Toxicity Rank Order (TRO) As a New Approach for Toxicity Prediction by QSAR Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources
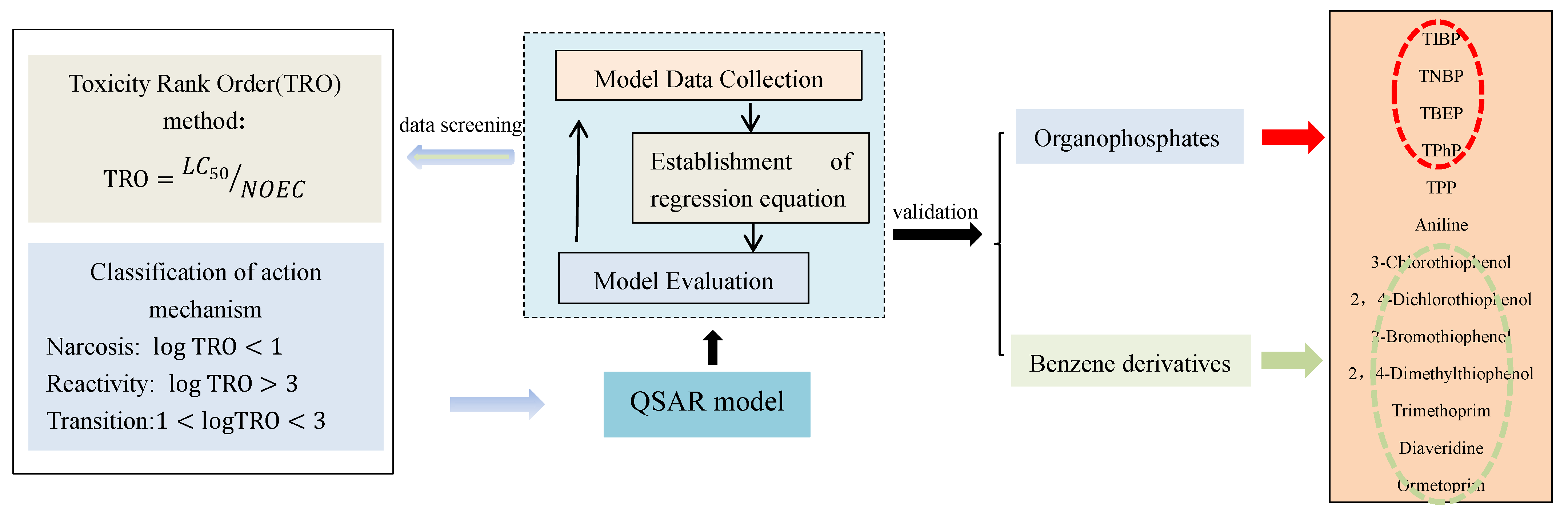
2.2. Theory and Methodology
2.3. Approach Details
- Reviewed and collected the relevant toxicity literature for the QSAR model, identified the toxicity endpoints of model prediction, and used the TRO method to filter the modeling data.
- Summarized the modeling rules of QSAR and established the principles for parameter selection of QSAR models.
- Selected environmental contaminants according to QSAR model parameter selection principles for validation.
