Examining the New-Member Effect to an Established Community-Based Physical Activity Program for Older Adults in England
Abstract
1. Introduction
Public Health Program Background
2. Materials and Methods
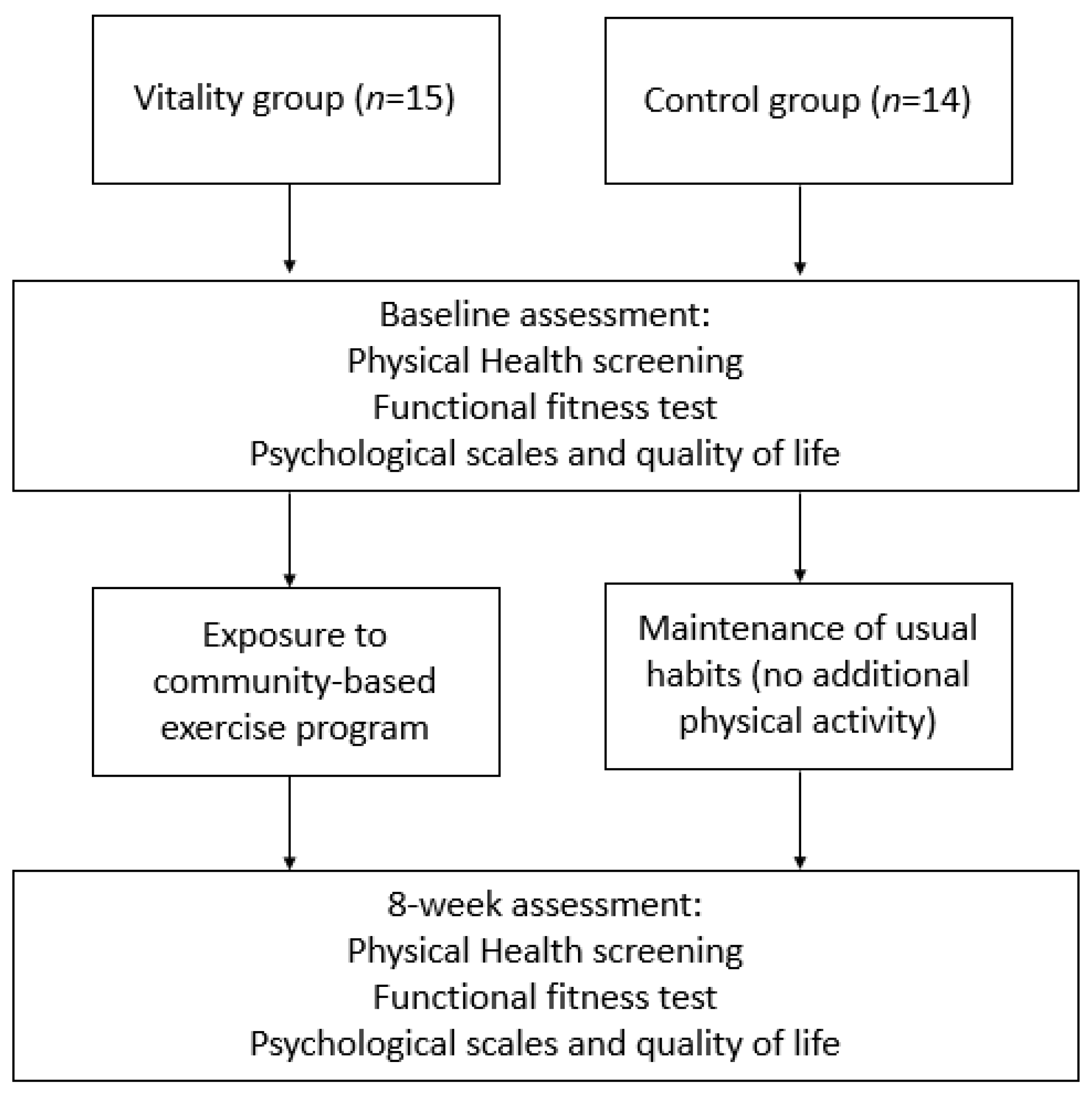
2.1. Ethics and Participant Recruitment
2.2. Procedures
2.3. Basic Physical Health Measures
2.4. Functional Fitness Assessments
2.5. Psychological Scales and Quality of Life
2.5.1. Enjoyment
2.5.2. Self-Efficacy
2.5.3. Quality of Life
2.6. Data Analysis
3. Results
3.1. Basic Physical Health Measurements
3.2. Functional Fitness Measurements
3.3. Psychological Scales and QOL
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Session Phase | Exercise | Example Instructions |
|---|---|---|
| Warm up & mobility: | Massage joints | Gently, massage/rub your joints in this order: hands/wrists; upper forearm; around elbows; upper arm; around shoulders; knuckles into chest and down to torso; front and sides of upper leg, and; around knees and hips (no leaning forward). |
| March | Then rock your feet from heels to toes and then march your feet moving one each at a time into a rhythm. Once up to speed—Keep it up! | |
| Upper body: | Finger taps | Relax arms and hands. Tap thumb to the top of every finger (index to little) and reverse. Make sure you exaggerate the movements. Ensure you tap the finger and open the hand every time. |
| Hand flashes | Relax arms and hands. With the palms down, open the hands, stretch them in a star shape, hold for 3 s. Remember not to hold your breath, while you hold the stretch. Rest/relax the hand, do not make a fist. Repeat exercise again with palms up | |
| Shoulder Rolls | Slowly ease both shoulders up towards your ears. Gently ease them back and down. Make these movements a continuous circle. Pause in the down position for a moment before releasing and starting again. | |
| Reaching Forward | Starting with single arms, push your hand forward from your chest to the front and return. Repeat with the other arm. Sit tall, don’t lean forward. When you feel ready try doing the exercise with both arms together. | |
| Tap & Clap | Sit on the chair. Tap both hands gently on your thighs and then clap your hands together in front of you. Repeat 3 times. Without leaning forward, gradually take your clap higher towards the ceiling. Clap low, middle and high. Repeat starting the claps low again. | |
| Lower body: | The Can-Can | Without leaning back, lift one knee—taking your foot off the floor and lower back down. Then with the same leg, lift and straighten the leg from the knee, try to feel the squeeze on the front of the thigh, don’t lock the knee out, and keep it soft. Repeat with the other leg. |
