Ozone Efficacy for the Disinfection of Ambulances Used to Transport Patients during the COVID-19 Pandemic in Peru
Abstract
1. Introduction
2. Materials and Methods
2.1. Type of Study
2.2. Ozone Generation Prototype
2.3. In Vitro Tests
2.4. Field Testing with Experimental Inoculation in Conventional Ambulances
2.5. Exploratory Field Testing in Ambulances Transporting Patients with COVID-19
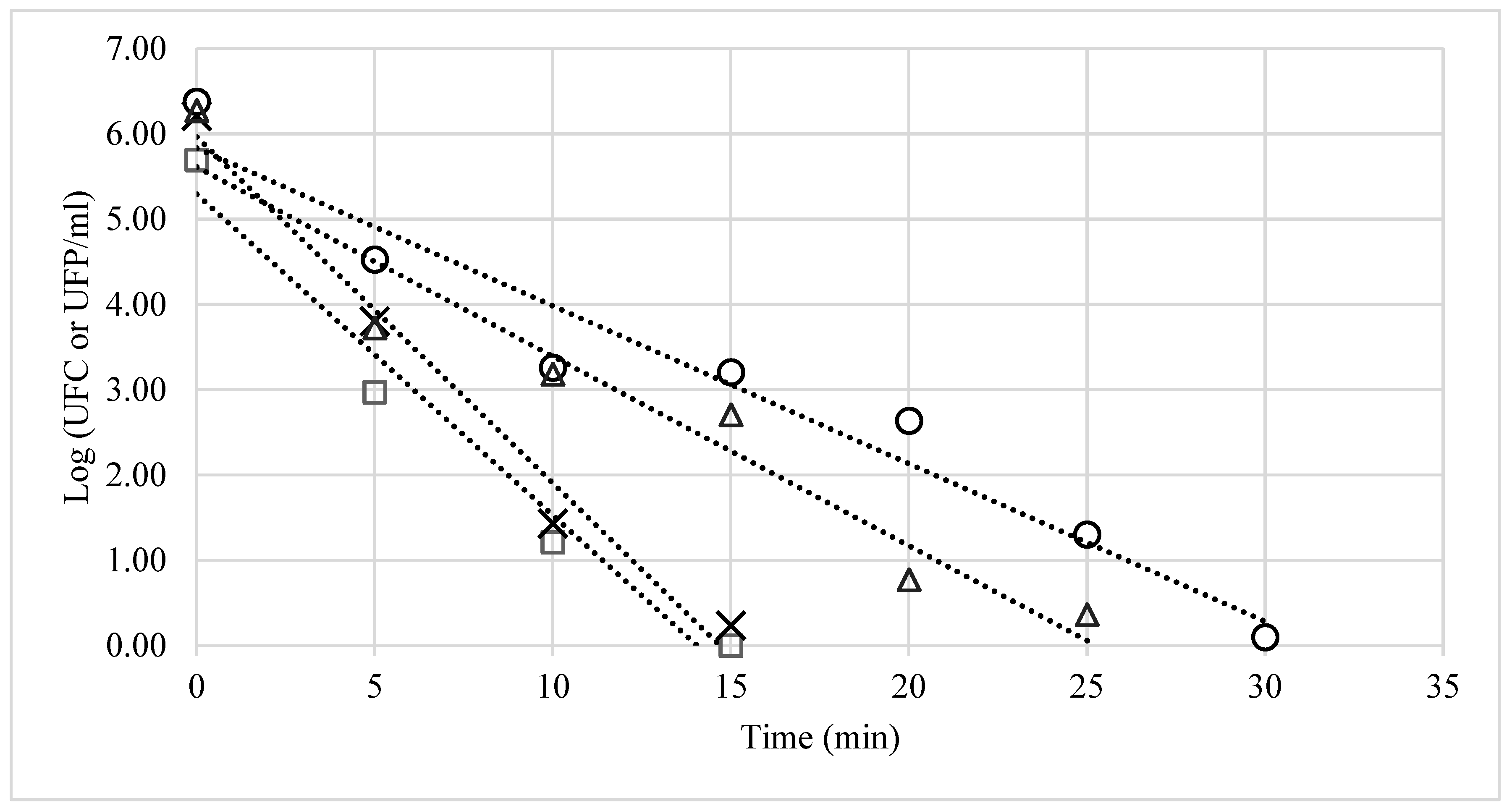
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chin, A.W.H.; Chu, J.T.S.; Perera, M.R.A.; Hui, K.P.Y.; Yen, H.L.; Chan, M.C.W.; Peiris, M.; Poon, L.L.M. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe 2020, 1, E10. [Google Scholar] [CrossRef] [PubMed]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and surface stability of HCoV-19 (SARS-CoV-2) compared to SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef] [PubMed]
- Silva-Jaimes, M. SARS-CoV-2 and other emerging viruses and their relationship to safety in the food chain. Sci. Agropecu. 2020, 11, 267–277. [Google Scholar] [CrossRef]
- Oner, M.E.; Demirci, A. Ozone for food decontamination: Theory and applications. In Handbook of Hygiene Control in the Food Industry; Lelieveld, H., Holah, J., Gabric, D., Eds.; Woodhead Publishing: Sawston, PA, USA, 2016; pp. 491–501. [Google Scholar] [CrossRef]
- Naddeo, V.; Liu, H. Editorial Perspectives: 2019 novel coronavirus (SARS-CoV-2): What is its fate in urban water cycle and how can the water research community respond? Environ. Sci. Water Res. Technol. 2020, 6, 1213–1216. [Google Scholar] [CrossRef]
- Hudson, J.B.; Sharma, M.; Vimalanathan, S. Development of a practical method for using ozone gas as a virus decontaminating agent. Ozone Sci. Eng. 2009, 31, 216–223. [Google Scholar] [CrossRef]
- Clavo, B.; Córdoba-Lanús, E.; Rodríguez-Esparragón, F.; Cazorla-Rivero, S.E.; García-Pérez, O.; Piñero, J.E.; Villar, J.; Blanco, A.; Torres-Ascensión, C.; Martín-Barrasa, J.L.; et al. Effects of ozone treatment on personal protective equipment contaminated with sars-cov-2. Antioxidants 2020, 9, 1222. [Google Scholar] [CrossRef]
- Yano, H.; Nakano, R.; Suzuki, Y.; Nakano, A.; Kasahara, K.; Hosoi, H. Inactivation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by gaseous ozone treatment. J. Hosp. Infect. 2020, 106, 837–838. [Google Scholar] [CrossRef]
- Jamalludeen, N.; Johnson, R.P.; Friendship, R.; Kropinski, A.M.; Lingohr, E.J.; Gyles, C.L. Isolation and characterization of nine bacteriophages that lyse O149 enterotoxigenic Escherichia coli. Vet. Microbiol. 2007, 124, 47–57. [Google Scholar] [CrossRef]
