Fast Air-to-Liquid Sampler Detects Surges in SARS-CoV-2 Aerosol Levels in Hospital Rooms
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
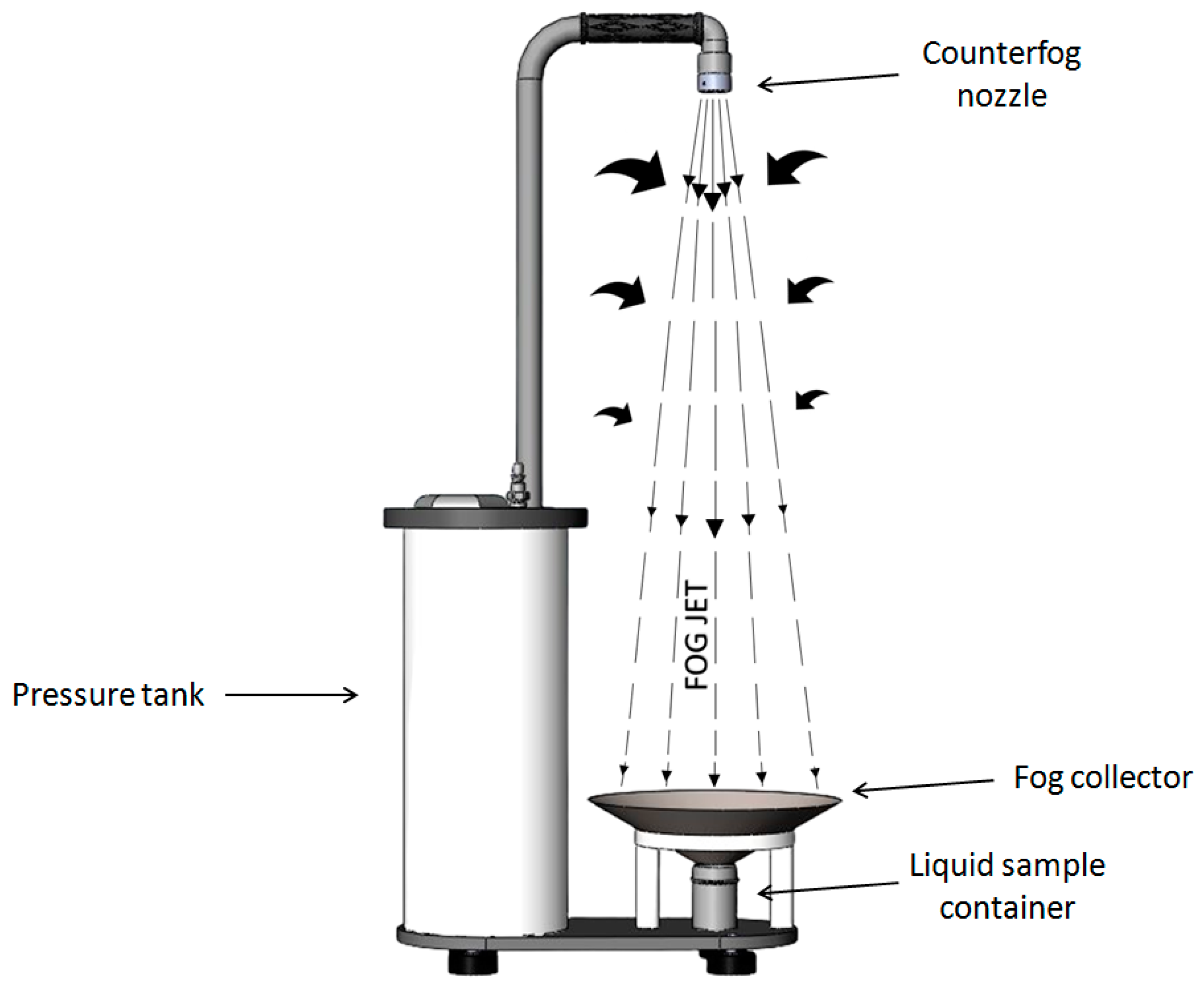
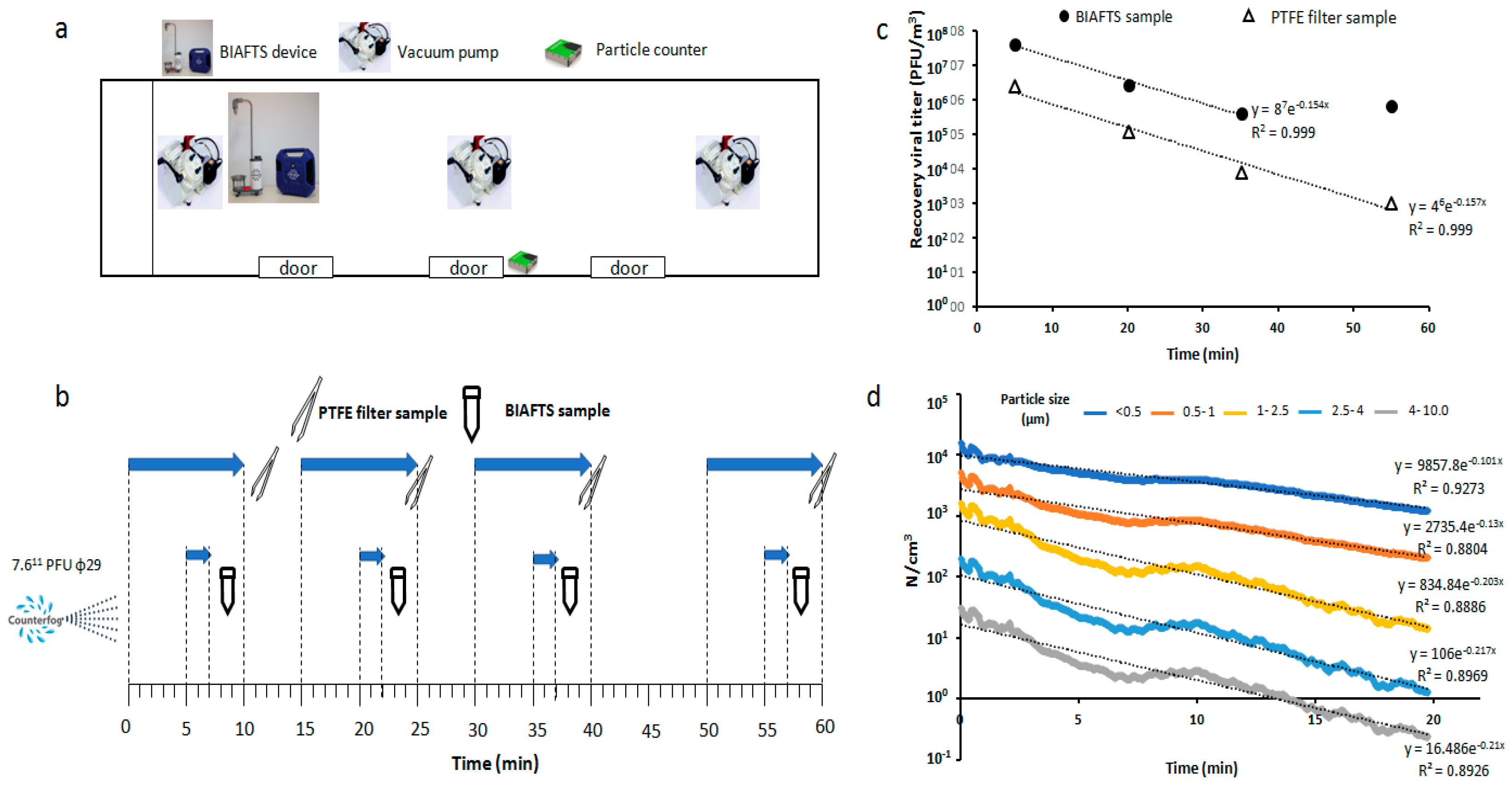
2.2. Aerosolization and Sampling of Test Bacteriophage ϕ29
2.3. Field Tests with SARS-CoV-2
2.4. Analytical Methods
2.5. Data Analysis
2.6. Calculation of Bacteriophage ϕ29 Aerosol Concentration
3. Results
3.1. Calibration Tests of BIAFTS Using Bacteriophage ϕ29
3.2. Bacteriophage ϕ29 Aerosol Evolution over Time
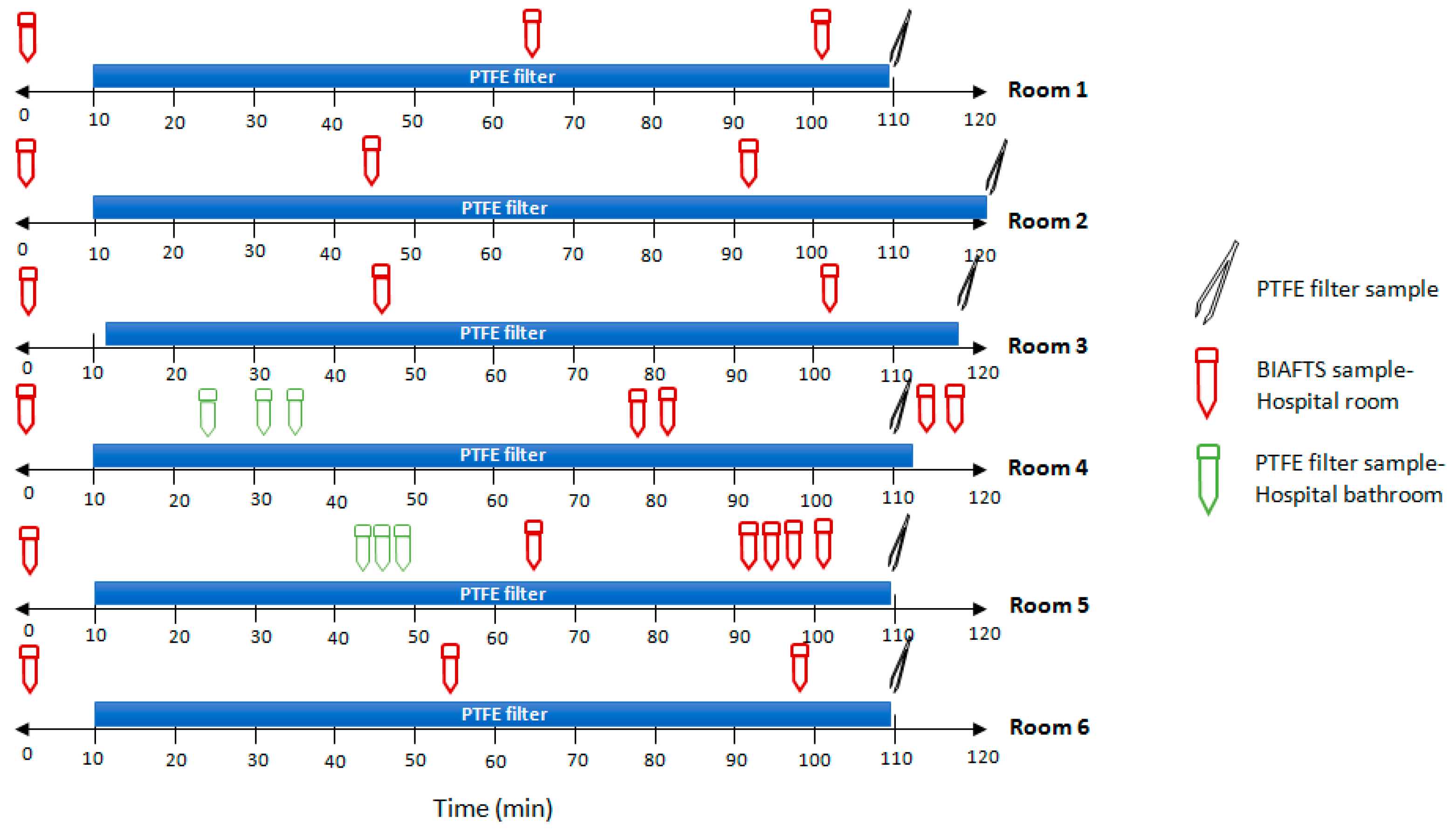
3.3. Real SARS-CoV-2 Bioaerosols Detection in Hospital Rooms
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, C.C.; Prather, K.A.; Sznitman, J.; Jimenez, J.L.; Lakdawala, S.S.; Tufekci, Z.; Marr, L.C. Airborne Transmission of Respiratory Viruses. Science 2021, 373, abd9149. [Google Scholar] [CrossRef] [PubMed]
- Lednicky, J.A.; Lauzard, M.; Fan, Z.H.; Jutla, A.; Tilly, T.B.; Gangwar, M.; Usmani, M.; Shankar, S.N.; Mohamed, K.; Eiguren-Fernandez, A.; et al. Viable SARS-CoV-2 in the Air of a Hospital Room with COVID-19 Patients. Int. J. Infect. Dis. 2020, 100, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Kutter, J.S.; de Meulder, D.; Bestebroer, T.M.; Lexmond, P.; Mulders, A.; Richard, M.; Fouchier, R.A.M.; Herfst, S. SARS-CoV and SARS-CoV-2 Are Transmitted through the Air between Ferrets over More than One Meter Distance. Nat. Commun. 2021, 12, 1653. [Google Scholar] [CrossRef] [PubMed]
