Coexistence of Anaemia and Stunting among Children Aged 6–59 Months in Ethiopia: Findings from the Nationally Representative Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting, Data Source, and Study Design
2.2. Data Collection
2.3. Variable and Measurements
2.4. Independent Variable
2.5. Data Analysis
3. Results
3.1. Sociodemographic Characteristics of the Sample
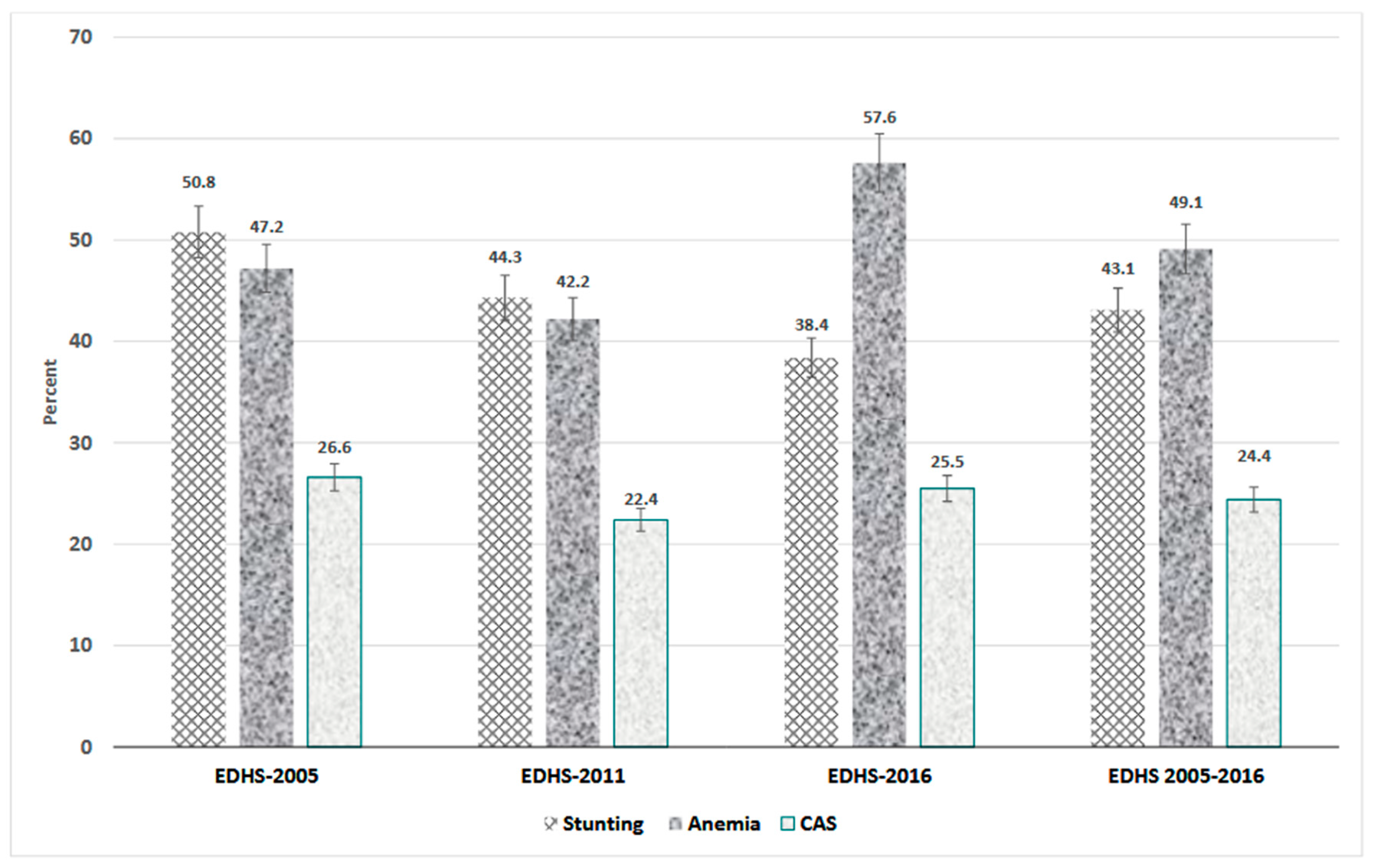
3.2. Prevalence of Anaemia, Stunting, and Concurrent Anaemia and Stunting (CAS)
3.3. Factors Associated with Concurrent Anaemia and Stunting (CAS)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Development Initiatives. 2018 Global Nutrition Report: Shining a Light to Spur Action on Nutrition; Global Nutrition: Bristol, UK, 2018. [Google Scholar]
- WHO. WHO Fact Sheets—Malnutrition. 2022. Available online: https://www.who.int/news-room/fact-sheets (accessed on 16 March 2022).
- World Health Organization (WHO). Malnutrition. 2019. Available online: https://www.who.int/news-room/fact-sheets/detail/malnutrition (accessed on 25 March 2022).
- Anaemia in Women and Children. Available online: https://www.who.int/data/gho/data/themes/topics/anaemia_in_women_and_children (accessed on 9 June 2022).