| Prepare to stand | Set posture—follow posture set up instructions with your feet slightly further back towards the chair, heels on the floor. Hold on to the side of the chair. Lean forward slightly. Take your weight onto your feet and legs. Lift bottom off the seat 3–4 inches. Then lower back down to sit. Keep looking forward, ‘chin on shelf’ parallel to the floor. | |
| Heel, Toe, Heel | Sit tall, don’t look down. Hold the chair for support if needed. Ease one foot forward so the heel is level with the toes on the other leg. Pull toes up so the heel is to the floor. Now replace the heel with the toe, aim for the same spot on the floor. Then alternate from the heel to toe. Place firmly but avoid banging the heel down. Repeat on the other foot. | |
| Ankle Rolls | Without leaning back, lift one foot slightly off the floor and circle the ankle 3 times clockwise. Place the foot down and repeat on the other leg. Then go back to your first ankle and repeat anticlockwise. Then repeat on the other foot. | |
| Chair Marching | March the feet—lift your knees up and down, taking your foot off the floor. Ensure you alternate legs. Swing your arms backwards and forwards in time with your legs. | |
| Cool down & stretches: | March | Then rock your feet from heels to toes and then march your feet moving one each at a time into a rhythm. Start to slow your speed every 10 s until you are just tapping the floor with your toes. |
| Spine Twist | Place hands together into a prayer position. Place your hands on your breastbone. Keep your feet firmly on the floor and your bottom on the chair with nose and chin over your hands; twist your upper body very slowly to the right with your head following the movement. Slowly return to the centre, repeat to the left. | |
| Side bends | With your shoulders back and your hands resting down by your sides. Keep your feet firmly on the floor and your bottom on the chair. Slowly reach down to the right. Return to centre, reset your posture; repeat to the left. Only go as far as is comfortable. Try not to lean forward. | |
| Triceps Stretch | Place right hand towards right shoulder. Let the right-hand slide over the right shoulder. Feel the stretch in the back of the upper arm. If possible, use your left hand to encourage the elbow up. Repeat on the other side. | |
| Hamstring Stretch | Move to sit on the front 1/3 of the chair. Straighten one leg and place the heel on the floor. Keep the other knee bent and foot on the floor. Sit up tall and lean slightly forward from the hip. Feel the stretch down the back of the straight leg. Repeat on the other side. | |
