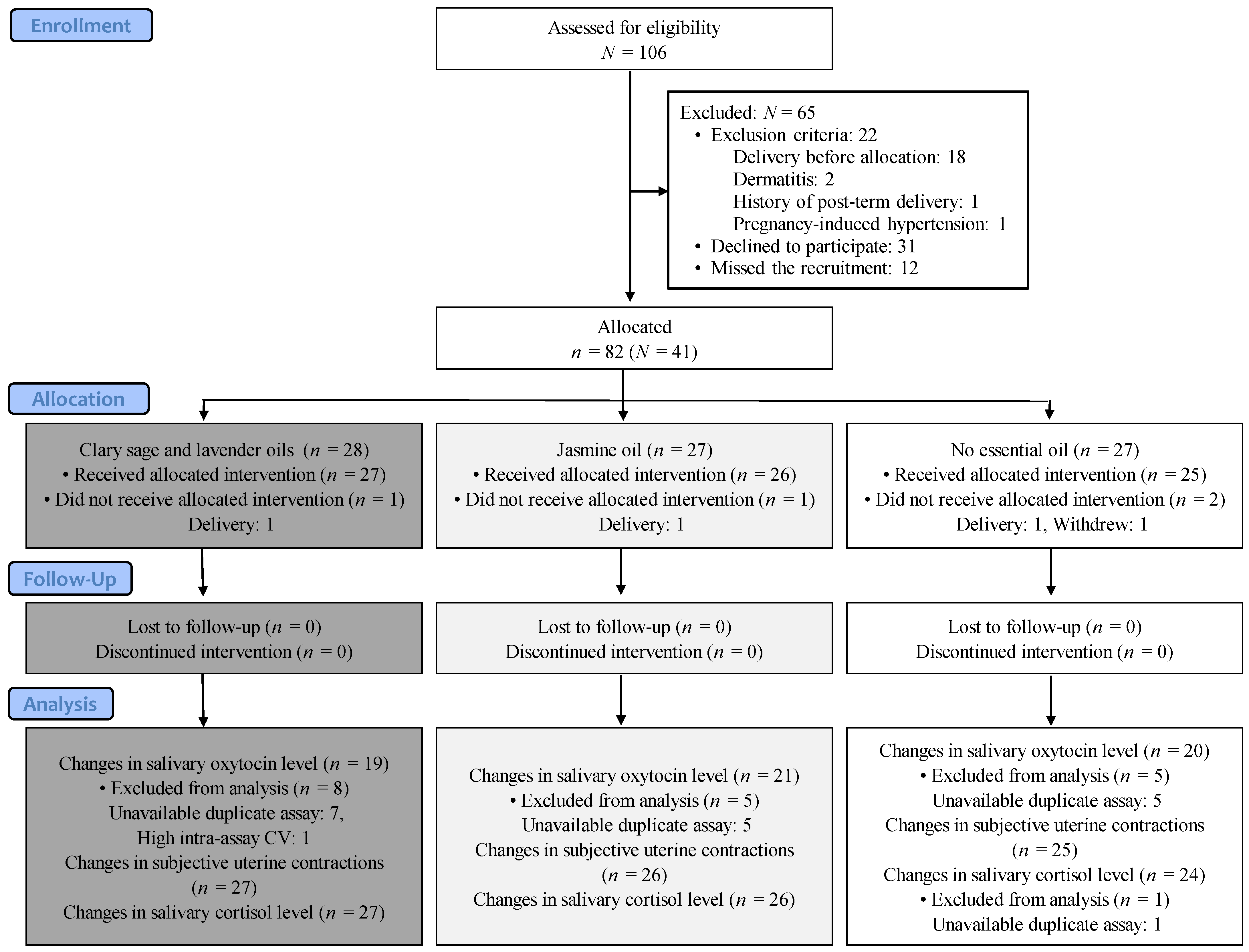
2.2. Participants and Setting
2.3. Masking
2.4. Sample Size
2.5. Interventions
2.5.1. Footbath Preparations in Experimental and Control Groups
2.5.2. Procedures
2.6. Outcome Measures
2.6.1. Primary Outcome: Changes in the Salivary Oxytocin Level before and after Using the Footbath
2.6.2. Secondary Outcome: Changes in the Subjective Uterine Contraction and Salivary Cortisol Level before and after Using the Footbath
2.6.3. Arrangement in Measurement of Salivary Oxytocin and Cortisol Levels
2.6.4. Adverse Events
2.7. Statistical Analysis
3. Results
3.1. Primary Outcome: Changes in Salivary Oxytocin Level before and after Using the Footbath
3.2. Secondary Outcomes: Changes in Subjective Uterine Contraction and Salivary Cortisol Level before and after Using the Aromatherapy Footbath
3.3. Adverse Events
4. Discussion
4.1. Primary Outcome: Changes in Salivary Oxytocin Level
4.2. Secondary Outcomes: Changes in Subjective Uterine Contraction and Salivary Cortisol Level
4.3. Implication for Clinical Practice
4.4. Limitations and Strengths
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American College of Nurse-Midwives; Midwives Alliance of North America; National Association of Certified Professional Midwives. Supporting healthy and normal physiologic childbirth: A consensus statement by the American College of Nurse-Midwives, Midwives Alliance of North America, and the National Association of Certified Professional Midwives. J. Midwifery Womens Health 2012, 57, 529–532. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.A.; Hamilton, B.E.; Osterman, M.J.K.; Driscoll, A.K. Births: Final data for 2019. Natl. Vital Stat. Rep. 2021, 70, 1–51. [Google Scholar] [PubMed]
- World Health Organization. WHO Recommendations for Induction of Labour. Available online: http://whqlibdoc.who.int/publications/2011/9789241501156_eng.pdf?ua=1 (accessed on 22 November 2021).
- Schwarz, C.; Gross, M.M.; Heusser, P.; Berger, B. Women’s perceptions of induction of labour outcomes: Results of an online-survey in Germany. Midwifery 2016, 35, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Munstedt, K.; Brenken, A.; Kalder, M. Clinical indications and perceived effectiveness of complementary and alternative medicine in departments of obstetrics in Germany: A questionnaire study. Eur. J. Obs. Gynecol. Reprod. Biol. 2009, 146, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Burns, E.; Blamey, C.; Ersser, S.J.; Lloyd, A.J.; Barnetson, L. The use of aromatherapy in intrapartum midwifery practice an observational study. Complement. Nurs. Midwifery 2000, 6, 33–34. [Google Scholar] [CrossRef]
- Weston, M.; Grabowska, C. Complementary therapy for induction of labour. Pract. Midwife 2013, 16, S16–S18. [Google Scholar]
- Evans, M. Postdates pregnancy and complementary therapies. Complement. Clin. Pract. 2009, 15, 220–224. [Google Scholar] [CrossRef]