- Adams, M.H. Bacteriophages; Interscience Publishers: New York, NY, USA, 1959. [Google Scholar]
- International Commission on Microbiological Specifications for Foods. Microorganisms in Foods 6: Microbial Ecology of Food Commodities, 2nd ed.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2005. [Google Scholar]
- Mazzola, P.G.; Penna, T.C.; Martins, A.M. Determination of decimal reduction time (D value) of chemical agents used in hospitals for disinfection purposes. BMC Infect. Dis. 2003, 3, 24. [Google Scholar] [CrossRef]
- Lang, M.M.; Harris, L.J.; Beuchat, L.R. Evaluation of inoculation method and inoculum drying time for their effects on survival and efficiency of recovery of Escherichia coli O157:H7, Salmonella, and Listeria monocytogenes inoculated on the surface of tomatoes. J. Food Prot. 2004, 67, 732–741. [Google Scholar] [CrossRef]
- Mohammad, Z.; Kalbasi-Ashtari, A.; Riskowski, G.; Castillo, A. Reduction of Salmonella and Shiga toxin-producing Escherichia coli on alfalfa seeds and sprouts using an ozone generating system. Int. J. Food Microbiol. 2019, 289, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Ersoy, Z.G.; Barisci, S.; Dinc, O. Mechanisms of the Escherichia coli and Enterococcus faecalis inactivation by ozone. LWT Food Sci. Technol. 2019, 100, 306–313. [Google Scholar] [CrossRef]
- Chang, R.; Pandey, P.; Li, Y.; Venkitasamy, C.; Chen, Z.; Gallardo, R.; Weimer, B.; Jay-Russell, M.; Weimer, B. Assessment of gaseous ozone treatment on Salmonella typhimurium and Escherichia coli O157:H7 reductions in poultry litter. Waste Manag. 2020, 117, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Percivalle, E.; Clerici, M.; Cassaniti, I.; Nepita, E.V.; Marchese, P.; Olivati, D.; Catelli, C.; Berri, A.; Baldanti, F.; Marone, P.; et al. SARS-CoV-2 viability on different surfaces after gaseous ozone treatment: A preliminary evaluation. J. Hosp. Infect. 2021, 110, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Traoré, O.; Springthorpe, V.S.; Sattar, S.A. A quantitative study of the survival of two species of Candida on porous and non-porous environmental surfaces and hands. J. Appl. Microbiol. 2002, 92, 549–555. [Google Scholar] [CrossRef]
