Psychometric Evaluation of the Chinese Version of Mild Cognitive Impairment Questionnaire among Older Adults with Mild Cognitive Impairment
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Participants and Settings
2.3. Instruments
2.3.1. MCQ
2.3.2. Chinese Version of WHOQOL-OLD
2.3.3. FRAIL Assessment
2.3.4. Beijing Version of Montreal Cognitive Assessment
2.4. Data Collection and Ethical Consideration
2.5. Data Analyses
3. Results
3.1. Descriptive Characteristics of the Participants
3.2. Reliability
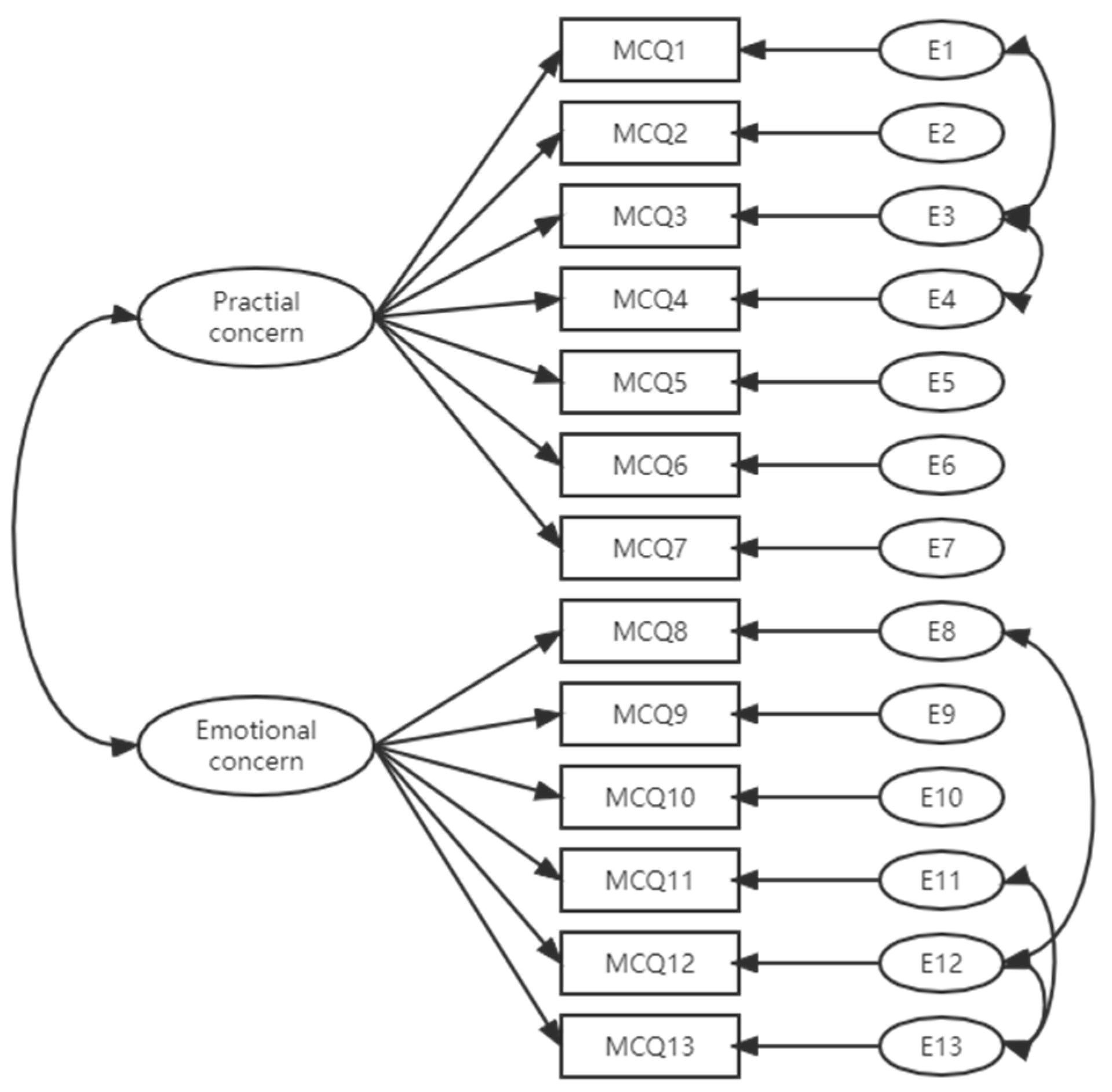
3.3. Construct Validity
3.4. Convergent Validity
3.5. Discriminant Validity
4. Discussion
4.1. Clinical Implication
4.2. Methodological Considerations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jia, L.; Du, Y.; Chu, L.; Zhang, Z.; Li, F.; Lyu, D.; Li, Y.; Li, Y.; Zhu, M.; Jiao, H.; et al. Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: A cross-sectional study. Lancet Public Health 2020, 5, e661–e671. [Google Scholar] [CrossRef]
- Hussenoeder, F.S.; Conrad, I.; Roehr, S.; Fuchs, A.; Pentzek, M.; Bickel, H.; Moesch, E.; Weyerer, S.; Werle, J.; Wiese, B.; et al. Mild cognitive impairment and quality of life in the oldest old: A closer look. Qual. Life Res. 2020, 29, 1675–1683. [Google Scholar] [CrossRef]
- Samy, A.L.; Kamaruzzaman, S.B.; Krishnaswamy, S.; Low, W.Y. Predictors of quality of life among older people with mild cognitive impairment attending urban primary care clinics. Clin. Gerontol. 2020, 43, 441–454. [Google Scholar] [CrossRef]
- Clement-Carbonell, V.; Cabañero-Martínez, M.J.; Fernández-Alcántara, M.; Ruiz-Robledillo, N.; Escribano, S.; Congost-Maestre, N.; Ferrer-Cascales, R. Psychometric properties of the Spanish version of the mild cognitive impairment questionnaire. Res. Nurs. Health 2020, 43, 284–293. [Google Scholar] [CrossRef]
- Dean, K.; Jenkinson, C.; Wilcock, G.; Walker, Z. The development and validation of a patient-reported quality of life measure for people with mild cognitive impairment. Int. Psychogeriatr. 2014, 26, 487–497. [Google Scholar] [CrossRef]
- Song, R.; Gang, M.; Park, M.; Park, M.; Jang, M.; Hwang, I.O.; Kim, J.L. Validity and reliability of K-MCQ to assess quality of life of older adults with mild cognitive impairment. J. Korean Gerontol. Nurs. 2021, 23, 164–175. [Google Scholar] [CrossRef]