- Kaviani, M.; Maghbool, S.; Azima, S.; Tabaei, M.H. Comparison of the effect of aromatherapy with Jasminum officinale and Salvia officinale on pain severity and labor outcome in nulliparous women. Iran. J. Nurs. Midwifery Res. 2014, 19, 666–672. [Google Scholar]
- Musil, A. Labor encouragement with essential oils. Midwifery Today Int. Midwife 2013, 107, 57–58. [Google Scholar]
- Kamel, R.M. The onset of human parturition. Arch. Gynecol. Obstet. 2010, 281, 975–982. [Google Scholar] [CrossRef]
- Buckley, S.J.H. Hormonal Physiology of Childbearing: Evidence and Implications for Women, Babies, and Maternity Care; Childbirth Connection Programs, National Partnership for Women & Families: Washington, DC, USA, 2015; pp. 3–34. [Google Scholar]
- Tadokoro, Y.; Horiuchi, S.; Takahata, K.; Shuo, T.; Sawano, E.; Shinohara, K. Changes in salivary oxytocin after inhalation of clary sage essential oil scent in term-pregnant women: A feasibility pilot study. BMC Res. Notes 2017, 10, 717. [Google Scholar] [CrossRef]
- Tarumi, W.; Shinohara, K. The effects of essential oil on salivary oxytocin concentration in postmenopausal women. J. Altern. Complement. Med. 2020, 26, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Mansour Lamadah, S. The effect of aromatherapy massage using lavender oil on the level of pain and anxiety during labour among primigravida women. Am. J. Nurs. Sci. 2016, 5, 37–44. [Google Scholar] [CrossRef]
- Zahra, A.; Leila, M.S. Lavender aromatherapy massages in reducing labor pain and duration of labor: A randomized controlled trial. Afr. J. Pharm. Pharmacol. 2013, 7, 426–430. [Google Scholar] [CrossRef]
- Vakilian, K.; Keramat, A. The effect of breathing technique with and without aromatherapy on the length of active phase and second stage of labour. Nurs. Midwifery Stud. 2013, 2, 115–119. [Google Scholar] [CrossRef]
- Perry, R.; Terry, R.; Watson, L.K.; Ernst, E. Is lavender an anxiolytic drug? A systematic review of randomised clinical trials. Phytomedicine 2012, 19, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Yokoyama, Y.; Irahara, M.; Aono, T. Influence of psychological stress on suckling-induced pulsatile oxytocin release. Obs. Gynecol. 1994, 84, 259–262. [Google Scholar]
- Posadzki, P.; Alotaibi, A.; Ernst, E. Adverse effects of aromatherapy: A systematic review of case reports and case series. Int. J. Risk Saf. Med. 2012, 24, 147–161. [Google Scholar] [CrossRef]
- Tisserand, R.; Young, R. Essential Oil Safety: A Guide for Health Care Professionals [Kindle 2nd version]. In Churchill Livingstone; Elsevier Health Sciences: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Kino, H. Obstetrics II. In Handbook of Aromatherapy for Medical Staffs, 1st ed.; Kawabata, K., Samejima, K., Onomura, K., Eds.; Medicus Shuppan, publishers Co., Ltd.: Osaka, Japan, 1999; pp. 64–67. (In Japanese) [Google Scholar]
- Ibata, M.; Uemura, M. Ninpu/Sanpu/Jokufu [Pregnant women/Parturient woman/puerperant]. In Beginner for Aromatherapy: Holistic Approach for Daily Nursing, 1st ed.; Imanishi, J., Arakawa, S., Eds.; Japanese Nursing Association Publishing Company: Tokyo, Japan, 2010; pp. 125–132. (In Japanese) [Google Scholar]