- Hamner, L.; Dubbel, P.; Capron, I.; Ross, A.; Jordan, A.; Lee, J.; Lynn, J.; Ball, A. High SARS-CoV-2 Attack Rate Following Exposure at a Choir Practice. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 606–610. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qian, H.; Hang, J.; Chen, X.; Cheng, P.; Ling, H.; Wang, S.; Liang, P.; Li, J.; Xiao, S.; et al. Probable Airborne Transmission of SARS-CoV-2 in a Poorly Ventilated Restaurant. Build. Environ. 2021, 196, 107788. [Google Scholar] [CrossRef] [PubMed]
- Moschovis, P.P.; Yonker, L.M.; Shah, J.; Singh, D.; Demokritou, P.; Kinane, T.B. Aerosol Transmission of SARS-CoV-2 by Children and Adults during the COVID-19 Pandemic. Pediatr. Pulmonol. 2021, 56, 1389–1394. [Google Scholar] [CrossRef]
- WHO. Infection Prevention and Control of Epidemic- and Pandemic-Prone Acute Respiratory Infections in Health Care; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- Gralton, J.; Tovey, E.; McLaws, M.L.; Rawlinson, W.D. The Role of Particle Size in Aerosolised Pathogen Transmission: A Review. J. Infect. 2011, 62, 1–13. [Google Scholar] [CrossRef]
- Sánchez García-Casarrubios, J.; Llerena-Aguilar, F.-J.; Pérez-Díaz, J.-L. Fog Dynamics. In Enhancing CBRNE Safety & Security: Proceedings of the SICC 2017 Conference; Springer: Cham, Switzerland, 2018; pp. 81–86. [Google Scholar]
- The National Academies of Sciences, Engineering, and Medicine. Video 31—CQ1 Reflection and Syntheses: Identifying Opportunities and Gaps on the Path Ahead by Kim Prather. In Proceedings of the Airborne Transmission of SARSCoV-2: A Virtual Workshop, Virtual, 26–27 August 2020. [Google Scholar]
- Watanabe, T.; Bartrand, T.A.; Weir, M.H.; Omura, T.; Haas, C.N. Development of a Dose-Response Model for SARS Coronavirus. Risk Anal. 2010, 30, 1129–1138. [Google Scholar] [CrossRef]
- Ke, Z.; Oton, J.; Qu, K.; Cortese, M.; Zila, V.; McKeane, L.; Nakane, T.; Zivanov, J.; Neufeldt, C.J.; Cerikan, B.; et al. Structures and Distributions of SARS-CoV-2 Spike Proteins on Intact Virions. Nature 2020, 588, 498–502. [Google Scholar] [CrossRef]
