Formation of Recalcitrant Compounds during Anaerobic Digestion of Thermally Pre-Treated Sludge: A Critical Macromolecular and Structural Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrates
2.2. Operation of the TH-AD and CAD Digesters
2.3. Anaerobic Biodegradability of Digestates
2.4. Analytical Methods
3. Results
3.1. Operation of TH-AD and CAD Digesters
3.2. Analysis of Macromolecules in CAD and TH-AD Processes
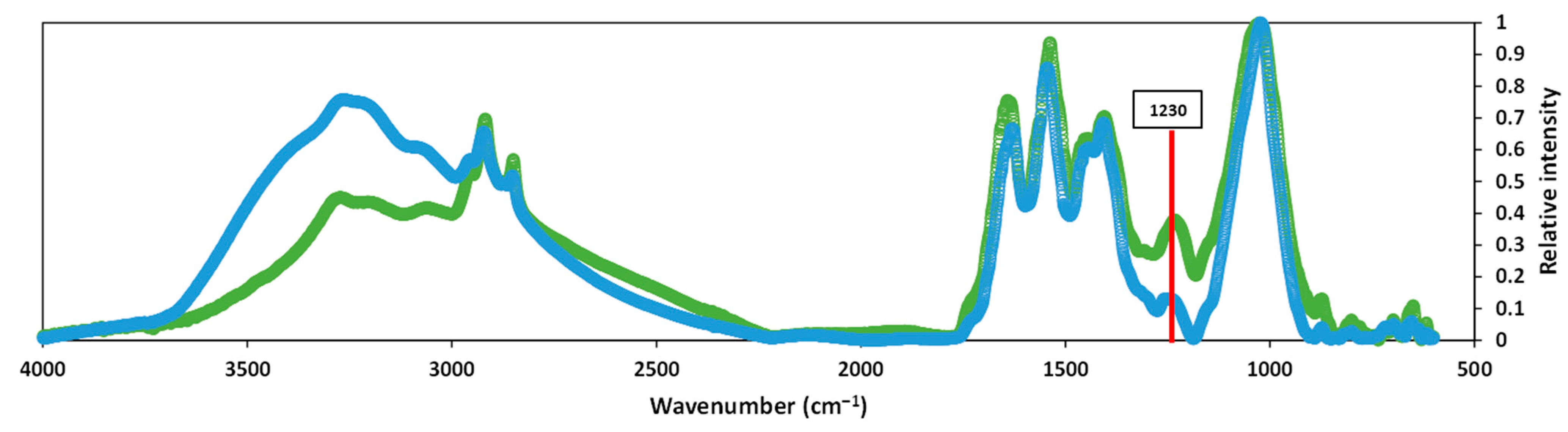
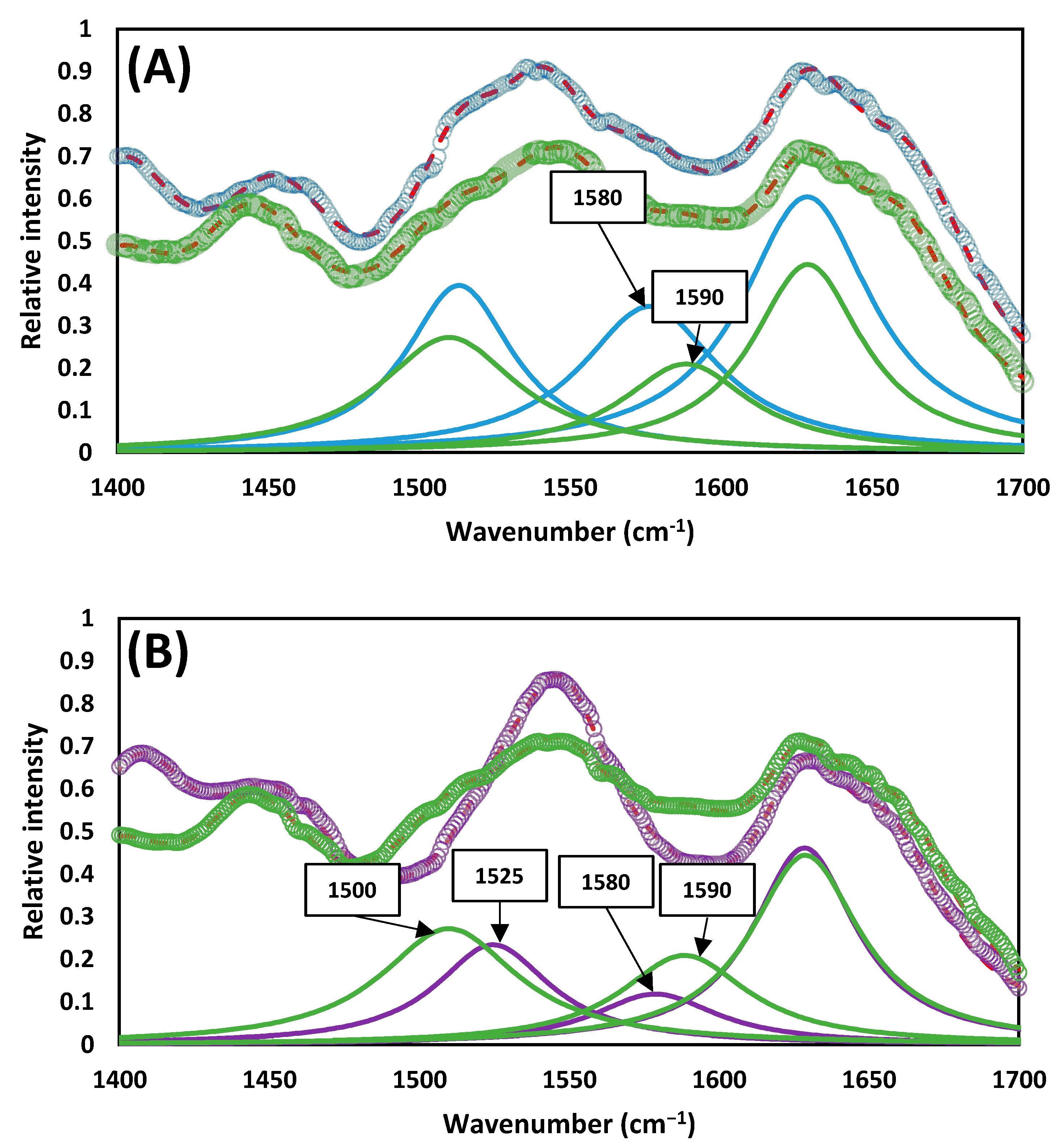
3.3. Fourier-Transform Infrared Spectroscopy Analysis
4. Discussion
4.1. Performance of the Operation of TH-AD and CAD Digesters
4.2. Macromolecular and Structural Study of the Sludges Produced by TH-AD and CAD Processes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carrère, H.; Bougrier, C.; Castets, D.; Delgenès, J.P. Impact of Initial Biodegradability on Sludge Anaerobic Digestion Enhancement by Thermal Pretreatment. J. Environ. Sci. Health—Part A Toxic/Hazard. Subst. Environ. Eng. 2008, 43, 1551–1555. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lee, K.; Park, K.Y. Enhancement of Biogas Production from Anaerobic Digestion of Waste Activated Sludge by Hydrothermal Pre-Treatment. Int. Biodeterior. Biodegrad. 2015, 101, 42–46. [Google Scholar] [CrossRef]
- Carrère, H.; Dumas, C.; Battimelli, A.; Batstone, D.J.; Delgenès, J.P.; Steyer, J.P.; Ferrer, I. Pretreatment Methods to Improve Sludge Anaerobic Degradability: A Review. J. Hazard. Mater. 2010, 183, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Donoso-Bravo, A.; Pérez-Elvira, S.I.; Aymerich, E.; Fdz-Polanco, F. Assessment of the Influence of Thermal Pre-Treatment Time on the Macromolecular Composition and Anaerobic Biodegradability of Sewage Sludge. Bioresour. Technol. 2011, 102, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Abu-Orf, M.; Goss, T. Comparing Thermal Hydrolysis Processes (CAMBITM and EXELYSTM) for Solids Pretreatment Prior to Anaerobic Digestion. Residuals Biosolid 2012, 1024–1036. [Google Scholar] [CrossRef]
- Gonzalez, A.; Hendriks, A.T.W.M.; van Lier, J.B.; de Kreuk, M. Pre-Treatments to Enhance the Biodegradability of Waste Activated Sludge: Elucidating the Rate Limiting Step. Biotechnol. Adv. 2018, 36, 1434–1469. [Google Scholar] [CrossRef]
- Yang, S.; McDonald, J.; Hai, F.I.; Price, W.E.; Khan, S.J.; Nghiem, L.D. Effects of Thermal Pre-Treatment and Recuperative Thickening on the Fate of Trace Organic Contaminants during Anaerobic Digestion of Sewage Sludge. Int. Biodeterior. Biodegrad. 2017, 124, 146–154. [Google Scholar] [CrossRef]