2.4. Validation Analysis
3. Results and Discussion
3.1. Definition and Calculation of Toxicity Rank Order
3.2. Classification of Toxicity Mechanism Based on TRO
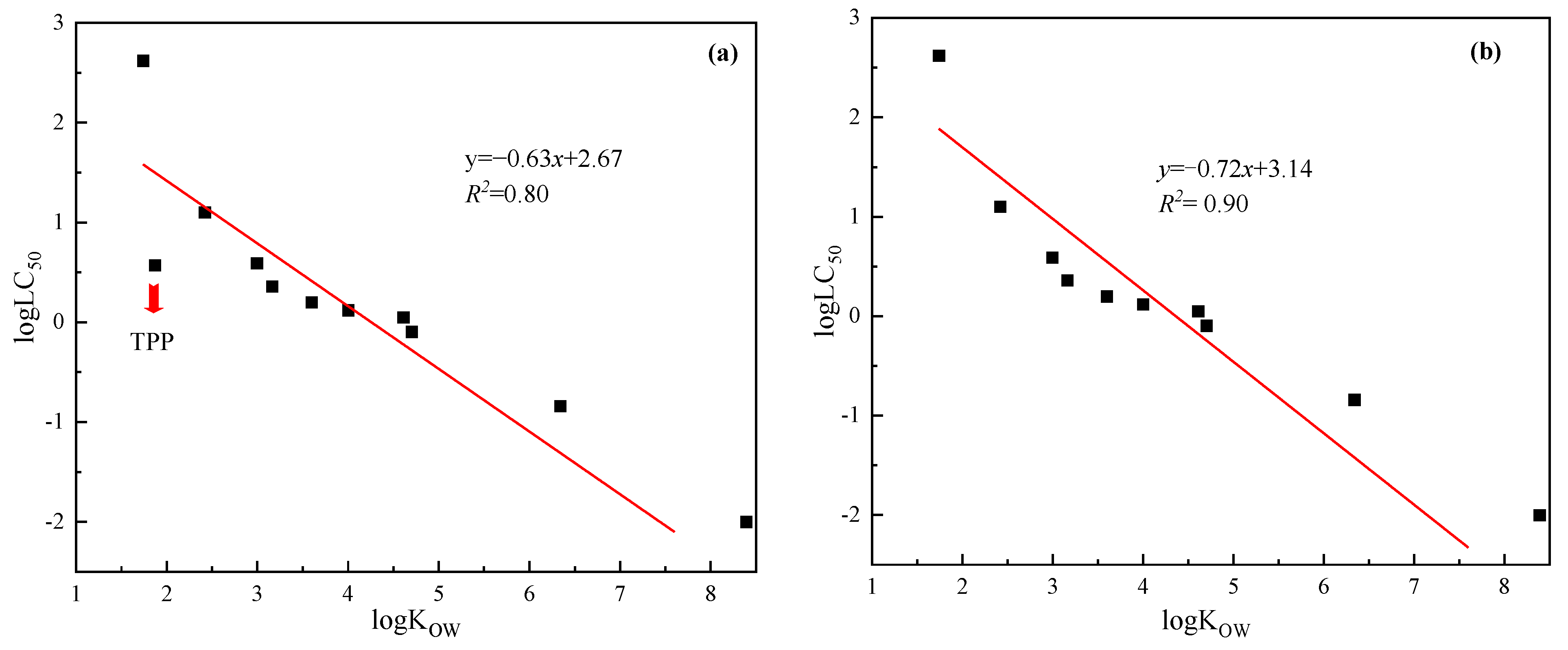
3.3. Rule of Parameter Selection
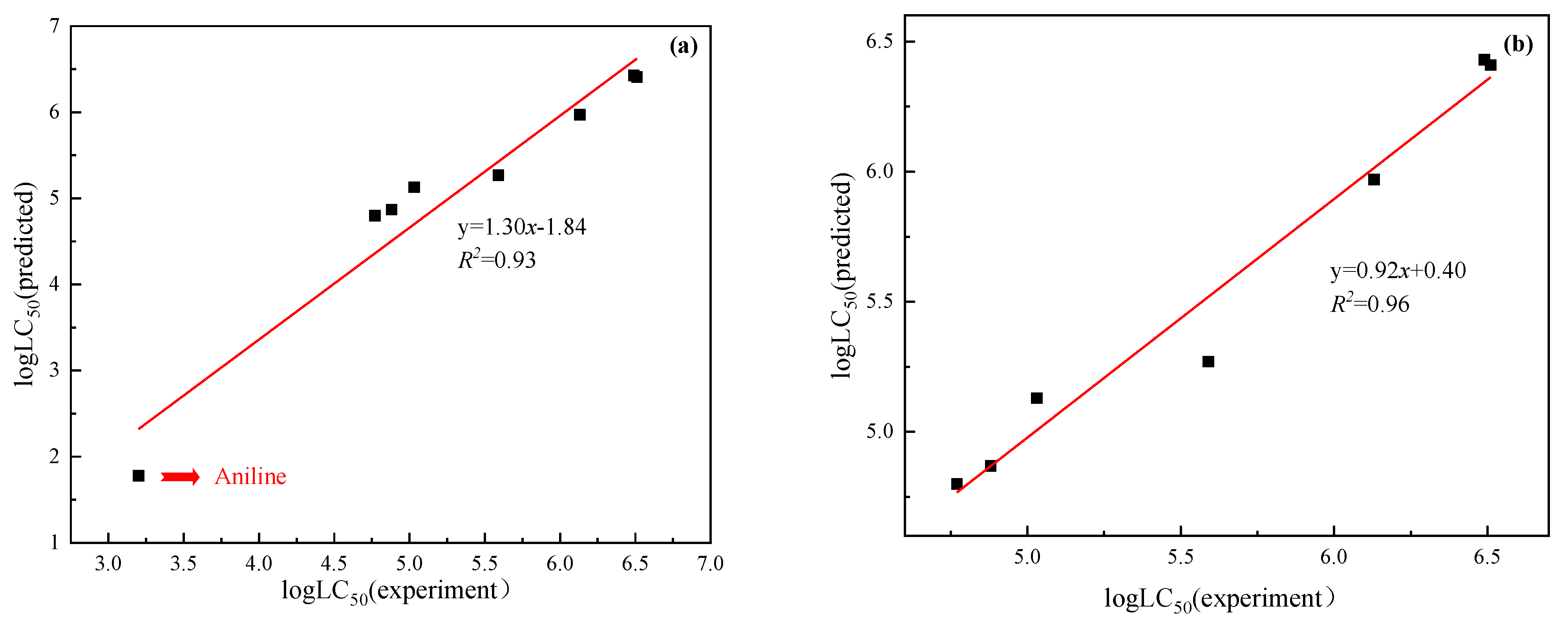
3.4. Validation of Application in Selected Case
3.5. Comparison of Model Performance with TRO
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, W.; Qing, X.; Wang, J.; He, T.; Fan, R.; Huang, Y. Bioaccumulation and Potential Risk of Organophosphate Flame Retardants in Coral Reef Fish from the Nansha Islands, South China Sea. Chemosphere 2022, 287, 132125. [Google Scholar] [CrossRef] [PubMed]
- Percy, Z.; Vuong, A.M.; Xu, Y.; Xie, C.; Ospina, M.; Calafat, A.M.; Hoofnagle, A.; Lanphear, B.P.; Braun, J.M.; Cecil, K.M. Maternal Urinary Organophosphate Esters and Alterations in Maternal and Neonatal Thyroid Hormones. Am. J. Epidemiol. 2021, 190, 1793–1802. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Hua, Z.; Chu, K.; Gu, L.; Liu, Y.; Liu, X. Distribution Behavior and Risk Assessment of Emerging Perfluoroalkyl Acids in Multiple Environmental Media at Luoma Lake, East China. Environ. Res. 2021, 194, 110733. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.M.; Burch, R. Principle of Human Experimental Techniques; Methuen Publishing: London, UK, 1959; pp. 1–229. [Google Scholar]
- Nath, A.; De, P.; Roy, K. QSAR Modelling of Inhalation Toxicity of Diverse Volatile Organic Molecules Using No Observed Adverse Effect Concentration (NOAEC) as the Endpoint. Chemosphere 2022, 287, 131954. [Google Scholar] [CrossRef]
- Yang, L.Z.; Liu, M. A Double-Activity (Green Algae Toxicity and Bacterial Genotoxicity) 3D-QSAR Model Based on the Comprehensive Index Method and Its Application in Fluoroquinolones’ Modification. Int. J. Environ. Res. Public Health 2020, 17, 942. [Google Scholar] [CrossRef]
- Ren, Z.; Wang, Y.; Xu, H.; Li, Y.; Han, S. Fuzzy Comprehensive Evaluation Assistant 3D-QSAR of Environmentally Friendly FQs to Reduce ADRs. Int. J. Environ. Res. Public Health 2019, 16, 3161. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Toxicity Changes of Wastewater during Various Advanced Oxidation Processes Treatment: An Overview. J. Clean. Prod. 2021, 315, 128202. [Google Scholar] [CrossRef]
- Haghshenas, H.; Kaviani, B.; Firouzeh, M.; Tavakol, H. Developing a Variation of 3D-QSAR/MD Method in Drug Design. J. Comput. Chem. 2021, 42, 917–929. [Google Scholar] [CrossRef]