- Laue, M.; Kauter, A.; Hoffmann, T.; Möller, L.; Michel, J.; Nitsche, A. Morphometry of SARS-CoV and SARS-CoV-2 Particles in Ultrathin Plastic Sections of Infected Vero Cell Cultures. Sci. Rep. 2021, 11, 3515. [Google Scholar] [CrossRef]
- Haig, C.W.; Mackay, W.G.; Walker, J.T.; Williams, C. Bioaerosol Sampling: Sampling Mechanisms, Bioefficiency and Field Studies. J. Hosp. Infect. 2016, 93, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Verreault, D.; Moineau, S.; Duchaine, C. Methods for Sampling of Airborne Viruses. Microbiol. Mol. Biol. Rev. 2008, 72, 413–444. [Google Scholar] [CrossRef] [PubMed]
- Chia, P.Y.; Coleman, K.K.; Tan, Y.K.; Ong, S.W.X.; Gum, M.; Lau, S.K.; Lim, X.F.; Lim, A.S.; Sutjipto, S.; Lee, P.H.; et al. Detection of Air and Surface Contamination by SARS-CoV-2 in Hospital Rooms of Infected Patients. Nat. Commun. 2020, 11, 2800. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ning, Z.; Chen, Y.; Guo, M.; Liu, Y.; Gali, N.K.; Sun, L.; Duan, Y.; Cai, J.; Westerdahl, D.; et al. Aerodynamic Analysis of SARS-CoV-2 in Two Wuhan Hospitals. Nature 2020, 582, 557–560. [Google Scholar] [CrossRef]
- Zhou, J.; Otter, J.A.; Price, J.R.; Cimpeanu, C.; Garcia, D.M.; Kinross, J.; Boshier, P.R.; Mason, S.; Bolt, F.; Holmes, A.H.; et al. Investigating SARS-CoV-2 Surface and Air Contamination in an Acute Healthcare Setting during the Peak of the COVID-19 Pandemic in London. Clin. Infect. Dis. 2020, 73, e1870–e1877. [Google Scholar] [CrossRef]
- Ratnesar-Shumate, S.; Bohannon, K.; Williams, G.; Holland, B.; Krause, M.; Green, B.; Freeburger, D.; Dabisch, P. Comparison of the Performance of Aerosol Sampling Devices for Measuring Infectious SARS-CoV-2 Aerosols. Aerosol Sci. Technol. 2021, 55, 975–986. [Google Scholar] [CrossRef]