- Sapkaite, I.; Barrado, E.; Fdz-Polanco, F.; Pérez-Elvira, S.I. Optimization of a Thermal Hydrolysis Process for Sludge Pre-Treatment. J. Environ. Manag. 2017, 192, 25–30. [Google Scholar] [CrossRef]
- Barber, W. Thermal Hydrolysis for Sewage Treatment: A Critical Review. Water Res. 2016, 104, 53–71. [Google Scholar] [CrossRef]
- Liu, X.; Wang, W.; Gao, X.; Zhou, Y.; Shen, R. Effect of Thermal Pretreatment on the Physical and Chemical Properties of Municipal Biomass Waste. Waste Manag. 2012, 32, 249–255. [Google Scholar] [CrossRef]
- Zhang, D.; Feng, Y.; Huang, H.; Khunjar, W.; Wang, Z.W. Recalcitrant Dissolved Organic Nitrogen Formation in Thermal Hydrolysis Pretreatment of Municipal Sludge. Environ. Int. 2020, 138, 105629. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, K.; Miotto, D. Honey Melanoidins: Analysis of the Compositions of the High Molecular Weight Melanoidins Exhibiting Radical-Scavenging Activity. Food Chem. 2011, 127, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Tamanna, N.; Mahmood, N. Food Processing and Maillard Reaction Products: Effect on Human Health and Nutrition. Int. J. Food Sci. 2015, 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhang, S.; Cheng, K.; Antonietti, M. A Hydrothermal Process to Turn Waste Biomass into Artificial Fulvic and Humic Acids for Soil Remediation. Sci. Total Environ. 2019, 686, 1140–1151. [Google Scholar] [CrossRef] [PubMed]
- Faixo, S.; Gehin, N.; Balayssac, S.; Gilard, V.; Mazeghrane, S.; Haddad, M.; Gaval, G.; Paul, E.; Garrigues, J.-C. Current Trends and Advances in Analytical Techniques for the Characterization and Quantification of Biologically Recalcitrant Organic Species in Sludge and Wastewater: A Review. Anal. Chim. Acta 2021, 1152, 338284. [Google Scholar] [CrossRef]
- Ortega-martínez, E.; Chamy, R.; Jeison, D. Thermal Pre-Treatment: Getting Some Insights on the Formation of Recalcitrant Compounds and Their Effects on Anaerobic Digestion. J. Environ. Manag. 2021, 282, 111940. [Google Scholar] [CrossRef]
- Penaud, V.; Delgenès, J.P.; Moletta, R. Characterization of Soluble Molecules from Thermochemically Pretreated Sludge. J. Environ. Eng. 2000, 126, 397–402. [Google Scholar] [CrossRef]
- Bougrier, C.; Delgenès, J.P.; Carrère, H. Impacts of Thermal Pre-Treatments on the Semi-Continuous Anaerobic Digestion of Waste Activated Sludge. Biochem. Eng. J. 2007, 34, 20–27. [Google Scholar] [CrossRef]
- Dwyer, J.; Starrenburg, D.; Tait, S.; Barr, K.; Batstone, D.J.; Lant, P. Decreasing Activated Sludge Thermal Hydrolysis Temperature Reduces Product Colour, without Decreasing Degradability. Water Res. 2008, 42, 4699–4709. [Google Scholar] [CrossRef]