- Patel, H.M.; Noolvi, M.N.; Sharma, P.; Jaiswal, V.; Bansal, S.; Lohan, S.; Kumar, S.S.; Abbot, V.; Dhiman, S.; Bhardwaj, V. Quantitative Structure-Activity Relationship (QSAR) Studies as Strategic Approach in Drug Discovery. Med. Chem. Res. 2014, 23, 4991–5007. [Google Scholar] [CrossRef]
- Liu, D.; Van Belleghem, J.D.; de Vries, C.R.; Burgener, E.; Chen, Q.; Manasherob, R.; Aronson, J.R.; Amanatullah, D.F.; Tamma, P.D.; Suh, G.A. The Safety and Toxicity of Phage Therapy: A Review of Animal and Clinical Studies. Viruses 2021, 13, 1268. [Google Scholar] [CrossRef]
- Wu, X.; Guo, J.; Dang, G.; Sui, X.; Zhang, Q. Prediction of Acute Toxicity to Daphnia Magna and Interspecific Correlation: A Global QSAR Model and a Daphnia-Minnow QTTR Model. SAR QSAR Environ. Res. 2022, 33, 583–600. [Google Scholar] [CrossRef] [PubMed]
- Raevsky, O.A.; Grigor’ev, V.Y.; Dearden, J.C.; Weber, E.E. Classification and Quantification of the Toxicity of Chemicals to Guppy, Fathead Minnow, and Rainbow Trout. Part 2. Polar Narcosis Mode of Action. QSAR Comb. Sci. 2009, 28, 163–174. [Google Scholar] [CrossRef]
- De, P.; Roy, K. QSAR and QSAAR Modeling of Nitroimidazole Sulfonamide Radiosensitizers: Application of Small Dataset Modeling. Struct. Chem. 2021, 32, 631–642. [Google Scholar] [CrossRef]
- Lavado, G.J.; Baderna, D.; Carnesecchi, E.; Toropova, A.P.; Toropov, A.A.; Dorne, J.L.C.M.; Benfenati, E. QSAR Models for Soil Ecotoxicity: Development and Validation of Models to Predict Reproductive Toxicity of Organic Chemicals in the Collembola Folsomia Candida. J. Hazard. Mater. 2022, 423, 127236. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Wang, Y.; Chu, D.; Yan, H. 4D-QSAR and MIA-QSAR Study on the Bruton’s Tyrosine Kinase (Btk) Inhibitors. J. Mol. Graph. Model. 2019, 92, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Amiri, R.; Messadi, D.; Bouakkadia, A. QSAR Study of the Octanol/Water Partition Coefficient of Organophosphorus Compounds: The Hybrid GA/MLR and GA/ANN Approaches. J. Serb. Chem. Soc. 2020, 85, 467–480. [Google Scholar] [CrossRef]
- Liu, S. Bin Conceptual Density Functional Theory and Some Recent Developments. Wuli Huaxue Xuebao/Acta Phys.-Chim. Sin. 2009, 25, 590–600. [Google Scholar] [CrossRef]