- Mamiya, M.; Hamada, Y.; Yabumoto, S.; Sakamoto, Y.; Asahi, K.; Nomura, A.; Inoue, H.; Sawatari, Y.; Sawatari, Y. Case report: Delivery through onset of labor stimulated by aromatherapy. Osaka Bosei Eisei Gakkai Zasshi 2000, 36, 40–41. [Google Scholar]
- Smith, C.A.; Levett, K.M.; Collins, C.T.; Dahlen, H.G.; Ee, C.C.; Suganuma, M. Massage, reflexology and other manual methods for pain management in labour. Cochrane Database Syst. Rev. 2018, 3, CD009290. [Google Scholar] [CrossRef]
- Kheirkhah, M.; Vali Pour, N.S.; Nisani, L.; Haghani, H. Comparing the effects of aromatherapy with rose oils and warm foot bath on anxiety in the first stage of labor in nulliparous women. Iran. Red Crescent Med. J. 2014, 16, e14455. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-F.; Wang, C.-H.; Chan, P.-T.; Chiang, H.-W.; Hu, T.-M.; Tam, K.-W.; Loh, E.-W. Labour pain control by aromatherapy: A meta-analysis of randomised controlled trials. Women Birth 2019, 32, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Alhafez, L.; Berghella, V. Evidence-based labor management: First stage of labor (part 3). Am. J. Obs. Gynecol. MFM 2020, 2, 100185. [Google Scholar] [CrossRef]
- Omoto, C. Maternal Nursing and Aromatherapy. In Nursing Research in Japanese Society of Aromatherapy, Aromatherapy for Nurse, 1st ed.; Medicus Shuppan Publishers Co., Ltd.: Osaka, Japan, 2005; pp. 133–144. (In Japanese) [Google Scholar]
- Mizoe, K.; Tanaka, H.; Ogahara, M.; Kasahara, S.; Ohno, Y. Introduction of aromatherapy to outpatient pregnant women (the second repot). Hyogoken Bosei Eisei Gakkaish. 2005, 14, 10–15. (In Japanese) [Google Scholar]
- Pauley, T.; Percival, R. Reducing post-dates induction numbers with post-dates complementary therapy clinics. Br. J. Midwifery 2014, 22, 630–633. [Google Scholar] [CrossRef]
- Tree of Life Co., Ltd. Table of Composition: Clary Sage Essential Oil, Lot No. 57; Tree of Life Co., Ltd.: Tokyo, Japan, 2015. (In Japanese) [Google Scholar]
- Tree of Life Co., Ltd. Table of Composition: Lavender Essential Oil, Lot No. 73; Tree of Life Co., Ltd.: Tokyo, Japan, 2016. (In Japanese) [Google Scholar]
- Tree of Life Co., Ltd. Table of Composition: Jasmine Essential Oil, Lot No. 39; Tree of Life Co., Ltd.: Tokyo, Japan, 2016. (In Japanese) [Google Scholar]
- Salimetrics, L.L.C.; SalivaBio, L.C.C. Saliva Collection and Handling Advice, 3rd ed. 2015; p. 5. Available online: https://www.salimetrics.com/assets/documents/Saliva_Collection_Handbook.pdf (accessed on 18 October 2016).
- Cyranowski, J.M.; Hofkens, T.L.; Frank, E.; Seltman, H.; Cai, H.-M.; Amico, J.A. Evidence of dysregulated peripheral oxytocin release among depressed women. Psychosom Med. 2008, 70, 967–975. [Google Scholar] [CrossRef]
- Skrundz, M.; Bolten, M.; Nast, I.; Hellhammer, D.H.; Meinlschmidt, G. Plasma oxytocin concentration during pregnancy is associated with development of postpartum depression. Neuropsychopharmacology 2011, 36, 1886–1893. [Google Scholar] [CrossRef] [PubMed]