- Burton, N.C.; Grinshpun, S.A.; Reponen, T. Physical Collection Efficiency of Filter Materials for Bacteria and Viruses. Ann. Occup. Hyg. 2007, 51, 143–151. [Google Scholar] [CrossRef]
- Grimes, S.; Jardine, P.J.; Anderson, D. Bacteriophage Φ29 DNA Packaging. Adv. Virus Res. 2002, 58, 281–294. [Google Scholar] [CrossRef]
- Pérez Díaz, J.L.; Sánchez García-Casarrubios, J.; Méndez-Vigo Carranza, P.; Ruiz Navas, E.M.; Iliev Petrov, M.; Alcamí Pertejo, A.; Vázquez, Á.; Rastrojo, A.; Archilla, V.; Sánchez García, M.; et al. Fast Surface Disinfection with COUNTERFOG® SDR-F05A+. Eur. Phys. J. Plus 2021, 136, 393. [Google Scholar] [CrossRef]
- Santos, S.B.; Carvalho, C.M.; Sillankorva, S.; Nicolau, A.; Ferreira, E.C.; Azeredo, J. The Use of Antibiotics to Improve Phage Detection and Enumeration by the Double-Layer Agar Technique. BMC Microbiol. 2009, 9, 148. [Google Scholar] [CrossRef]
- Pérez-Díaz, J.L.; Llerena-Aguilar, F.J.; Martín-Pérez, T.; Sánchez-García-Casarrubios, J.; Ruiz-Navas, E. Decontamination of Diesel Particles from Air by Using the Counterfog® System. Air Qual. Atmos. Health 2019, 12, 305–310. [Google Scholar] [CrossRef]
- Tharayil, A.; Rajakumari, R.; Mozetic, M.; Primc, G.; Thomas, S. Contact Transmission of SARS-CoV-2 on Fomite Surfaces: Surface Survival and Risk Reduction. Interface Focus 2021, 12, 48. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.G.; Wallace, L.; Rim, D. Contributions of Coagulation, Deposition, and Ventilation to the Removal of Airborne Nanoparticles in Indoor Environments. Environ. Sci. Technol. 2021, 55, 9730–9739. [Google Scholar] [CrossRef] [PubMed]