- Lu, D.; Sun, F.; Zhou, Y. Insights into Anaerobic Transformation of Key Dissolved Organic Matters Produced by Thermal Hydrolysis Sludge Pretreatment. Bioresour. Technol. 2018, 266, 60–67. [Google Scholar] [CrossRef]
- Koch, K. Calculating the Degree of Degradation of the Volatile Solids in Continuously Operated Bioreactors. Biomass Bioenergy 2015, 74, 79–83. [Google Scholar] [CrossRef]
- Angelidaki, I.; Sanders, W. Assessment of the Anaerobic Biodegradability of Macropollutants. Rev. Environ. Sci. Biotechnol. 2004, 3, 117–129. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewaters, 21st ed.; APHA: Washington, DC, USA, 2005. [Google Scholar]
- Ripley, A.L.E.; Boyle, W.C.; Converse, J.C. Improved Alkalimetric for Anaerobic Digestion Wastes Monitoring of Municipal Sludge. Water Pollut. Contron Fed. 1986, 58, 406–411. [Google Scholar]
- Le, C.; Stuckey, D.C. Colorimetric Measurement of Carbohydrates in Biological Wastewater Treatment Systems: A Critical Evaluation. Water Res. 2016, 94, 280–287. [Google Scholar] [CrossRef]
- Josefsson, B.; Uppström, L.; Östling, G. Automatic Spectrophotometric Procedures for the Determination of the Total Amount of Dissolved Carbohydrates in Sea Water. Deep. -Sea Res. Oceanogr. Abstr. 1972, 19, 385–395. [Google Scholar] [CrossRef]
- Frølund, B.; Griebe, T.; Nielsen, P.H. Enzymatic Activity in the Activated-Sludge Floc Matrix. Appl. Microbiol. Biotechnol. 1995, 43, 755–761. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2006. [Google Scholar]
- Kowalski, M.; Kowalska, K.; Wiszniowski, J.; Turek-Szytow, J. Qualitative Analysis of Activated Sludge Using FT-IR Technique. Chem. Pap. 2018, 72, 2699–2706. [Google Scholar] [CrossRef]
- Han, M.M.; Yi, Y.; Wang, H.X.; Huang, F. Investigation of the Maillard Reaction between Polysaccharides and Proteins from Longan Pulp and the Improvement in Activities. Molecules 2017, 22, 938. [Google Scholar] [CrossRef]
- Ioannou, A.; Varotsis, C. Real Time Monitoring the Maillard Reaction Intermediates by HPLC- FTIR. J. Phys. Chem. Biophys. 2016, 6, 6–10. [Google Scholar] [CrossRef]
- Montha, S.; Suwandittakul, P.; Poonsrisawat, A.; Oungeun, P.; Kongkaew, C. Maillard Reaction in Natural Rubber Latex: Characterization and Physical Properties of Solid Natural Rubber. Adv. Mater. Sci. Eng. 2016, 2016, 7807524. [Google Scholar] [CrossRef][Green Version]
- Rozenberg, M.; Lansky, S.; Shoham, G. Spectroscopic FTIR and NMR Study of the Interaction of Sugars with Proteins. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 222, 116861. [Google Scholar] [CrossRef]