- Takele, B.A.; Gezie, L.D.; Alamneh, T.S. Pooled prevalence of stunting and associated factors among children aged 6–59 months in Sub-Saharan Africa countries: A Bayesian multilevel approach. PLoS ONE 2022, 17, e0275889. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.D.; Biggs, B.A.; Holton, S.; Nguyen, H.T.M.; Hanieh, S.; Fisher, J. Co-morbid anaemia and stunting among children of pre-school age in low- and middle-income countries: A syndemic. Public Health Nutr. 2019, 22, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Gosdin, L.; Martorell, R.; Bartolini, R.M.; Mehta, R.; Srikantiah, S.; Young, M.F. The Co-Occurrence of Anaemia and Stunting in Young Children. Matern. Child Nutr. 2018, 14, e12597. [Google Scholar] [CrossRef] [PubMed]
- Popkin, B.M.; Corvalan, C.; Grummer-Strawn, L.M. Dynamics of the double burden of malnutrition and the changing nutrition reality. Lancet 2020, 395, 65–74. [Google Scholar] [CrossRef]
- Castejon, H.V.; Ortega, P.; Amaya, D.; Gomez, G.; Leal, J.; Castejon, O.J. Co-existence of ia, Vitamin A Deficiency and Growth Retardation among Children 24–84 Months Old in Maracaibo, Venezuela. Nutr. Neurosci. 2004, 7, 113–119. [Google Scholar] [CrossRef]
- Orsango, A.Z.; Loha, E.; Lindtjørn, B.; Engebretsen, I.M.S. Co-morbid anaemia and stunting among children 2–5 years old in southern Ethiopia: A community-based cross-sectional study. BMJ Paediatr. Open 2021, 5, e001039. [Google Scholar] [CrossRef]
- Mohammed, S.H.; Larijani, B.; Esmaillzadeh, A. Concurrent anemia and stunting in young children: Prevalence, dietary and non-dietary associated factors. Nutr. J. 2019, 18, 10. [Google Scholar] [CrossRef]
- Soliman, A.T.; De Sanctis, V.; Kalra, S. Anemia and growth. Indian J. Endocrinol. Metab. 2014, 18 (Suppl. S1), S1–S5. [Google Scholar] [CrossRef]
- Bradley, A.; Woodruff, J.P.; Wirth, I.N.-T.; Beaulière, J.M.; Mamady, D.; Ayoya, M.A.; Rohner, F. Determinants of Stunting, Wasting, and Anemia in Guinean Preschool-Age Children: An Analysis of DHS Data From 1999, 2005, and 2012. 2018. Food Nutr Bull 2018, 39, 39–53. [Google Scholar]
- Lopez, A.; Cacoub, P.; Macdougall, I.C.; Peyrin-Biroulet, L. Iron deficiency anaemia. Lancet 2016, 387, 907–916. [Google Scholar] [CrossRef]
- EPHI; ICF. Ethiopia MiniDemographic and Health Survey 2019: Key Indicators; EPHI: Rockville, MD, USA; ICF: Rockville, MD, USA, 2019. [Google Scholar]
- United Nations Children’s Fund (UNICEF); United Nations University (UNU); World Health Organization (WHO). Iron Deficiency Anaemia: Assessment, Prevention, and Control. A Guide for Programme Managers; UNICEF, UNU and WHO: Geneva, Switzerland, 2001. [Google Scholar]
- De Onis, M.; Borghi, E.; Arimond, M.; Webb, P.; Croft, T.; Saha, K.; De-Regil, L.M.; Thuita, F.; Heidkamp, R.; Krasevec, J.; et al. Prevalence thresholds for wasting, overweight and stunting in children under 5 years. Public Health Nutr. 2019, 22, 175–179. [Google Scholar] [CrossRef]
- Federal Democratic Republic of Ethiopia (FDRE). 2013–2015. National Nutrition Programme; FDRE: Addis Ababa, Ethiopia, 2013. [Google Scholar]
- Federal Democratic Republic of Ethiopia. National Food and Nutrition Strategy; The Federal Democratic Republic of Ethiopia: Addis Ababa, Ethiopia, 2021. [Google Scholar]
- Ethiopian Public Health Institute (EPHI); ICF. Ethiopia Mini Demographic and Health Survey 2019: Final Report; EPHI: Rockville, MD, USA; ICF: Rockville, MD, USA, 2021. [Google Scholar]
- Sahiledengle, B.; Mwanri, L.; Petrucka, P.; Kumie, A.; Beressa, G.; Atlaw, D.; Tekalegn, Y.; Zenbaba, D.; Desta, F.; Teferu, Z.; et al. Determinants of undernutrition among young children in Ethiopia. Sci. Rep. 2022, 12, 20945. [Google Scholar] [CrossRef] [PubMed]
- Amare, H.H.; Lindtjorn, B. Concurrent anemia and stunting among schoolchildren in Wonago district in southern Ethiopia: A cross-sectional multilevel analysis. PeerJ 2021, 9, e11158. [Google Scholar] [CrossRef] [PubMed]
- EDHS; Central Statistical Agency (CSA) [Ethiopia]; ICF. Ethiopia Demographic and Health Survey 2016; CSA: Addis Ababa, Ethiopia; ICF: Rockville, MD, USA, 2016. [Google Scholar]
- EDHS; Central Statistical Agency [Ethiopia]; ORC Macro. Ethiopia Demographic and Health Survey 2005; Central Statistical Agency: Addis Ababa, Ethiopia; ORC Macro: Calverton, NY, USA, 2006.