| Calf Stretch | Stand behind your chair. Place both hands on the back of the chair for support. Keeping both feet facing the back of the chair. Take a big step back with one leg. Press your back heel to the floor and keep the front knee slightly bent. You should feel the stretch in the back of the straight leg. Then repeat with the other leg | |
| Chest Stretch | Move to sit on the front 1/3 of the chair. Keeping your tummy tight take arms backwards and hold onto the back of the chair. Open the chest pulling the tummy in, then lean slightly forwards and upwards. Draw the shoulders back until you feel a gentle stretch across the chest. |
Appendix B. The Study Protocol
References
- Chatterji, S.; Byles, J.; Cutler, D.; Seeman, T.; Verdes, E. Health, functioning, and disability in older adults-present status and future implications. Lancet 2015, 385, 563–575. [Google Scholar] [CrossRef]
- Sowa, A.; Tobiasz-Adamczyk, B.; Topór-Mądry, R.; Poscia, A.; Ignazio la Milia, D. Predictors of healthy ageing: Public health policy targets. BMC Health Serv. Res. 2016, 16, 289. [Google Scholar] [CrossRef] [PubMed]
- Wright, N.C.; Looker, A.C.; Saag, K.G.; Curtis, J.R.; Delzell, E.S.; Randall, S.; Dawson-Hughes, B. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J. Bone Miner. Res. 2014, 29, 2520–2526. [Google Scholar] [CrossRef]
- Jindai, K.; Nielson, C.M.; Vorderstrasse, B.A.; Quiñones, A.R. Multimorbidity and Functional Limitations Among Adults 65 or Older, NHANES 2005–2012. Prev. Chronic Dis. 2016, 13, 160174. [Google Scholar] [CrossRef]
- Milanović, Z.; Pantelić, S.; Trajković, N.; Sporiš, G.; Kostić, R.; James, N. Age-related decrease in physical activity and functional fitness among elderly men and women. Clin. Interv. Aging 2013, 8, 549–556. [Google Scholar] [CrossRef]
- Seguin, R.A.; Heidkamp-Young, E.; Kuder, J.; Nelson, M.E. Improved physical fitness among older female participants in a nationally disseminated, community-based exercise program. Health Educ. Behav. 2012, 39, 183–190. [Google Scholar] [CrossRef]
- Vaughan, L.; Leng, X.; La Monte, M.J.; Tindle, H.A.; Cochrane, B.B.; Shumaker, S.A. Functional Independence in Late-Life: Maintaining Physical Functioning in Older Adulthood Predicts Daily Life Function after Age 80. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2016, 71, S79–S86. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.Y.; Skirbekk, V.F.; Tyrovolas, S.; Kassebaum, N.J.; Dieleman, J.L. Measuring population ageing: An analysis of the Global Burden of Disease Study 2017. Lancet Public Health 2019, 4, e159–e167. [Google Scholar] [CrossRef] [PubMed]
- Imison, C.; Naylor, C.; Buck, D.; Curry, N.; Addicott, R.; Zollinger-Read, P. Transforming Our Healthcare System: Ten Priorities for Commissioners. 2015. Available online: https://www.kingsfund.org.uk/sites/default/files/field/field_publication_file/10PrioritiesFinal2.pdf (accessed on 26 April 2023).