- Karbasi, M.; Askari, G.; Madadlou, A. Surface Decoration of Whey Protein Microgels through the Maillard Conjugation with Maltodextrin. Food Hydrocoll. 2019, 91, 190–197. [Google Scholar] [CrossRef]
- Liu, Q.; Kong, B.; Han, J.; Sun, C.; Li, P. Structure and Antioxidant Activity of Whey Protein Isolate Conjugated with Glucose via the Maillard Reaction under Dry-Heating Conditions. Food Struct. 2014, 1, 145–154. [Google Scholar] [CrossRef]
- Rubinsztain, Y.; Yariv, S.; Ioselis, P.; Aizenshtat, Z.; Ikan, R. Characterization of Melanoidins by IR Spectroscopy-I. Galactose-Glycine Melanoidins. Org. Geochem. 1986, 9, 117–125. [Google Scholar] [CrossRef]
- Su, J.F.; Huang, Z.; Yuan, X.Y.; Wang, X.Y.; Li, M. Structure and Properties of Carboxymethyl Cellulose/Soy Protein Isolate Blend Edible Films Crosslinked by Maillard Reactions. Carbohydr. Polym. 2010, 79, 145–153. [Google Scholar] [CrossRef]
- Xu, Z.Z.; Huang, G.Q.; Xu, T.C.; Liu, L.N.; Xiao, J.X. Comparative Study on the Maillard Reaction of Chitosan Oligosaccharide and Glucose with Soybean Protein Isolate. Int. J. Biol. Macromol. 2019, 131, 601–607. [Google Scholar] [CrossRef]
- Valo, A.; Carrère, H.; Delgenès, J.P. Thermal, Chemical and Thermo-Chemical Pre-Treatment of Waste Activated Sludge for Anaerobic Digestion. J. Chem. Technol. Biotechnol. 2004, 79, 1197–1203. [Google Scholar] [CrossRef]
- Oosterhuis, M.; Ringoot, D.; Hendriks, A.; Roeleveld, P. Thermal Hydrolysis of Waste Activated Sludge at Hengelo Wastewater Treatment Plant, the Netherlands. Water Sci. Technol. 2014, 70, 1–7. [Google Scholar] [CrossRef]
- Bougrier, C.; Delgenès, J.P.; Carrère, H. Combination of Thermal Treatments and Anaerobic Digestion to Reduce Sewage Sludge Quantity and Improve Biogas Yield. Process Saf. Environ. Prot. 2006, 84, 280–284. [Google Scholar] [CrossRef]
- Jorobekova, S.; Kydralieva, K. Plant Growth Biostimulants from By-Products of Anaerobic Digestion of Organic Substances. In Organic Fertilizers—History, Production and Applications; IntechOpen: London, UK, 2019; pp. 1–16. [Google Scholar]
- Helal, A.A.; Murad, G.A.; Helal, A.A. Characterization of Different Humic Materials by Various Analytical Techniques. Arab. J. Chem. 2011, 4, 51–54. [Google Scholar] [CrossRef]
- Ribeiro, J.S.; Ok, S.S.; Garrigues, S.; De la Guardia, M. FTIR Tentative Characterization of Humic Acids Extracted from Organic Materials. Spectrosc. Lett. 2001, 34, 179–190. [Google Scholar] [CrossRef]
- Amir, S.; Hafidi, M.; Merlina, G.; Hamdi, H.; Jean-Claude, R. Elemental Analysis, FTIR and 13C-NMR of Humic Acids from Sewage Sludge Composting. Agronomie 2004, 24, 13–18. [Google Scholar] [CrossRef]