- Heim, C.; Young, L.J.; Newport, D.J.; Mletzko, T.; Miller, A.H.; Nemeroff, C.B. Lower CSF oxytocin concentrations in women with a history of childhood abuse. Mol. Psychiatry 2009, 14, 954–958. [Google Scholar] [CrossRef] [PubMed]
- Spong, C.Y. Defining “term” pregnancy: Recommendations from the Defining “Term” Pregnancy Workgroup. JAMA 2013, 309, 2445–2446. [Google Scholar] [CrossRef]
- Campbell, M.K.; Ostbye, T.; Irgens, L.M. Post-term birth: Risk factors and outcomes in a 10-year cohort of Norwegian births. Obs. Gynecol. 1997, 89, 543–548. [Google Scholar] [CrossRef]
- Gordon, I.; Zagoory-Sharon, O.; Schneiderman, I.; Leckman, J.F.; Weller, A.; Feldman, R. Oxytocin and cortisol in romantically unattached young adults: Associations with bonding and psychological distress. Psychophysiology 2008, 45, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Oberg, A.S.; Frisell, T.; Svensson, A.C.; Iliadou, A.N. Maternal and fetal genetic contributions to postterm birth: Familial clustering in a population-based sample of 475,429 Swedish births. Am. J. Epidemiol. 2013, 177, 531–537. [Google Scholar] [CrossRef]
- Stotland, N.E.; Washington, A.E.; Caughey, A.B. Prepregnancy body mass index and the length of gestation at term. Am. J. Obs. Gynecol. 2007, 197, e1–e5. [Google Scholar] [CrossRef] [PubMed]
- Light, K.C.; Grewen, K.M.; Amico, J.A. More frequent partner hugs and higher oxytocin levels are linked to lower blood pressure and heart rate in premenopausal women. Biol. Psychol. 2005, 69, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Shima, S. CES-D Scale: Utsubyo (Yokuutsujotai) Jikohyoka Syakudo [CES-D Scale: Self-Administered Scale for Depression (Depressive State)], 1st ed.; Chiba Test Center Co., Ltd.: Tokyo, Japan, 1998; pp. 1–12. (In Japanese) [Google Scholar]
- Shimizu, H.; Imae, K. State-Trait Anxiety Inventory nonihonngobann (daigakuseyo) nosakusei [Development of the Japanese edition of the State-Trait Anxiety Inventory (STAI) for student use]. Jpn. J. Educ. Psychol. 1981, 29, 348–353. [Google Scholar]
- Daughters, K.; Manstead, A.S.R.; Hubble, K.; Rees, A.; Thapar, A.; van Goozen, S.H.M. Salivary oxytocin concentrations in males following intranasal administration of oxytocin: A double-blind, cross-over study. PLoS ONE 2015, 10, e0145104. [Google Scholar] [CrossRef]
- Goebel-Stengel, M.; Stengel, A.; Taché, Y.; Reeve, J.R. The importance of using the optimal plasticware and glassware in studies involving peptides. Anal. Biochem. 2011, 414, 38–46. [Google Scholar] [CrossRef]
- Enzo Life Sciences Inc. Product Manual Oxytocin ELISA Kit; Enzo Life Sciences Inc.: Farmingdale, NY, USA, 2015. [Google Scholar]
- Salimetrics, L.L.C. Expanded Range, High Sensitivity, Salivary Coritosl Enzyme Immnoassay Kit. 2016. Available online: https://www.salimetrics.com/assets/documents/1-3002n.pdf (accessed on 29 August 2017).