- Soriano, T.A.; DeCherrie, L.V.; Thomas, D.C. Falls in the community-dwelling older adult: A review for primary-care providers. Clin. Interv. Aging. 2007, 2, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Sekaran, N.K.; Choi, H.; Hayward, R.A.; Langa, K.M. Fall-Associated Difficulty with Activities of Daily Living in Functionally Independent Individuals Aged 65 to 69 in the United States: A Cohort Study. J. Am. Geriatr. Soc. 2013, 61, 96–100. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. Physical Activity Guidelines for Americans: 2nd Edition. 2018. Available online: https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf (accessed on 26 April 2023).
- Chief Medical Officer, UK. UK Chief Medical Officers’ Physical Activity Guidelines. 2020. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/832868/uk-chief-medical-officers-physical-activity-guidelines.pdf (accessed on 26 April 2023).
- NHS Digital and the Office for National Statistics. Health Survey for England 2018. Adult’s Health-Related Behaviours. 2019. Available online: https://files.digital.nhs.uk/B5/771AC5/HSE18-Adult-Health-Related-Behaviours-rep-v3.pdf (accessed on 26 April 2023).
- Hupin, D.; Roche, F.; Gremeaux, V.; Chatard, J.C.; Oriol, M.; Gaspoz, J.M.; Barthélémy, J.; Edouard, P. Even a low-dose of moderate-to-vigorous physical activity reduces mortality by 22% in adult’s aged ≥ 60 years: A systematic review and meta-analysis. Br. J. Sport. Med. 2015, 49, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Keadle, S.K.; McKinnon, R.; Graubard, B.I.; Troiano, R.P. Prevalence and trends in physical activity among older adults in the United States: A comparison across three national surveys. Prev. Med. 2016, 89, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, L.D.; Robertson, M.C.; Gillespie, W.J.; Sherrington, C.; Gates, S.; Clemson, L.M.; Lamb, S. Interventions for preventing falls in older people living in the community. Cochrane Database Sys. Rev. 2012, 9, CD007146. [Google Scholar] [CrossRef]
- Franco, M.R.; Pereira, L.S.; Ferreira, P.H. Exercise interventions for preventing falls in older people living in the community. Br. J. Sport. Med. 2014, 48, 867–868. [Google Scholar] [CrossRef]
- Belza, B.; Shumway-Cook, A.; Phelan, E.A.; Williams, B.; Snyder, S.J.; LoGerfo, J.P. The effects of a community-based exercise program on function and health in older adults: The EnhanceFitness Program. J. Appl. Gerontol. 2006, 25, 291–306. [Google Scholar] [CrossRef]
- Hambrook, R.; Middleton, G.; Bishop, D.; Crust, L.; Broom, D. Time to speed up, not slow down: A narrative review on the importance of community-based physical activity among older people. J. Health Soc. Sci. 2020, 5, 91–102. [Google Scholar] [CrossRef]
- Killingback, C.; Tsofliou, F.; Clark, C. Older adults’s adherence to community-based group exercise programs: A multiple-case study. BMC Public Health 2017, 17, 115–127. [Google Scholar] [CrossRef]
- Windle, G.; Hughes, D.; Linck, P.; Russell, I.; Woods, B. Is exercise effective in promoting mental well-being in older age? A systematic review. Aging. Ment. Health 2010, 14, 652–669. [Google Scholar] [CrossRef]
- Van Der Bij, A.K.; Laurant, M.G.; Wensing, M. Effectiveness of physical activity interventions for older adults: A review. Am. J. Prev. Med. 2002, 22, 120–133. [Google Scholar] [CrossRef]
- Farrance, C.; Tsofliou, F.; Clark, C. Adherence to community-based group exercise interventions for older people: A mixed-methods systematic review. Prev. Med. 2016, 87, 155–166. [Google Scholar] [CrossRef]
- World Health Organization. Global Action Plan on Physical Activity 2018–2030: More Active People for a Healthier World. Available online: https://www.who.int/publications/i/item/9789241514187 (accessed on 26 April 2023).