- Pal, R.; Chattaraj, P.K. Chemical Reactivity from a Conceptual Density Functional Theory Perspective. J. Indian Chem. Soc. 2021, 98, 100008. [Google Scholar] [CrossRef]
- Hu, J.; Li, X.; Liu, F.; Fu, W.; Lin, L.; Li, B. Comparison of Chemical and Biological Degradation of Sulfonamides: Solving the Mystery of Sulfonamide Transformation. J. Hazard. Mater. 2022, 424, 127661. [Google Scholar] [CrossRef]
- Jiang, D.; Zhou, J.G.; Li, N.; Rao, K.F.; Li, X.; Hu, Y.; Ma, M. Quantitative Structure-Activity Relationships between acute toxicity of organophosphates and Vibrio qinghaiensis sp.-Q67. Asian J. Ecotoxicol. 2014, 9, 71–80. [Google Scholar] [CrossRef]
- Yu, Y. Investigation on the Relationship between Critical Body Residue and Toxicological Kinetics of Nitrobenzenes in Zebrafish. Master’s Thesis, Northeast Normal University, Changchun, China, 2017. [Google Scholar]
- Shi, J.Y.; Jia, Q.Z.; Wang, Q. Predicting Toxic Effects of Benzene Derivatives on V. fischeri with Norm Descriptors. J. Tianjin Univ. Sci. Technol 2020, 35, 29–36. [Google Scholar] [CrossRef]
- Yang, L.C. Investigation on Mode of Action of Pesticides to Daphnia Magna and HepG2, and Their Comparison. Master’s Thesis, Northeast Normal University, Changchun, China, 2019. [Google Scholar]
- Shi, Y. Support Vector Regression-Based QSAR Models for Prediction of Antioxidant Activity of Phenolic Compounds. Sci. Rep. 2021, 11, 8806. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Lu, P.; Li, Y.; Wang, J.; Zhao, H. Prediction of Phthalate Acid Esters Degradation in Soil Using QSAR Model: A Combined Consideration of Soil Properties and Quantum Chemical Parameters. Ecotoxicol. Environ. Saf 2021, 226, 112830. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Xu, X.; Hu, Y.; Si, H.; Zhai, H. Novel Quantitative Structure–Activity Relationship Model to Predict Activities of Natural Products against COVID-19. Chem. Biol. Drug Des. 2021, 97, 978–983. [Google Scholar] [CrossRef]
- Lavado, G.J.; Baderna, D.; Gadaleta, D.; Ultre, M.; Roy, K.; Benfenati, E. Ecotoxicological QSAR Modeling of the Acute Toxicity of Organic Compounds to the Freshwater Crustacean Thamnocephalus Platyurus. Chemosphere 2021, 280, 130652. [Google Scholar] [CrossRef]
- Zhao, P.Y. Prediction of Toxicity for Organic Pollutants to Fathead Minnow and Rat. Master’s Thesis, Tianjin University of Science & Technology, Tianjin, China, 2019. [Google Scholar]