- Kavanagh, J.; Kelly, A.J.; Thomas, J. Breast stimulation for cervical ripening and induction of labour. Cochrane Database Syst. Rev. 2005, 2010, CD003392. [Google Scholar] [CrossRef]
- Smith, C.A.; Armour, M.; Dahlen, H.G. Acupuncture or acupressure for induction of labour. Cochrane Database Syst. Rev. 2017, 10, CD002962. [Google Scholar] [CrossRef]
- Kelly, A.J.; Kavanagh, J.; Thomas, J. Castor oil, bath and/or enema for cervical priming and induction of labour. Cochrane Database Syst. Rev. 2013, 7, CD003099. [Google Scholar] [CrossRef]
- Bowman, R.; Taylor, J.; Muggleton, S.; Davis, D. Biophysical effects, safety and efficacy of raspberry leaf use in pregnancy: A systematic integrative review. BMC Complement. Med. Ther. 2021, 21, 56. [Google Scholar] [CrossRef] [PubMed]
- Takahata, K.; Horiuchi, S.; Tadokoro, Y.; Sawano, E.; Shinohara, K. Oxytocin levels in low-risk primiparas following breast stimulation for spontaneous onset of labor: A quasi-experimental study. BMC Pregnancy Childbirth 2019, 19, 351. [Google Scholar] [CrossRef] [PubMed]
- Amico, J.A.; Finley, B.E. Breast stimulation in cycling women, pregnant women and a woman with induced lactation: Pattern of release of oxytocin, prolactin and luteinizing hormone. Clin. Endocrinol. 1986, 25, 97–106. [Google Scholar] [CrossRef]
- Christensson, K.; Nilsson, B.A.; Stock, S.; Matthiesen, A.S.; Uvnäs-Moberg, K. Effect of nipple stimulation on uterine activity and on plasma levels of oxytocin in full term, healthy, pregnant women. Acta Obs. Gynecol. Scand. 1989, 68, 205–210. [Google Scholar] [CrossRef]
- Finley, B.E.; Amico, J.; Castillo, M.; Seitchik, J. Oxytocin and prolactin responses associated with nipple stimulation contraction stress tests. Obs. Gynecol. 1986, 67, 836–839. [Google Scholar] [CrossRef] [PubMed]
- Yazdkhasti, M.; Pirak, A. The effect of aromatherapy with lavender essence on severity of labor pain and duration of labor in primiparous women. Complement. Ther. Clin. Pract. 2016, 25, 81–86. [Google Scholar] [CrossRef]
- Prevost, M.; Zelkowitz, P.; Tulandi, T.; Hayton, B.; Feeley, N.; Carter, C.S.; Joseph, L.; Pournajafi-Nazarloo, H.; Ping, E.Y.; Abenhaim, H.; et al. Oxytocin in pregnancy and the postpartum: Relations to labor and its management. Front. Public Health 2014, 2, 1–9. [Google Scholar] [CrossRef]
- Feldman, R.; Weller, A.; Zagoory-Sharon, O.; Levine, A. Evidence for a neuroendocrinological foundation of human affiliation: Plasma oxytocin levels across pregnancy and the postpartum period predict mother-infant bonding. Psychol. Sci. 2007, 18, 965–970. [Google Scholar] [CrossRef]
- McCullough, M.E.; Churchland, P.S.; Mendez, A.J. Problems with measuring peripheral oxytocin: Can the data on oxytocin and human behavior be trusted? Neurosci. Biobehav. Rev. 2013, 37, 1485–1492. [Google Scholar] [CrossRef]