- EDHS; Central Statistical Agency [Ethiopia]; ICF International. Ethiopia Demographic and Health Survey 2011; Central Statistical Agency: Addis Ababa, Ethiopia; ICF International: Reston, VA, USA, 2012. [Google Scholar]
- World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Vitamin and Mineral Nutrition Information System; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- WHO. Child Growth Standards. Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age. 2006. Available online: https://www.who.int/publications/i/item/924154693X (accessed on 25 February 2022).
- Mohammed, S.H.; Esmaillzadeh, A. The relationships among iron supplement use, Hb concentration and linear growth in young children: Ethiopian Demographic and Health Survey. Br. J. Nutr. 2017, 118, 730–736. [Google Scholar] [CrossRef]
- Yin, S.; Zhou, Y.; Li, H.; Cheng, Z.; Zhang, Y.; Zhang, L.; Liu, J.; Liu, J. Association of maternal BMI during early pregnancy with infant anemia: A large Chinese birth cohort. Nutr. Metab. 2020, 17, 32. [Google Scholar] [CrossRef]
- Oliveira, M.d.N.; Martorell, R.; Phuong, N. Risk factors associated with hemoglobin levels and nutritional status among Brazilian children attending daycare centers in Sao Paulo city, Brazil. Arch. Latinoam. Nutr. 2010, 60, 23–29. [Google Scholar]
- Afolabi, R.F.; Palamuleni, M.E. Exploring determinants of under-5 stunting in Malawi using a generalised linear mixed model. S. Afr. J. Child Health 2021, 15, 18–24. [Google Scholar] [CrossRef]
- Ali, Z.; Saaka, M.; Adams, A.G.; Kamwininaang, S.K.; Abizari, A.R. The effect of maternal and child factors on stunting, wasting and underweight among preschool children in Northern Ghana. BMC Nutr. 2017, 3, 31. [Google Scholar] [CrossRef] [PubMed]
- Addo, O.Y.; Stein, A.D.; Fall, C.H.; Gigante, D.P.; Guntupalli, A.M.; Horta, B.L.; Kuzawa, C.W.; Lee, N.; Norris, S.A.; Prabhakaran, P.; et al. Maternal height and child growth patterns. J. Pediatr. 2013, 163, 549–554. [Google Scholar] [CrossRef]
- Felisbino-Mendes, M.S.; Villamor, E.; Velasquez-Melendez, G. Association of maternal and child nutritional status in Brazil: A population based cross-sectional study. PLoS ONE 2014, 9, e87486. [Google Scholar] [CrossRef]
- Prendergast, A.J.; Humphrey, J.H. The stunting syndrome in developing countries. Paediatr. Int. Child Health 2014, 34, 250–265. [Google Scholar] [CrossRef]
- Crane, R.J.; Jones, K.D.J.; Berkley, J.A. Environmental Enteric Dysfunction: An Overview. Food Nutr. Bull. 2015, 36 (Suppl. S1), S76–S87. [Google Scholar] [CrossRef]
- Prevention CC for DC and CDC—Hookworm—Disease. 2019. Available online: https://www.cdc.gov/parasites/hookworm/disease.html (accessed on 19 April 2023).
- Oliveira, D.; Ferreira, F.S.; Atouguia, J.; Fortes, F.; Guerra, A.; Centeno-Lima, S. Infection by Intestinal Parasites, Stunting and Anemia in School-Aged Children from Southern Angola. PLoS ONE 2015, 10, e0137327. [Google Scholar] [CrossRef]
- Brito, L.L.; Barreto, M.L.; Silva, R.D.C.R.; Assis, A.M.O.; Reis, M.G.; Parraga, I.M.; Blanton, R.E. Moderate- and low-intensity co-infections by intestinal helminths and Schistosoma mansoni, dietary iron intake, and anemia in Brazilian children. Am. J. Trop. Med. Hyg. 2006, 75, 939–944. [Google Scholar] [CrossRef]