- Gray, S.M.; McKay, H.A.; Nettlefold, L.; Race, D.; Macdonald, H.M.; Naylor, P.J.; Sims-Gould, J. Physical activity is good for older adults—But is programme implementation being overlooked? A systematic review of intervention studies that reported frameworks or measures of implementation. Br. J. Sport. Med. 2021, 55, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.; Walsh, S.; Kwok, W.; Pinheiro, M.B.; De Oliveira, J.S.; Hassett, L.; Bauman, A.; Bull, F.; Tiedemann, A.; Sherrington, C. A scoping review of physical activity interventions for older adults. Int. J. Behav. Nutr. Phys. Act. 2021, 18, 82. [Google Scholar] [CrossRef] [PubMed]
- Handley, M.A.; Lyles, C.R.; McCulloch, C.; Cattamanchi, A. Selecting and improving quasi-experimental designs in effectiveness and implementation research. Annu. Rev. Public Health 2018, 39, 5–25. [Google Scholar] [CrossRef] [PubMed]
- INVOLVE; The National Institute for Health Research. Strategies for Diversity and Inclusion in Public Involvement: Supplement to the Briefing Notes for Researchers. Available online: http://www.invo.org.uk/wp-content/uploads/2012/06/INVOLVEInclusionSupplement1.pdf (accessed on 26 April 2023).
- Stewart, A.L.; Mills, K.M.; King, A.C.; Haskell, W.L.; Gillis, D.; Ritter, P.L. CHAMPS physical activity questionnaire for older adults: Outcomes for interventions. Med. Sci. Sport. Exerc. 2001, 33, 1126–1141. [Google Scholar] [CrossRef] [PubMed]
- American College of Sports Medicine Exercise. Preparticipation Health Screening Recommendations. 2015. Available online: https://www.acsm.org/docs/default-source/default-document-library/read-research/acsm-risk-stratification-chart.pdf?sfvrsn=7b8b1dcd_6 (accessed on 26 April 2023).
- Rikli, R.E.; Jones, C.J. Senior Fitness Test Manual, 2nd ed.; Human Kinetics: Leeds, UK, 2013. [Google Scholar]
- Kendzierski, D.; DeCarlo, K.J. Physical activity enjoyment scale: Two validation studies. Int. J. Sport Exerc. Psychol. 1991, 13, 50–64. [Google Scholar] [CrossRef]
- Resnick, B.; Jenkins, L.S. Testing the reliability and validity of the self-efficacy for exercise scale. Nurs. Res. 2000, 49, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Lyons, R.A.; Perry, I.M.; Littlepage, B.N. Evidence for the validity of the Short-form 36 Questionnaire (SF-36) in an elderly population. Age Ageing 1994, 23, 182–184. [Google Scholar] [CrossRef]
- Mänttäri, A.; Suni, J.; Sievänen, H.; Husu, P.; Vähä-Ypyä, H.; Valkeinen, H.; Tokola, K.; Vasankari, T. Six-minute walk test: A tool for predicting maximal aerobic power (VO2max) in healthy adults. Clin. Physiol. Funct. Imaging 2018, 38, 1038–1045. [Google Scholar] [CrossRef]
- Pang, M.Y.; Eng, J.J.; Dawson, A.S.; McKay, H.A.; Harris, J.E. A community-based fitness and mobility exercise program for older adults with chronic stroke: A randomized, controlled trial. J. Am. Geriatr. Soc. 2005, 53, 1667–1674. [Google Scholar] [CrossRef]