- Valstad, M.; Alvares, G.A.; Egknud, M.; Matziorinis, A.M.; Andreassen, O.A.; Westlye, L.T.; Quintana, D.S. The correlation between central and peripheral oxytocin concentrations: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2017, 78, 117–124. [Google Scholar] [CrossRef]
- Uvnäs-Moberg, K.; Ekström-Bergström, A.; Berg, M.; Buckley, S.; Pajalic, Z.; Hadjigeorgiou, E.; Kotłowska, A.; Lengler, L.; Kielbratowska, B.; Leon-Larios, F.; et al. Maternal plasma levels of oxytocin during physiological childbirth—A systematic review with implications for uterine contractions and central actions of oxytocin. BMC Pregnancy Childbirth 2019, 19, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, A.R.; Fuchs, F.; Husslein, P.; Soloff, M.S. Oxytocin receptors in the human uterus during pregnancy and parturition. Am. J. Obs. Gynecol. 1984, 150, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Takemura, M.; Nomura, S.; Nobunaga, T.; Kubota, Y.; Inoue, T.; Hashimoto, K.; Kumazawa, I.; Ito, Y.; Ohashi, K.; et al. Expression of oxytocin receptor in human pregnant myometrium. Endocrinology 1996, 137, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Toda, M.; Morimoto, K.; Nagasawa, S.; Kitamura, K. Change in salivary physiological stress markers by spa bathing. Biomed. Res. 2006, 27, 11–14. [Google Scholar] [CrossRef]
| Clary Sage and Lavender Oil Group (n = 27) | Jasmine Oil Group (n = 26) | No Essential Oil Group (n = 25) | p-Value (vs. No Essential Oil Group) | |||||
|---|---|---|---|---|---|---|---|---|
| Clary Sage and Lavender Oil Group | Jasmine Oil Group | |||||||
| Age (years) * | 31.9 | [4.1] | 32.8 | [4.0] | 32.9 | [2.8] | 0.298 | 0.909 |
| Gestation weeks * | 38.6 | [0.5] | 38.8 | [0.5] | 38.6 | [0.4] | 0.894 | 0.163 |
| Due date confirmed by USG ≤12 GW | 25 | (92.6) | 25 | (96.2) | 25 | (100) | 0.491 | 1 |
| Primiparas | 19 | (70.4) | 12 | (46.2) | 16 | (64.0) | 0.625 | 0.2 |
| Married | 27 | (100) | 26 | (100) | 25 | (100) | - | - |
| BMI before pregnancy ≥ 29 | 0 | (0) | 0 | (0) | 0 | (0) | - | - |
| Country of origin: Japan | 27 | (100) | 26 | (100) | 25 | (100) | - | - |
| Education ≥ 12 years | 27 | (100) | 24 | (92.3) | 23 | (92.0) | 0.226 | 1 |
| Depression (CES-D score > 15) | 1 | (3.7) | 1 | (3.8) | 0 | (0) | 0.519 | 0.51 |
| Trait anxiety score (STAI) * | 37.4 | [7.3] | 38.1 | [6.8] | 35.5 | [5.3] | 0.293 | 0.137 |
| State anxiety score (STAI) * | 32.5 | [8.1] | 34.3 | [7.9] | 32.4 | [6.9] | 0.97 | 0.363 |
| Mean | SD | 95% CI | MD | SD | 95% CI | t | df | p-Value | |
|---|---|---|---|---|---|---|---|---|---|
| Clary sage and lavender oil group (n = 19) | |||||||||
| Pre | 139.5 | 106.5 | [88.1, 190.8] | 12.5 | 23.9 | [1.0, 24.1] | 2.29 | 18 | 0.035 |
| Post | 152.0 | 115.2 | [96.5, 207.5] | ||||||
| Jasmine oil group (n = 21) | |||||||||