- Midzi, N.; Mtapuri-Zinyowera, S.; Mapingure, M.P.; Sangweme, D.; Chirehwa, M.T.; Brouwer, K.C.; Mudzori, J.; Hlerema, G.; Mutapi, F.; Kumar, N.; et al. Consequences of polyparasitism on anaemia among primary school children in Zimbabwe. Acta Trop. 2010, 115, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Accinelli, R.A.; Leon-Abarca, J.A. Solid fuel use is associated with anemia in children. Environ. Res. 2017, 158, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Retherford, R.D. Does biofuel smoke contribute to anaemia and stunting in early childhood? Int. J. Epidemiol. 2007, 36, 117–129. [Google Scholar] [CrossRef]
- Tarekegn, B.T.; Assimamaw, N.T.; Atalell, K.A.; Kassa, S.F.; Muhye, A.B.; Techane, M.A.; Alemu, T.G.; Wubneh, C.A.; Belay, G.M.; Tamir, T.T.; et al. Prevalence and associated factors of double and triple burden of malnutrition among child-mother pairs in Ethiopia: Spatial and survey regression analysis. BMC Nutr. 2022, 8, 34. [Google Scholar] [CrossRef] [PubMed]
| Variables | Total Weighted Frequency | Percent |
|---|---|---|
| Individual-level factors | ||
| Child factors | ||
| Sex | ||
| Male | 10,824 | 51.1 |
| Female | 10,348 | 48.9 |
| Age (months) | ||
| 6–11 | 2479 | 11.7 |
| 12–23 | 4504 | 21.3 |
| 24–35 | 4471 | 21.1 |
| 36–59 | 9719 | 45.9 |
| Birth order | ||
| Firstborn | 3698 | 17.5 |
| 2–4 | 9206 | 43.5 |
| 5 or higher | 8267 | 39.0 |
| Birth interval | ||
| <33 months | 14,566 | 68.8 |
| ≥33 months | 6606 | 31.2 |
| Perceived size of a child at birth | ||
| Larger | 6792 | 32.2 |
| Average | 8574 | 40.6 |
| Small | 5739 | 27.2 |
| Currently breastfeeding | ||
| Yes | 14,936 | 70.5 |
| No | 6236 | 29.5 |
| Received measles | ||
| Yes | 8086 | 47.5 |
| No | 8925 | 52.5 |
| Full vaccination | ||
| Yes | 4156 | 28.9 |
| No | 12,546 | 75.1 |
| Diarrhea | ||
| Yes | 3065 | 14.5 |
| No | 18,077 | 85.5 |
| Fever | ||
| Yes | 3564 | 16.9 |
| No | 17,569 | 83.1 |
| Children age 6–59 months given iron supplement | ||
| Yes | 1336 | 6.3 |
| No | 19,836 | 93.7 |
| Children age 6–59 months given deworming medication | ||
| Yes | 2982 | 14.1 |
| No | 18,190 | 85.9 |
| Parental factors | ||
| Mother’s age | ||
| <18 | 108 | 0.5 |
| 18–24 | 4524 | 21.4 |
| 25–34 | 11,146 | 52.6 |
| ≥35 | 5394 | 25.5 |
| Mother’s education | ||
| No education | 14,862 | 70.2 |
| Primary | 5321 | 25.1 |
| Secondary | 678 | 3.2 |
| Higher | 311 | 1.5 |
| Mother’s occupation | ||
| Not working | 11,184 | 53.0 |
| Non agriculture | 4659 | 22.1 |
| Agriculture | 5239 | 24.8 |
| ANC Visit | ||
| None | 7081 | 51.4 |
| 1–3 | 3438 | 24.9 |
| 4–7 | 2929 | 21.3 |
| 8+ | 318 | 2.3 |
| Maternal BMI (kg/m2) | ||
| <18.5 | 4416 | 21.0 |
| 18.5 to 24.9 | 15,609 | 74.2 |
| 25+ | 1001 | 4.8 |
| Any anaemia | ||
| Yes | 5070 | 24.5 |
| No | 15,601 | 75.5 |
| Maternal stature | ||
| Very short (<145 cm) | 466 | 2.2 |
| Short (145 to <155 cm) | 7337 | 34.9 |
| Normal/Tall (155 to <200 cm) | 13,237 | 62.9 |
| Listening to radio | ||
| Not at all | 13,182 | 62.3 |
| Yes | 7983 | 37.7 |
| Watching television | ||
| Not at all | 16,532 | 78.1 |
| Yes | 4625 | 21.9 |
| Household factors | ||
| Wealth index | ||
| Poor | 9640 | 45.5 |
| Middle | 4439 | 21.0 |
| Rich | 7094 | 33.5 |
| Household Size | ||
| 1–4 | 5001 | 23.6 |
| ≥5 | 16,171 | 76.4 |
| Place of cooking | ||
| In the house | 8586 | 49.7 |
| In separate building | 7089 | 41.1 |
| Outdoors | 1585 | 9.2 |
| Type of cooking fuel | ||
| Clean fuels | 349 | 1.7 |
| Solid fuels | 20,441 | 98.3 |
| Toilet facility | ||
| Improved | 2127 | 10.2 |
| Unimproved | 9368 | 44.8 |
| Open defecation | 9421 | 45.0 |
| Source of drinking water | ||