- Vergnoux, A.; Guiliano, M.; Di Rocco, R.; Domeizel, M.; Théraulaz, F.; Doumenq, P. Quantitative and Mid-Infrared Changes of Humic Substances from Burned Soils. Environ. Res. 2011, 111, 205–214. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Li, C. Evolution of Humic Substances during Anaerobic Sludge Digestion. Environ. Eng. Manag. J. 2017, 16, 1577–1582. [Google Scholar] [CrossRef]
- Liu, R.; Hao, X.; van Loosdrecht, M.C.M.; Zhou, P.; Li, J. Dynamics of Humic Substance Composition during Anaerobic Digestion of Excess Activated Sludge. Int. Biodeterior. Biodegrad. 2019, 145, 104771. [Google Scholar] [CrossRef]
- el Fels, L.; Zamama, M.; el Asli, A.; Hafidi, M. Assessment of Biotransformation of Organic Matter during Co-Composting of Sewage Sludge-Lignocelullosic Waste by Chemical, FTIR Analyses, and Phytotoxicity Tests. Int. Biodeterior. Biodegrad. 2014, 87, 128–137. [Google Scholar] [CrossRef]
- Mendez, L.; Mahdy, A.; Demuez, M.; Ballesteros, M.; González-Fernández, C. Effect of High Pressure Thermal Pretreatment on Chlorella Vulgaris Biomass: Organic Matter Solubilisation and Biochemical Methane Potential. Fuel 2014, 117, 674–679. [Google Scholar] [CrossRef]
- Rodríguez-Abalde, Á.; Gómez, X.; Blanco, D.; Cuetos, M.J.; Fernández, B.; Flotats, X. Study of Thermal Pre-Treatment on Anaerobic Digestion of Slaughterhouse Waste by TGA-MS and FTIR Spectroscopy. Waste Manag. Res. 2013, 31, 1195–1202. [Google Scholar] [CrossRef]
- Chandra, R.; Kumar, V.; Tripathi, S. Evaluation of Molasses-Melanoidin Decolourisation by Potential Bacterial Consortium Discharged in Distillery Effluent. 3 Biotech 2018, 8, 187. [Google Scholar] [CrossRef]
- Arimi, M.M.; Zhang, Y.; Götz, G.; Geißen, S.U. Treatment of Melanoidin Wastewater by Anaerobic Digestion and Coagulation. Environ. Technol. 2015, 36, 2410–2418. [Google Scholar] [CrossRef]
- Mallick, S.P.; Mallick, Z.; Mayer, B.K. Meta-Analysis of the Prevalence of Dissolved Organic Nitrogen (DON) in Water and Wastewater and Review of DON Removal and Recovery Strategies. Sci. Total Environ. 2022, 828, 154476. [Google Scholar] [CrossRef]
- Cui, R.; Gong, H.; Xu, Y.; Xu, E.; Yang, D.; Gu, G.; Dai, X. One-Stage Partial Nitritation-Anammox Treatment of Reject Water from High-Solid-Sludge Anaerobic Digestion with Thermal Hydrolysis Pretreatment: Inhibition and System Recovery. J. Environ. Chem. Eng. 2022, 10, 107958. [Google Scholar] [CrossRef]
- Yan, W.; Xu, H.; Lu, D.; Zhou, Y. Effects of Sludge Thermal Hydrolysis Pretreatment on Anaerobic Digestion and Downstream Processes: Mechanism, Challenges and Solutions. Bioresour. Technol. 2022, 344, 126248. [Google Scholar] [CrossRef]