- Sanderson, H.; Thomsen, M. Comparative Analysis of Pharmaceuticals versus Industrial Chemicals Acute Aquatic Toxicity Classification According to the United Nations Classification System for Chemicals. Assessment of the (Q)SAR Predictability of Pharmaceuticals Acute Aquatic Toxicity and Their Predominant Acute Toxic Mode-of-Action. Toxicol. Lett. 2009, 187, 84–93. [Google Scholar] [CrossRef]
- Wang, D.; Lin, Z.; Huo, Z.; Wang, T.; Yao, Z.; Cong, Y. Mechanism-Based QSAR Models for the Toxicity of Quorum Sensing Inhibitors to Gram-Negative and Gram-Positive Bacteria. Bull. Environ. Contam. Toxicol. 2016, 97, 145–150. [Google Scholar] [CrossRef]
- Carnesecchi, E.; Toma, C.; Roncaglioni, A.; Kramer, N.; Benfenati, E.; Dorne, J.L.C.M. Integrating QSAR Models Predicting Acute Contact Toxicity and Mode of Action Profiling in Honey Bees (A. Mellifera): Data Curation Using Open Source Databases, Performance Testing and Validation. Sci. Total Environ. 2020, 735, 139243. [Google Scholar] [CrossRef]
- Li, J.J.; Zhang, Y.J.; Zhao, Y.H. Discrimination of the mechanisms of toxic action for organic pollutants to aqueous organisms and its influencing factors. Environ. Chem. 2013, 32, 1236–1245. [Google Scholar]
- Klüver, N.; Vogs, C.; Altenburger, R.; Escher, B.I.; Scholz, S. Development of a General Baseline Toxicity QSAR Model for the Fish Embryo Acute Toxicity Test. Chemosphere 2016, 164, 164–173. [Google Scholar] [CrossRef]
- Van Wezel, A.P.; Opperhuizen, A. Narcosis Due to Environmental Pollutants in Aquatic Organisms: Residue-Based Toxicity, Mechanisms, and Membrane Burdens. Crit. Rev. Toxicol. 1995, 25, 255–279. [Google Scholar] [CrossRef]
- Ahlers, J.; Riedhammer, C.; Vogliano, M.; Ebert, R.U.; Kühne, R.; Schüürmann, G. Acute to Chronic Ratios in Aquatic Toxicity—Variation across Trophic Levels and Relationship with Chemical Structure. Environ. Toxicol. Chem. 2006, 25, 2937–2945. [Google Scholar] [CrossRef] [PubMed]
- Nendza, M.; Müller, M. Discriminating Toxicant Classes by Mode of Action: 2. Physico-Chemical Descriptors. Quant. Struct. Relatsh. 2001, 19, 581–598. [Google Scholar] [CrossRef]
- Yang, L.; Sang, C.; Wang, Y.; Liu, W.; Hao, W.; Chang, J.; Li, J. Development of QSAR Models for Evaluating Pesticide Toxicity against Skeletonema costatum. Chemosphere 2021, 285, 131456. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Zhang, Y.; Pei, L.; Lou, Y.; Mu, Y.; Yun, J.; Li, F.; Wang, Y.; Hao, Z.; Xi, S.; et al. Chemometric QSAR Modeling of Acute Oral Toxicity of Polycyclic Aromatic Hydrocarbons (PAHs) to Rat Using Simple 2D Descriptors and Interspecies Toxicity Modeling with Mouse. Ecotoxicol. Environ. Saf. 2021, 222, 112525. [Google Scholar] [CrossRef]