| Improved | 9571 | 45.8 |
| Unimproved | 11,338 | 54.2 |
| Household flooring | ||
| Improved | 1892 | 8.9 |
| Unimproved | 19,274 | 91.1 |
| Time to reach a water source | ||
| On premises | 1489 | 7.0 |
| ≤30 min | 11,863 | 56.0 |
| 31–60 min | 4520 | 21.4 |
| >60 min | 3300 | 15.6 |
| Community Level Factors | ||
| Residence | ||
| Urban | 2279 | 10.8 |
| Rural | 18,894 | 89.2 |
| Region # | ||
| Large centrals | 19,459 | 91.9 |
| Small peripherals | 1220 | 5.8 |
| Metropolis | 493 | 2.3 |
| Ecological Zone | ||
| Tropical zone | 3148 | 14.9 |
| Subtropical zone | 14,924 | 70.5 |
| Cool zone | 3100 | 14.6 |
| EDHS | ||
| 2005 | 3898 | 18.4 |
| 2011 | 8943 | 42.2 |
| 2016 | 8332 | 39.4 |
| Variables | Concurrent Anaemia and Stunting (CAS), n (%) | Crude OR | p-Value | |
|---|---|---|---|---|
| Yes | No | |||
| Child factors | ||||
| Sex | ||||
| Male | 2789 (54.0) | 8035 (50.2) | 1.14 (1.07–1.22) | p < 0.001 |
| Female | 2376 (46.0) | 7972 (49.8) | Ref. | |
| Age (months) | ||||
| 6–11 | 380 (7.4) | 2098 (13.1) | Ref. | |
| 12–23 | 1326 (25.7) | 3178 (19.9) | 2.69 (2.34–3.09) | p < 0.001 |
| 24–35 | 1297 (25.1) | 3173 (19.8) | 2.95 (2.56–3.39) | p < 0.001 |
| 36–59 | 2163 (41.9) | 7556 (47.2) | 1.89 (1.65–2.16) | p < 0.001 |
| Birth order | ||||
| First born | 812 (15.7) | 2886 (18.0) | Ref. | |
| 2–4 | 2186 (42.3) | 7020 (43.9) | 1.18 (1.07–1.29) | 0.001 |
| 5 or higher | 2167 (41.9) | 6100 (38.1) | 1.40 (1.27–1.55) | p < 0.001 |
| Birth interval | ||||
| <33 months | 3569 (69.1) | 10,996 (68.7) | 0.98 (0.92–1.06) | 0.767 |
| ≥33 months | 1596 (30.9) | 5010 (31.3) | Ref. | |
| Perceived size of a child at birth | ||||
| Larger | 1555 (30.2) | 5.236 (32.8) | Ref. | |
| Average | 1969 (38.2) | 6604 (41.4) | 1.09 (1.01–1.19) | 0.03 |
| Small | 1627 (31.6) | 4112 (25.8) | 1.45 (1.33–1.58) | p < 0.001 |
| Currently breastfeeding | ||||
| Yes | 3809 (73.7) | 11,127 (69.5) | 1.08 (1.01–1.16) | 0.022 |
| No | 1356 (26.3) | 4879 (30.5) | Ref. | |
| Full vaccination | ||||
| Yes | 941 (23.0) | 3215 (25.5) | Ref. | |
| No | 3153 (77.0) | 9393 (74.5) | 1.21 (1.11–1.32) | p < 0.001 |
| Diarrhea | ||||
| Yes | 841 (16.3) | 2224 (13.9) | 1.25 (1.14–1.37) | p < 0.001 |
| No | 4319 (83.7) | 13,758 (86.1) | Ref. | |
| Fever | ||||
| Yes | 959 (18.6) | 2605 (16.3) | 1.19 (1.09–1.29) | p < 0.001 |
| No | 4196 (81.4) | 13,373 (83.7) | Ref. | |
| Children age 6–59 months given iron supplement | ||||
| Yes | 309 (6.0) | 1026 (6.4) | Ref. | |
| No | 4856 (94.0) | 14,980 (93.6) | 1.03 (0.91–1.18) | 0.623 |
| Children age 6–59 months given deworming medication | ||||
| Yes | 652 (12.6) | 2330 (14.6) | Ref. | |
| No | 4513 (87.4) | 13,677 (85.4) | 1.18 (1.07–1.31) | 0.001 |
| Parental factors | ||||
| Mother’s age | ||||
| <18 | 33 (0.6) | 75 (0.5) | 0.90 (0.56–1.44) | 0.665 |
| 18–24 | 1110 (21.5) | 3413 (21.3) | 0.96 (0.87–1.06) | 0.490 |
| 25–34 | 2727 (52.8) | 8419 (52.6) | 0.99 (0.92–1.08) | 0.981 |
| ≥35 | 1295 (25.1) | 4099 (25.6) | Ref. | |
| Mother’s education | ||||
| No education | 3881 (75.1) | 10,980 (68.6) | 6.06 (3.95–9.29) | p < 0.001 |
| Primary | 1159 (22.4) | 4.162 (26.0) | 4.35 (2.83–6.69) | p < 0.001 |
| Secondary | 102 (2.0) | 576 (3.6) | 2.65 (1.67–4.22) | p < 0.001 |
| Higher | 23 (0.5) | 287 (1.8) | Ref. | |
| Mother’s occupation | ||||
| Not working | 2828 (54.9) | 8355 (52.4) | 1.29 (1.18–1.41) | p < 0.001 |
| Agriculture | 1312 (25.5) | 3927 (24.6) | 1.24 (1.11–1.39) | p < 0.001 |