- Somvanshi, S.B.; Kharat, P.B.; Saraf, T.S.; Somwanshi, S.B.; Shejul, S.B.; Jadhav, K.M. Multifunctional nano-magnetic particles assisted viral RNA-extraction protocol for potential detection of COVID-19. Mater. Res. Innov. 2021, 25, 169–174. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Makison Booth, C.; Clayton, M.; Crook, B.; Gawn, J.M. Effectiveness of surgical masks against influenza bioaerosols. J. Hosp. Infect. 2013, 84, 22–26. [Google Scholar] [CrossRef]
- Booth, T.F.; Kournikakis, B.; Bastien, N.; Ho, J.; Kobasa, D.; Stadnyk, L.; Li, Y.; Spence, M.; Paton, S.; Henry, B.; et al. Detection of airborne severe acute respiratory syndrome (SARS) coronavirus and environmental contamination in SARS outbreak units. J. Infect. Dis. 2005, 191, 1472–1477. [Google Scholar] [CrossRef]
- Ijaz, M.K.; Brunner, A.H.; Sattar, S.A.; Nair, R.C.; Johnson-Lussenburg, C.M. Survival characteristics of airborne human coronavirus 229E. J. Gen. Virol. 1985, 66, 2743–2748. [Google Scholar] [CrossRef]
- Hudson, J.B.; Sharma, M.; Petric, M. Inactivation of Norovirus by ozone gas in conditions relevant to healthcare. J. Hosp. Infect. 2007, 66, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Quevedo-león, R.; Bastías-Montes, J.; Espinoza-Tellez, T.; Ronceros, B.; Balic, I.; Muñoz, O. Inactivation of coronaviruses in food industry: The use of inorganic and organic disinfectants, ozone, and UV radiation. Sci. Agropecu. 2020, 11, 257–266. [Google Scholar] [CrossRef]
- Blanchard, E.L.; Lawrence, J.D.; Noble, J.A.; Xu, M.; Joo, T.; Ng, N.L.; Schmidt, B.E.; Santangelo, P.J.; Finn, M.G. Enveloped virus inactivation on personal protective equipment by exposure to ozone. medRxiv, 2020; Preprint. [Google Scholar] [CrossRef]
- Dubuis, M.E.; Dumont-Leblond, N.; Laliberté, C.; Veillette, M.; Turgeon, N.; Jean, J.; Duchaine, C. Ozone efficacy for the control of airborne viruses: Bacteriophage and norovirus models. PLoS ONE 2020, 15, e0231164. [Google Scholar] [CrossRef]
- Vyskocil, J.M.; Turgeon, N.; Turgeon, J.G.; Duchaine, C. Ozone treatment in a wind tunnel for the reduction of airborne viruses in swine buildings. Aerosol. Sci. Technol. 2020, 54, 1471–1478. [Google Scholar] [CrossRef]
- Fathizadeh, H.; Maroufi, P.; Momen-Heravi, M.; Dao, S.; Köse, Ş.; Ganbarov, K.; Pagliano, P.; Esposito, S.; Kafil, H.S. Protection and disinfection policies against SARS-CoV-2 (COVID-19). Infez. Med. 2020, 28, 185–191. [Google Scholar]
- Bayarri, B.; Cruz-Alcalde, A.; López-Vinent, N.; Micó, M.M.; Sans, C. Can ozone inactivate SARS-CoV-2? A review of mechanisms and performance on viruses. J. Hazard. Mater. 2021, 415, 125658. [Google Scholar] [CrossRef]