- Taylor, A.W.; Johnson, M.J. Physiology of Exercise and Healthy Ageing; Human Kinetics: Leeds, UK, 2008. [Google Scholar]
- Hawkins, S.A.; Wiswell, R.A. Rate and mechanism of maximal oxygen consumption decline with ageing. Sport. Med. 2003, 33, 877–888. [Google Scholar] [CrossRef]
- Taylor, A.H.; Cable, N.T.; Faulkner, G.; Hillsdon, M.; Narici, M.; Van Der Bij, A.K. Physical activity and older adults: A review of health benefits and the effectiveness of interventions. J. Sport. Sci. 2004, 22, 703–725. [Google Scholar] [CrossRef]
- Chodzko-Zajko, W.; Schwingel, A.; Park, C.H. Successful ageing: The role of physical activity. Am. J. Lifestyle Med. 2009, 3, 20–28. [Google Scholar] [CrossRef]
- Chen, K.M.; Tseng, W.S.; Chang, Y.H.; Huang, H.T.; Li, C.H. Feasibility appraisal of an elastic band exercise program for older adults in wheelchairs. Geriatr. Nurs. 2013, 34, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Klonizakis, M.; Gumber, A.; McIntosh, E.; King, B.; Middleton, G.; Michaels, J.A.; Tew, G. Exercise fidelity and progression in a supervised exercise program for adults with venous leg ulcers. Int. Wound J. 2018, 15, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Dorling, J.; Broom, D.R.; Burns, S.F.; Clayton, D.J.; Deighton, K.; James, L.J.; Miyashita, M.; Thackray, A.E.; Batterham, R.L.; Stensel, D.J. Acute and chronic effects of exercise on appetite, energy intake and appetite-related hormones: The modulating effect of adiposity, sex and habitual physical activity. Nutrients 2018, 10, 1140. [Google Scholar] [CrossRef] [PubMed]
| Assessment/Group | Baseline | After 8 Weeks | Difference (+/−) | Effect Size (Cohen’s d) |
|---|---|---|---|---|
| Stature (cm) | ||||
| Vitality: | 159.7 ± 3.9 | 159.3 ± 4.0 | −0.4 | |
| Control: | 166.3 ± 11.1 | 166.5 ± 11.2 | +0.2 | |
| Body mass (kg) | ||||
| Vitality: | 73.3 ± 8.6 | 71.8 ± 8.5 * | −1.5 ** | 0.17 |
| Control: | 76.8 ± 20.6 | 76.6 ± 20.4 | −0.2 | 0.01 |
| Body Mass Index (kg/m2) | ||||
| Vitality: | 28.8 ± 3.4 | 28.3 ± 3.3 * | −0.5 | 0.15 |
| Control: | 27.5 ± 6.0 | 27.4 ± 5.9 | −0.1 | 0.01 |
| Resting Heart rate (b/min) | ||||
| Vitality: | 74.8 ± 15.4 | 76.5 ± 11.8 | +1.7 | 0.01 |
| Control: | 76.4 ± 9.7 | 75.5 ± 12.0 | −0.9 | 0.08 |
| Systolic pressure (mmHg) | ||||
| Vitality: | 145.8 ± 17.2 | 145.6 ± 21.3 | −0.2 | 0.09 |
| Control: | 138.1 ± 15.8 | 134.4 ± 14.1 | −3.7 | 0.24 |
| Diastolic pressure (mmHg) | ||||
| Vitality: | 79.5 ± 10.4 | 78.7 ± 10.3 | −0.8 | 0.07 |
| Control: | 80.0 ± 11.2 | 76.9 ± 6.6 | −3.1 | 0.33 |
| Assessment/Group | Baseline | After 8 Weeks | Difference (+/−) | Effect Size (Cohen’s d) |
|---|---|---|---|---|