| Non agriculture | 1004 (19.5) | 3656 (22.9) | Ref. | |
| ANC visit | ||||
| None | 1949 (57.1) | 5132 (49.6) | 2.11 (1.63–2.72) | p < 0.001 |
| 1–3 | 817 (23.9) | 2621 (25.3) | 1.71 (1.32–2.22) | p < 0.001 |
| 4–7 | 591 (17.3) | 2337 (22.6) | 1.18 (0.91–1.54) | 0.219 |
| 8+ | 54 (1.6) | 264 (2.5) | Ref. | |
| Maternal BMI (kg/m2) | ||||
| <18.5 | 1215 (23.7) | 3202 (20.1) | Ref. | |
| 18.5 to 24.9 | 3761 (73.4) | 11,848 (74.5) | 0.80 (0.74–0.87) | p < 0.001 |
| 25+ | 149 (2.9) | 852 (5.4) | 0.43 (0.36–0.51) | p < 0.001 |
| Any anaemia | ||||
| Yes | 1550 (30.4) | 3520 (22.6) | 1.42 (1.32–1.53) | p < 0.001 |
| No | 3543 (69.6) | 12,058 (77.4) | Ref. | |
| Maternal stature | ||||
| Very short (<145 cm) | 157 (3.1) | 309 (1.9) | 1.77 (1.41–2.22) | p < 0.001 |
| Short (145 to <155 cm) | 2059 (40.2) | 5278 (33.2) | 1.39 (1.29–1.49) | p < 0.001 |
| Normal/Tall (155 to <200 cm) | 2909 (56.7) | 10,328 (64.9) | Ref. | |
| Listening to radio | ||||
| Not at all | 3332 (64.6) | 9849 (61.5) | 1.25 (1.17–1.35) | p < 0.001 |
| Yes | 1828 (35.4) | 6155 (38.5) | Ref. | |
| Watching television | ||||
| Not at all | 4197 (81.3) | 12,335 (77.1) | 1.71 (1.56–1.87) | p < 0.001 |
| Yes | 968 (18.7) | 3657 (22.9) | Ref. | |
| Household factors | ||||
| Wealth index | ||||
| Poor | 2732 (52.9) | 6908 (43.2) | 1.90 (1.75–2.06) | p < 0.001 |
| Middle | 1048 (20.3) | 3391 (21.2) | 1.39 (1.25–1.55) | p < 0.001 |
| Rich | 1386 (26.8) | 5707 (35.6) | Ref. | |
| Household size | ||||
| 1–4 | 1114 (21.6) | 3888 (24.3) | 0.83 (0.77–0.90) | p < 0.001 |
| ≥5 | 4052 (78.4) | 12,119 (75.7) | Ref. | |
| Place of cooking | ||||
| In the house | 2324 (56.3) | 6262 (47.7) | Ref. | |
| In separate building | 1438 (34.9) | 5651 (43.0) | 0.72 (0.66–0.79) | p < 0.001 |
| Outdoors | 362 (8.8) | 1223 (9.3) | 0.79 (0.71–0.89) | p < 0.001 |
| Type of cooking fuel | ||||
| Clean fuels | 41 (0.8) | 308 (2.0) | Ref. | |
| Solid fuels | 5019 (99.2) | 15,421 (98.0) | 3.69 (2.72–5.01) | p < 0.001 |
| Toilet facility | ||||
| Improved | 352 (6.9) | 1774 (11.2) | Ref. | |
| Unimproved | 2147 (42.1) | 7221 (45.7) | 1.57 (1.39–1.77) | p < 0.001 |
| Open defecation | 2599 (51.0) | 6822 (43.1) | 2.18 (1.94–2.44) | p < 0.001 |
| Source of drinking water | ||||
| Improved | 2228 (43.7) | 7343 (46.4) | Ref. | |
| Unimproved | 2870 (56.3) | 8468 (53.6) | 1.24 (1.15–1.33) | p < 0.001 |
| Household flooring | ||||
| Improved | 251 (4.9) | 1641 (10.3) | Ref. | |
| Unimproved | 4915 (95.1) | 14,359 (89.7) | 2.28 (2.02–2.57) | p < 0.001 |
| Time to reach a water source | ||||
| On premises | 199 (3.9) | 1289 (8.1) | Ref. | |
| ≤30 min | 2889 (55.9) | 8973 (56.1) | 1.94 (1.70–2.23) | p < 0.001 |
| 31–60 min | 1197 (23.2) | 3322 (20.7) | 2.26 (1.95–2.63) | p < 0.001 |
| >60 min | 879 (17.0) | 2421 (15.1) | 2.46 (2.12–2.85) | p < 0.001 |
| Community Level Factors | ||||
| Residence | ||||
| Urban | 333 (6.4) | 1946 (12.2) | Ref. | |
| Rural | 4833 (93.6) | 14,060 (87.8) | 2.22 (1.98–2.48) | p < 0.001 |
| Region | ||||
| Large centrals | 4763 (92.2) | 14,696 (91.8) | 1.22 (1.09–1.37) | 0.001 |
| Small peripherals | 335 (6.5) | 885 (5.5) | 1.49 (1.32–1.69) | p < 0.001 |
| Metropolis | 67 (1.3) | 426 (2.7) | Ref. | |
| Ecological zone | ||||
| Tropical zone | 741 (14.3) | 2406 (15.0) | 0.92 (0.80–1.04) | 0.199 |
| Subtropical zone | 3532 (68.4) | 11,392 (71.2) | 0.71 (0.62–0.81) | p < 0.001 |
| Cool zone | 892 (17.3) | 2208 (13.8) | Ref. | |
| EDHS | ||||