- Cristiano, L. Could ozone be an effective disinfection measure against the novel coronavirus (SARS-CoV-2)? J. Prev. Med. Hyg. 2020, 61, E301–E303. [Google Scholar] [CrossRef]
- Iwamura, T.; Nagano, K.; Nogami, T.; Matsuki, N.; Kosaka, N.; Shintani, H.; Katoh, M. Confirmation of the sterilization effect using a high concentration of ozone gas for the Bio-Clean Room. Biocontrol Sci. 2013, 18, 9–20. [Google Scholar] [CrossRef]
- Carter, T.J.; Poppendieck, D.G.; Shaw, D.; Carslaw, N. A Modelling Study of Indoor Air Chemistry: The Surface Interactions of Ozone and Hydrogen Peroxide. Atmos. Environ. 2023, 297, 119598. [Google Scholar] [CrossRef]
| Seats | Gurneys | Shelves | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No | Nf | %SR | No | Nf | %SR | No | Nf | %SR | |
| SA | 5.3 ± 0.12 | 0.05 ± 0.16 | 1.0 | 5.7 ± 0.29 | 0.2 ± 0.27 | 3.1 | 6.0 ± 0.10 | 0.0 | 0.0 |
| EC | 5.3 ± 0.22 | 0.0 | 0.0 | 5.4 ± 0.30 | 0.0 | 0.0 | 6.3 ± 0.12 | 0.0 | 0.0 |
| CA | 5.0 ± 0.18 | 0.0 | 0.0 | 5.1 ± 0.09 | 0.0 | 0.0 | 5.3 ± 0.09 | 0.0 | 0.0 |
| Φ21 | 5.7 ± 0.26 | 0.0 | 0.0 | 6.2 ± 0.25 | 0.0 | 0.0 | 6.5 ± 0.23 | 0.3 ± 0.43 | 5.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Castillo, M.A.; Rivera Romero, C.; Reátegui-Ochoa, K.; Mamani Zapana, E.; Silva-Jaimes, M. Ozone Efficacy for the Disinfection of Ambulances Used to Transport Patients during the COVID-19 Pandemic in Peru. Int. J. Environ. Res. Public Health 2023, 20, 5776. https://doi.org/10.3390/ijerph20105776
Gómez-Castillo MA, Rivera Romero C, Reátegui-Ochoa K, Mamani Zapana E, Silva-Jaimes M. Ozone Efficacy for the Disinfection of Ambulances Used to Transport Patients during the COVID-19 Pandemic in Peru. International Journal of Environmental Research and Public Health. 2023; 20(10):5776. https://doi.org/10.3390/ijerph20105776
Chicago/Turabian StyleGómez-Castillo, Miguel Alejandro, Cristina Rivera Romero, Kevin Reátegui-Ochoa, Enrique Mamani Zapana, and Marcial Silva-Jaimes. 2023. "Ozone Efficacy for the Disinfection of Ambulances Used to Transport Patients during the COVID-19 Pandemic in Peru" International Journal of Environmental Research and Public Health 20, no. 10: 5776. https://doi.org/10.3390/ijerph20105776
APA StyleGómez-Castillo, M. A., Rivera Romero, C., Reátegui-Ochoa, K., Mamani Zapana, E., & Silva-Jaimes, M. (2023). Ozone Efficacy for the Disinfection of Ambulances Used to Transport Patients during the COVID-19 Pandemic in Peru. International Journal of Environmental Research and Public Health, 20(10), 5776. https://doi.org/10.3390/ijerph20105776