- Fears, A.C.; Klimstra, W.B.; Duprex, P.; Hartman, A.; Weaver, S.C.; Plante, K.C.; Mirchandani, D.; Plante, J.A.; Aguilar, P.V.; Fernández, D.; et al. Comparative Dynamic Aerosol Efficiencies of Three Emergent Coronaviruses and the Unusual Persistence of SARS-CoV-2 in Aerosol Suspensions. medRxiv 2020. [Google Scholar] [CrossRef]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef] [PubMed]

| Room | Ct Value | Result | Airborne SARS-CoV-2 (RNA Copies m−3 air) | Sampler Used |
|---|---|---|---|---|
| 1 | - | Negative | ND | BIAFTS |
| 33.49 | Positive | 547.09 | ||
| - | Negative | ND | ||
| 34.10 | Positive | 258.45 | PTFE filter | |
| 2 | 31.04 | Positive | 4235.00 | BIAFTS |
| 39.02 | Indeterminate | 9.55 | ||
| - | Negative | ND | ||
| 35.06 | Positive | 234.45 | PTFE filter | |
| 3 | 36.53 | Positive | 82.66 | BIAFTS |
| 36.10 | Positive | 146·28 | ||
| 36.54 | Positive | 99.00 | ||
| 33.64 | Positive | 650.74 | PTFE filter | |
| 4 | 36.45 | Indeterminate | 5.10 | BIAFTS |
| 35.83 | Positive | 14.39 | ||
| 36.31 | Positive | 6.93 | ||
| 35.71 | Positive | 2.31 | ||
| - | Negative | ND | ||
| 34.47 | Positive | 148.15 | PTFE filter | |
| Bathroom-Room 4 | 36.6 | Positive | 3.91 | BIAFTS |
| 35.9 | Positive | 10.89 | ||
| 37.7 | Indeterminate | 3.63 | ||
| 5 | 36.45 | Positive | 197.42 | BIAFTS |
| 37.36 | Positive | 172.40 | ||
| 37.56 | Indeterminate | 132.08 | ||
| 37.64 | Indeterminate | 183.73 | ||
| 36.85 | Positive | 250.38 | ||
| 34.17 | Positive | 157.68 | PTFE filter | |
| Bathroom-Room 5 | 38.32 | Positive | 272.06 | BIAFTS |
| 38.22 | Indeterminate | 230.39 | ||
| 38.96 | Indeterminate | 125.00 | ||
| 6 | - | Negative | ND | BIAFTS |
| - | Negative | ND | ||