| 2005 | 1038 (20.1) | 2859 (17.9) | 1.01 (0.91–1.10) | 0.954 |
| 2011 | 2004 (38.8) | 6939 (43.3) | 0.92 (0.85–0.99) | 0.029 |
| 2016 | 2124 (41.1) | 6208 (38.8) | Ref. | |
| Variables | Null Model | Model I a | Model II b | Model III c | Model IV d |
|---|---|---|---|---|---|
| AOR (95%CI) | AOR (95%CI) | AOR (95%CI) | AOR (95%CI) | ||
| Individual-Level Factors | |||||
| Child factors | |||||
| Sex | |||||
| Male | 0.80 (0.73–0.88) ** | 0.81 (0.73–0.91) ** | |||
| Female | Ref. | Ref. | |||
| Age (months) | |||||
| 6–11 | Ref. | Ref. | |||
| 12–23 | 2.83 (2.43–3.28) ** | 3.07 (2.58–3.66) ** | |||
| 24–35 | 3.55 (3.01–4.18) ** | 4.13 (3.40–5.01) ** | |||
| 36–59 | 2.17 (1.78–2.64) ** | 2.51 (1.97–3.20) ** | |||
| Birth order | |||||
| First born | Ref. | Ref. | |||
| 2–4 | 1.08 (0.94–1.24) | 1.01 (0.85–1.17) | |||
| 5 or higher | 1.18 (1.03–1.37) * | 1.09 (0.90–1.32) | |||
| Perceived size of a child at birth | |||||
| Larger | Ref. | Ref. | |||
| Average | 1.12 (1.01–1.26) * | 1.09 (0.95–1.25) | |||
| Small | 1.47 (1.30–1.66) ** | 1.47 (1.28–1.69) ** | |||
| Currently breastfeeding | |||||
| Yes | 1.13 (1.01–1.27) * | 1.11 (0.97–1.27) | |||
| No | Ref. | Ref. | |||
| Full vaccination | |||||
| Yes | Ref. | Ref. | |||
| No | 1.08 (0.97–1.21) | 1.02 (0.89–1.16) | |||
| Diarrhea | |||||
| Yes | 1.11 (0.98–1.25) | 1.09 (0.94–1.25) | |||
| No | Ref. | Ref. | |||
| Fever | |||||
| Yes | 1.07 (0.95–1.19) | 1.11 (0.97–1.27) | |||
| No | Ref. | Ref. | |||
| Children age 6–59 months given deworming medication | |||||
| Yes | Ref. | Ref. | |||
| No | 1.13 (0.98–1.30) | 1.11 (0.95–1.28) | |||
| Parental factors | |||||
| Mother’s education | |||||
| No education | 4.13 (2.25–7.57) ** | 3.66 (1.85–7.22) ** | |||
| Primary | 3.64 (1.99–6.65) ** | 3.48 (1.77–6.84) ** | |||
| Secondary | 2.95 (1.56–5.56) * | 3.13 (1.54–6.37) * | |||
| Higher | Ref. | Ref. | |||
| Mother’s occupation | |||||
| Not working | 1.15 (1.01–1.29) * | 1.03 (0.90–1.19) | |||
| Agriculture | 1.11 (0.96–1.28) | 0.98 (0.83–1.16) | |||
| Non agriculture | Ref. | Ref. | |||
| ANC visit | |||||
| None | 1.14 (0.83–1.55) | 0.86 (0.60–1.24) | |||
| 1–3 | 1.05 (0.77–1.44) | 0.81 (0.56–1.17) | |||
| 4–7 | 0.84 (0.62–1.14) | 0.71 (0.49–1.01) | |||
| 8+ | Ref. | Ref. | |||
| Maternal BMI (kg/m2) | |||||
| <18.5 | Ref. | Ref. | |||
| 18.5 to 24.9 | 0.81 (0.73–0.89) ** | 0.82 (0.73–0.92) * | |||
| 25+ | 0.53 (0.41–0.69) ** | 0.60 (0.45–0.81) * | |||
| Any anaemia | |||||
| Yes | 1.30 (1.17–1.44) ** | 1.25 (1.11–1.41) ** | |||
| No | Ref. | Ref. | |||
| Maternal stature | |||||
| Very short (<145 cm) | 1.93 (1.41–2.62) ** | 2.04 (1.44–2.91) ** | |||
| Short (145 to <155 cm) | 1.43 (1.29–1.57) ** | 1.48 (1.32–1.65) ** | |||
| Normal/Tall (155 to <200 cm) | Ref. | Ref. | |||
| Listening to radio | |||||
| Not at all | 0.96 (0.86–1.07) | 0.88 (0.78–1.01) | |||
| Yes | Ref. | Ref. | |||
| Watching television | |||||
| Not at all | 1.33 (1.16–1.52) ** | 1.07 (0.91–1.25) | |||
| Yes | Ref. | Ref. | |||
| Household factors | |||||
| Wealth index | |||||
| Poor | 1.38 (1.22–1.57) ** | 1.15 (0.95–1.37) | |||
| Middle | 1.09 (0.95–1.25) | 0.98 (0.81–1.18) | |||
| Rich | Ref. | Ref. | |||
| Household size | |||||
| 1–4 | 0.88 (0.81–0.97) * | 0.88 (0.77–1.02) | |||
| ≥5 | Ref. | Ref. | |||
| Place of cooking | |||||
| In the house | Ref. | Ref. | |||
| In separate building | 0.84 (0.77–0.93) ** | 0.90 (0.79–1.03) | |||
| Outdoors | 0.83 (0.74–0.94) * | 0.82 (0.69–0.97) * | |||