| Pre | 166.2 | 136.7 | [104.0, 228.4] | 4.8 | 51.8 | [−18.8, 28.4] | 0.43 | 20 | 0.676 |
| Post | 171.0 | 141.4 | [106.6, 253.4] | ||||||
| No essential oil group (n = 20) | |||||||||
| Pre | 141.6 | 118.3 | [86.3, 197.0] | −3.3 | 71.0 | [−36.5, 29.9] | −0.21 | 19 | 0.839 |
| Post | 138.4 | 91.4 | [95.6, 181.1] | ||||||
| p-Value | |||
|---|---|---|---|
| Clary sage and lavender oil group (n = 27) | |||
| Increased | 3 | (11.1) | 0.882 |
| Unchanged | 22 | (81.5) | |
| Decreased | 2 | (7.4) | |
| Jasmine oil group (n = 26) | |||
| Increased | 7 | (26.9) | 0.544 |
| Unchanged | 17 | (65.4) | |
| Decreased | 2 | (7.7) | |
| No essential oil group (n = 25) | |||
| Increased | 4 | (16.0) | - |
| Unchanged | 20 | (80.0) | |
| Decreased | 1 | (4.0) | |
| Mean | SD | 95% CI | MD | SD | 95% CI | t | df | p-Value | |
|---|---|---|---|---|---|---|---|---|---|
| Clary sage and lavender oil group (n = 27) | |||||||||
| Pre | 3.84 | 1.36 | [3.30, 4.38] | −0.42 | 0.48 | [−0.62, −0.24] | −4.61 | 26 | <0.001 |
| Post | 3.41 | 1.15 | [2.96, 3.87] | ||||||
| Jasmine oil group (n = 26) | |||||||||
| Pre | 3.80 | 1.01 | [3.39, 4.21] | −0.41 | 0.53 | [−0.63, −0.19] | −3.91 | 25 | 0.001 |
| Post | 3.39 | 0.80 | [3.07, 3.71] | ||||||
| No essential oil group (n = 24) | |||||||||
| Pre | 3.90 | 1.14 | [3.42, 4.38] | −0.40 | 0.39 | [−0.56, −0.24] | −5.03 | 23 | <0.001 |
| Post | 3.50 | 1.01 | [3.07, 3.93] | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tadokoro, Y.; Takahata, K.; Shuo, T.; Shinohara, K.; Horiuchi, S. Changes in Salivary Oxytocin Level of Term Pregnant Women after Aromatherapy Footbath for Spontaneous Labor Onset: A Non-Randomized Experimental Study. Int. J. Environ. Res. Public Health 2023, 20, 6262. https://doi.org/10.3390/ijerph20136262
Tadokoro Y, Takahata K, Shuo T, Shinohara K, Horiuchi S. Changes in Salivary Oxytocin Level of Term Pregnant Women after Aromatherapy Footbath for Spontaneous Labor Onset: A Non-Randomized Experimental Study. International Journal of Environmental Research and Public Health. 2023; 20(13):6262. https://doi.org/10.3390/ijerph20136262
Chicago/Turabian StyleTadokoro, Yuriko, Kaori Takahata, Takuya Shuo, Kazuyuki Shinohara, and Shigeko Horiuchi. 2023. "Changes in Salivary Oxytocin Level of Term Pregnant Women after Aromatherapy Footbath for Spontaneous Labor Onset: A Non-Randomized Experimental Study" International Journal of Environmental Research and Public Health 20, no. 13: 6262. https://doi.org/10.3390/ijerph20136262
APA StyleTadokoro, Y., Takahata, K., Shuo, T., Shinohara, K., & Horiuchi, S. (2023). Changes in Salivary Oxytocin Level of Term Pregnant Women after Aromatherapy Footbath for Spontaneous Labor Onset: A Non-Randomized Experimental Study. International Journal of Environmental Research and Public Health, 20(13), 6262. https://doi.org/10.3390/ijerph20136262