- Huang, R.; Yang, J.; Cao, Y.; Dionysiou, D.D.; Wang, C. Peroxymonosulfate Catalytic Degradation of Persistent Organic Pollutants by Engineered Catalyst of Self-Doped Iron/Carbon Nanocomposite Derived from Waste Toner Powder. Sep. Purif. Technol. 2022, 291, 120963. [Google Scholar] [CrossRef]



| Soluble COD (g L−1) | Total COD (g L−1) | Volatile Solids (g L−1) | |
|---|---|---|---|
| Thermal pre-treated thickened activated sludge | 54 ± 2 | 139 ± 12 | 84.6 ± 3.2 |
| Thickened activated sludge | 39 ± 3 | 221 ± 34 | 116 ± 18 |
| CAD | TH-AD | |
|---|---|---|
| pH | 8.4 ± 0.2 | 8.3 ± 0.4 |
| VS degradation (%) | 46.9 ± 3.3 | 49.5 ± 4.1 |
| COD degradation (%) | 41.8 ± 7.3 | 58.7 ± 5.4 |
| CODs degradation (%) | 23.8 ± 2.8 | 68.7 ± 3.5 |
| Methane volumetric productivity (mL L digester−1 d−1) | 230.3 ± 53.5 | 766.5 ± 88.2 |
| Biogas composition (% methane) | 62.7 ± 2.2 | 68.8 ± 2.1 |
| Specific methane yield (mL CH4 L digester−1 g VSadded−1) | 76.8 ± 17.8 | 225.4 ± 25.9 |
| Color (Pt-Co units) | 3250 ± 150 | 24800 ± 210 |
| Total ammoniacal nitrogen in the digestate (TAN) (mg TAN L−1) | 7440 ± 232 | 5280 ± 189 |
| TAN yield (mg TAN g VS of thickened activated sludge −1) | 38.7 | 43.0 |
| Volatile fatty acids/Alkalinity ratio (mg CH3COOH mg CaCO3−1) | 0.19 ± 0.02 | 0.08 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega-Martínez, E.; Chamy, R.; Jeison, D. Formation of Recalcitrant Compounds during Anaerobic Digestion of Thermally Pre-Treated Sludge: A Critical Macromolecular and Structural Study. Int. J. Environ. Res. Public Health 2023, 20, 558. https://doi.org/10.3390/ijerph20010558
Ortega-Martínez E, Chamy R, Jeison D. Formation of Recalcitrant Compounds during Anaerobic Digestion of Thermally Pre-Treated Sludge: A Critical Macromolecular and Structural Study. International Journal of Environmental Research and Public Health. 2023; 20(1):558. https://doi.org/10.3390/ijerph20010558
Chicago/Turabian StyleOrtega-Martínez, Eduardo, Rolando Chamy, and David Jeison. 2023. "Formation of Recalcitrant Compounds during Anaerobic Digestion of Thermally Pre-Treated Sludge: A Critical Macromolecular and Structural Study" International Journal of Environmental Research and Public Health 20, no. 1: 558. https://doi.org/10.3390/ijerph20010558
APA StyleOrtega-Martínez, E., Chamy, R., & Jeison, D. (2023). Formation of Recalcitrant Compounds during Anaerobic Digestion of Thermally Pre-Treated Sludge: A Critical Macromolecular and Structural Study. International Journal of Environmental Research and Public Health, 20(1), 558. https://doi.org/10.3390/ijerph20010558