| Type of cooking fuel | |||||
| Clean fuels | Ref. | Ref. | |||
| Solid fuels | 2.21 (1.48–3.31) ** | 1.29 (0.78–2.15) | |||
| Toilet facility | |||||
| Improved | Ref. | Ref. | |||
| Unimproved | 1.16 (1.01–1.34) * | 1.20 (0.98–1.47) | |||
| Open defecation | 1.41 (1.21–1.63) ** | 1.57 (1.27–1.92) ** | |||
| Source of drinking water | |||||
| Improved | Ref. | Ref. | |||
| Unimproved | 0.95 (0.86–1.05) | 0.88 (0.77–1.01) | |||
| Household flooring | |||||
| Improved | Ref. | Ref. | |||
| Unimproved | 1.28 (1.08–1.51) * | 1.13 (0.89–1.44) | |||
| Time to reach a water source | |||||
| On premises | Ref. | Ref. | |||
| ≤30 min | 1.08 (0.91–1.29) | 0.90 (0.69–1.16) | |||
| 31–60 min | 1.22 (1.02–1.47) * | 1.02 (0.78–1.34) | |||
| >60 min | 1.26 (1.04–1.53) * | 1.05 (0.79–1.38) | |||
| Community Level Factors | |||||
| Residence | |||||
| Urban | Ref. | Ref. | |||
| Rural | 2.34 (2.07–2.65) ** | 1.41 (1.10–1.82) * | |||
| Region | |||||
| Large centrals | 0.83 (0.73–0.95) * | 0.79 (0.63–0.98) * | |||
| Small peripherals | 1.01 (0.88–1.16) | 0.89 (0.71–1.12) | |||
| Metropolis | Ref. | Ref. | |||
| Ecological zone | |||||
| Tropical zone | 0.82 (0.71–0.97) * | 0.92 (0.71–1.19) | |||
| Subtropical zone | 0.70 (0.62–0.80) ** | 0.81 (0.65–1.01) | |||
| Cool zone | Ref. | Ref. | |||
| EDHS | |||||
| 2005 | 0.99 (0.90–1.10) | 0.98 (0.90–1.11) | |||
| 2011 | 0.93 (0.86–1.01) | 0.82 (0.72–0.93) * | |||
| 2016 | Ref. | Ref. | |||
| Random effect | |||||
| Variance (SE) | 0.1632 (0.0001) | 0.1177 (0.0014) | 0.1992 (0.0001) | 0.1426 (0.0006) | 0.1851 (0.0018) |
| ICC (%) | 4.72 | 3.45 | 5.71 | 4.15 | 5.32 |
| MOR | 1.59 | 1.49 | 1.68 | 1.55 | 1.65 |
| PCV (%) | Ref. | 27.87 | 22.05 | 12.62 | 13.41 |
| Model comparison | |||||
| LL | −10,915.60 | −5699.8017 | −8563.7301 | −10,772.627 | −4430.9835 |
| Deviance | 21,831.20 | 11,399.6 | 17,127.46 | 21,545.254 | 8861.96 |
| AIC | 21,835.2 | 11,459.6 | 17,157.46 | 21,563.25 | 8959.967 |
| BIC | 21,850.98 | 11,677.97 | 17,272.55 | 21,634.25 | 9305.986 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahiledengle, B.; Mwanri, L.; Petrucka, P.; Agho, K.E. Coexistence of Anaemia and Stunting among Children Aged 6–59 Months in Ethiopia: Findings from the Nationally Representative Cross-Sectional Study. Int. J. Environ. Res. Public Health 2023, 20, 6251. https://doi.org/10.3390/ijerph20136251
Sahiledengle B, Mwanri L, Petrucka P, Agho KE. Coexistence of Anaemia and Stunting among Children Aged 6–59 Months in Ethiopia: Findings from the Nationally Representative Cross-Sectional Study. International Journal of Environmental Research and Public Health. 2023; 20(13):6251. https://doi.org/10.3390/ijerph20136251
Chicago/Turabian StyleSahiledengle, Biniyam, Lillian Mwanri, Pammla Petrucka, and Kingsley Emwinyore Agho. 2023. "Coexistence of Anaemia and Stunting among Children Aged 6–59 Months in Ethiopia: Findings from the Nationally Representative Cross-Sectional Study" International Journal of Environmental Research and Public Health 20, no. 13: 6251. https://doi.org/10.3390/ijerph20136251
APA StyleSahiledengle, B., Mwanri, L., Petrucka, P., & Agho, K. E. (2023). Coexistence of Anaemia and Stunting among Children Aged 6–59 Months in Ethiopia: Findings from the Nationally Representative Cross-Sectional Study. International Journal of Environmental Research and Public Health, 20(13), 6251. https://doi.org/10.3390/ijerph20136251