| - | Negative | ND | ||
| 34.33 | Positive | 456.96 | PTFE filter |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
del Álamo, C.; Vázquez-Calvo, Á.; Sanchiz, Á.; Rodríguez-Caravaca, G.; Martín, R.; Hernáez, B.; Méndez-Vigo-Carranza, P.; Sánchez García-Casarrubios, J.; Alcamí, A.; Pérez-Díaz, J.L. Fast Air-to-Liquid Sampler Detects Surges in SARS-CoV-2 Aerosol Levels in Hospital Rooms. Int. J. Environ. Res. Public Health 2023, 20, 576. https://doi.org/10.3390/ijerph20010576
del Álamo C, Vázquez-Calvo Á, Sanchiz Á, Rodríguez-Caravaca G, Martín R, Hernáez B, Méndez-Vigo-Carranza P, Sánchez García-Casarrubios J, Alcamí A, Pérez-Díaz JL. Fast Air-to-Liquid Sampler Detects Surges in SARS-CoV-2 Aerosol Levels in Hospital Rooms. International Journal of Environmental Research and Public Health. 2023; 20(1):576. https://doi.org/10.3390/ijerph20010576
Chicago/Turabian Styledel Álamo, Cristina, Ángela Vázquez-Calvo, África Sanchiz, Gil Rodríguez-Caravaca, Rocío Martín, Bruno Hernáez, Pablo Méndez-Vigo-Carranza, Juan Sánchez García-Casarrubios, Antonio Alcamí, and José Luis Pérez-Díaz. 2023. "Fast Air-to-Liquid Sampler Detects Surges in SARS-CoV-2 Aerosol Levels in Hospital Rooms" International Journal of Environmental Research and Public Health 20, no. 1: 576. https://doi.org/10.3390/ijerph20010576
APA Styledel Álamo, C., Vázquez-Calvo, Á., Sanchiz, Á., Rodríguez-Caravaca, G., Martín, R., Hernáez, B., Méndez-Vigo-Carranza, P., Sánchez García-Casarrubios, J., Alcamí, A., & Pérez-Díaz, J. L. (2023). Fast Air-to-Liquid Sampler Detects Surges in SARS-CoV-2 Aerosol Levels in Hospital Rooms. International Journal of Environmental Research and Public Health, 20(1), 576. https://doi.org/10.3390/ijerph20010576