- Yang, H.; Du, Z.; Lv, W.J.; Zhang, X.Y.; Zhai, H.L. In Silico Toxicity Evaluation of Dioxins Using Structure–Activity Relationship (SAR) and Two-Dimensional Quantitative Structure–Activity Relationship (2D-QSAR). Arch. Toxicol. 2019, 93, 3207–3218. [Google Scholar] [CrossRef]
- Kang, J.X.; Zhu, J.; Li, A.X.; Xiao, Z.Y.; Li, K. 2-dimensional quantitative structure-activity relationship of MKC-442 and its analogues. Chin. J. New Drugs 2019, 28, 8. [Google Scholar]
- Ouyang, S.; Zhang, D.; Zhu, T.; Yu, S.; Wunier; Hu, Q.N.; Le, Y. Development of 3D-QSAR Models for Predicting the Activities of Chemicals to Stimulate Muscle Growth via Β2-Adrenoceptor. Toxicol. Vitr. 2021, 77, 105251. [Google Scholar] [CrossRef]
- Xu, H.L.; Su, J.; Wang, Z.S.; Hou, C.X.; Wu, P.C.; Xing, Y.; Li, X.S.; Zhu, X.L.; Lu, Y.C.; Xu, L.J. Synthesis, Design and Three-Dimensional Quantitative Structure Activity Relationship (3D-QSAR) Research of Phenylpyrrole. Fungic. Chin. J. Org. Chem. 2021, 41, 11. [Google Scholar] [CrossRef]
- Liu, H.; Huang, H.; Xiao, X.; Zhao, Z.; Liu, C. Effects of Phthalate Esters (Paes) on Cell Viability and Nrf2 of Hepg2 and 3d-Qsar Studies. Toxics 2021, 9, 134. [Google Scholar] [CrossRef]
- Mennillo, E.; Cappelli, F.; Arukwe, A. Biotransformation and Oxidative Stress Responses in Rat Hepatic Cell-Line (H4IIE) Exposed to Organophosphate Esters (OPEs). Toxicol. Appl. Pharmacol. 2019, 371, 84–94. [Google Scholar] [CrossRef] [PubMed]
| No. | Compound | Equation | R2 | Ref. |
|---|---|---|---|---|
| 1 | Volatile organic molecules | 0.74 | [5] | |
| 2 | Phenols | TEAC ** = ∑(αi – αi *) × exp[−0.6 × (|X − Xi|) 2] + 0.758 | 0.90 | [21] |
| 3 | Phthalate acid esters | 0.89 | [22] | |
| 4 | Natural Products | 0.91 | [23] | |
| 5 | Organic compounds | 0.82 | [24] |
| Compound | CAS | Abbreviation | log KOW | log LC50 (mg·L−1) | log NOEC (mg·L−1) | log TRO | Types |
|---|---|---|---|---|---|---|---|
| Triisobutyl phosphate | 126-71-6 | TIBP | 3.60 | 0.20 | 0.50 | 0.30 | N * |
| Tributyl phosphate | 126-73-8 | TNBP | 4.00 | 0.12 | 0.24 | 0.12 | N |
| Tributoxyethyl phosphate | 78-51-3 | TBEP | 3.00 | 0.59 | 1.40 | 0.81 | N |