| 6 min walk (m) | ||||
| Vitality: | 430.5 ± 38.1 | 473.4 ± 37.0 * | +42.8 ** | 1.14 |
| Control: | 521.8 ± 73.3 | 517.0 ± 69.8 | −4.8 | 0.06 |
| 30 s chair stand (repetitions) | ||||
| Vitality: | 11.20 ± 1.9 | 12.9 ± 2.1 * | +1.7 | 0.84 |
| Control: | 13.9 ± 2.8 | 14.6 ± 2.8 | +0.7 | 0.25 |
| 8 foot up and go (s): | ||||
| Vitality: | 6.5 ± 1.0 | 6.00 ± 1.1 | −0.5 | 0.47 |
| Control: | 6.1 ± 1.0 | 6.2 ± 1.2 | +0.1 | 0.09 |
| 30 s arm curl (repetitions) | ||||
| Vitality: | 14.1 ± 2.7 | 16.1 ± 2.1 * | +2.0 | 0.82 |
| Control: | 17.1 ± 3.3 | 18.0 ± 3.7 | +0.9 | 0.25 |
| Back scratch (cm) | ||||
| Vitality: | −7.2 ± 10.1 | −6.0 ± 10.0 | +1.2 | 0.11 |
| Control: | −5.9 ± 8.6 | −7.0 ± 8.6 | −1.1 | 0.12 |
| Chair sit and reach (cm) | ||||
| Vitality: | −2.3 ± 11.2 | 0.6 ± 10.00 * | +2.9 ** | 0.27 |
| Control: | −4.1 ± 10.0 | −4.9 ± 9.5 | −0.8 | 0.08 |
| Assessment/Group | Baseline | After 8 Weeks | Difference (+/−) | Effect Size (Cohen’s d) |
|---|---|---|---|---|
| Self-efficacy (SEE) questionnaire (9-item score) | ||||
| Vitality: | 6.38 ± 1.45 | 6.88 ± 2.02 | +0.50 | 0.28 |
| Control: | 6.84 ± 1.74 | 6.67 ± 2.07 | −0.17 | 0.08 |
| Enjoyment (PACES) questionnaire (18-item score) | ||||
| Vitality: | 5.80 ± 0.89 | 6.13 ± 1.0 | +0.33 | 0.35 |
| Control: | 5.77 ± 1.20 | 5.95 ± 0.70 | +0.18 | 0.18 |
| SF-36 (QOL) questionnaire (36-item score) | ||||
| Vitality: | 75.2 ± 14.6 | 78.7 ± 14.3 | +3.5 | 0.24 |
| Control: | 80.9 ± 7.6 | 82.7 ± 7.3 | +1.8 | 0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Middleton, G.; Hambrook, R.; Bishop, D.C.; Crust, L.; Broom, D.R. Examining the New-Member Effect to an Established Community-Based Physical Activity Program for Older Adults in England. Int. J. Environ. Res. Public Health 2023, 20, 6161. https://doi.org/10.3390/ijerph20126161
Middleton G, Hambrook R, Bishop DC, Crust L, Broom DR. Examining the New-Member Effect to an Established Community-Based Physical Activity Program for Older Adults in England. International Journal of Environmental Research and Public Health. 2023; 20(12):6161. https://doi.org/10.3390/ijerph20126161
Chicago/Turabian StyleMiddleton, Geoff, Robyn Hambrook, Daniel C. Bishop, Lee Crust, and David R. Broom. 2023. "Examining the New-Member Effect to an Established Community-Based Physical Activity Program for Older Adults in England" International Journal of Environmental Research and Public Health 20, no. 12: 6161. https://doi.org/10.3390/ijerph20126161
APA StyleMiddleton, G., Hambrook, R., Bishop, D. C., Crust, L., & Broom, D. R. (2023). Examining the New-Member Effect to an Established Community-Based Physical Activity Program for Older Adults in England. International Journal of Environmental Research and Public Health, 20(12), 6161. https://doi.org/10.3390/ijerph20126161