| Triphenyl phosphate | 115-86-6 | TPHP | 4.70 | −0.10 | −0.73 | 0.63 | N |
| Diphenyl chlorophosphate | 2524-64-3 | - | 3.16 | 0.36 | 1.03 | 0.67 | N |
| Tripropyl phosphate | 513-08-6 | TPP | 1.87 | 0.57 | 1.90 | 1.36 | T ** |
| Diethyl phthalate | 84-66-2 | DEP | 2.42 | 1.10 | 0.79 | 0.30 | N |
| Di-n-butyl phthalate | 84-74-2 | DNBP | 4.61 | 0.05 | −0.25 | 0.30 | N |
| Bis(2-ethylhexyl)phthalate | 117-81-7 | BEHP | 8.39 | −2.0 | −2.30 | 0.30 | N |
| Bis(2-butoxyethyl) phosphate | 14260-97-0 | BBOEP | 1.74 | 2.62 | 2.32 | 0.30 | N |
| Tricresyl phosphate | 1330-78-5 | TCP | 6.34 | −0.84 | −8.15 | 0.30 | N |
| Diethyl phthalate | 84-66-2 | DEP | 2.42 | 1.10 | 0.79 | 0.30 | N |
| No. | Type | Compound | Equation | R2 | Ref. |
|---|---|---|---|---|---|
| 1 | 2D- QSAR | Dioxin | 0.91 | [40] | |
| 2 | MKC-442 | 0.79 | [41] | ||
| 3 | 3D- QSAR | β2-adrenocep | 0.99 | [42] | |
| 4 | Phenylpyrrole Fungicides | 0.99 | [43] | ||
| 5 | Phthalate Esters | 0.99 | [44] |
| No. | Compound | Equation | ||
|---|---|---|---|---|
| 1 | Volatile Organic Molecules | 0.74 | 0.81 | |
| 2 | Phenols | TEAC=∑(αi – αi*)×exp[−0.6 × (|X − Xi|) 2] + 0.758 | 0.90 | 0.91 |
| 3 | Phthalate Acid Esters | 0.89 | 0.92 | |
| 4 | Natural Products | 0.91 | 0.91 | |
| 5 | Freshwater Crustacean | 0.82 | 0.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Dong, Y.; Li, L.; Jiao, J.; Liu, S.; Zou, X. Toxicity Rank Order (TRO) As a New Approach for Toxicity Prediction by QSAR Models. Int. J. Environ. Res. Public Health 2023, 20, 701. https://doi.org/10.3390/ijerph20010701
Chen Y, Dong Y, Li L, Jiao J, Liu S, Zou X. Toxicity Rank Order (TRO) As a New Approach for Toxicity Prediction by QSAR Models. International Journal of Environmental Research and Public Health. 2023; 20(1):701. https://doi.org/10.3390/ijerph20010701
Chicago/Turabian StyleChen, Yuting, Yuying Dong, Le Li, Jian Jiao, Sitong Liu, and Xuejun Zou. 2023. "Toxicity Rank Order (TRO) As a New Approach for Toxicity Prediction by QSAR Models" International Journal of Environmental Research and Public Health 20, no. 1: 701. https://doi.org/10.3390/ijerph20010701
APA StyleChen, Y., Dong, Y., Li, L., Jiao, J., Liu, S., & Zou, X. (2023). Toxicity Rank Order (TRO) As a New Approach for Toxicity Prediction by QSAR Models. International Journal of Environmental Research and Public Health, 20(1), 701. https://doi.org/10.3390/ijerph20010701