- National Bureau of Statistics. China Population Census Yearbook 2020. 2022. Available online: http://www.stats.gov.cn/tjsj/pcsj/ (accessed on 21 July 2022).
- Petersen, R.C.; Smith, G.E.; Waring, S.C.; Ivnik, R.J.; Kokmen, E.; Tangelos, E.G. Aging, memory, and mild cognitive impairment. Int. Psychogeriatr. 1997, 9 (Suppl. 1), 65–69. [Google Scholar] [CrossRef]
- Petersen, R.C.; Negash, S. Mild cognitive impairment: An overview. CNS Spectr. 2008, 13, 45–53. [Google Scholar] [CrossRef]
- Oltra-Cucarella, J.; Ferrer-Cascales, R.; Alegret, M.; Gasparini, R.; Díaz-Ortiz, L.M.; Ríos, R.; Martínez-Nogueras, Á.L.; Onandia, I.; Pérez-Vicente, J.A.; Cabello-Rodríguez, L.; et al. Risk of progression to Alzheimer’s disease for different neuropsychological Mild Cognitive Impairment subtypes: A hierarchical meta-analysis of longitudinal studies. Psychol. Aging 2018, 33, 1007–1021. [Google Scholar] [CrossRef]
- Zakharov, V.V.; Gromova, D.O. Current approaches to management of patients with mild cognitive impairment. Z. Nevrol. Psikhiatrii Im. SS Korsakova 2017, 117, 107–112. [Google Scholar] [CrossRef]
- Ryu, S. The clinical significance of cognitive interventions for the patients with mild cognitive impairment. J. Korean Neuropsychiatr. Assoc. 2018, 57, 23–29. [Google Scholar] [CrossRef]
- Johansson, M.M.; Marcusson, J.; Wressle, E. Cognitive impairment and its consequences in everyday life: Experiences of people with mild cognitive impairment or mild dementia and their relatives. Int. Psychogeriatr. 2015, 27, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Balash, Y.; Mordechovich, M.; Shabtai, H.; Giladi, N.; Gurevich, T.; Korczyn, A.D. Subjective memory complaints in elders: Depression, anxiety, or cognitive decline? Acta Neurol. Scand. 2013, 127, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.Y.; Lee, S.B.; Kim, T.W.; Lee, T.J. Subjective memory complaints, depressive symptoms and instrumental activities of daily living in mild cognitive impairment. Int. Psychogeriatr. 2016, 28, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Yates, J.A.; Clare, L.; Woods, R.T.; MRC CFAS. Subjective memory complaints, mood and MCI: A follow-up study. Aging Ment. Health 2017, 21, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Pusswald, G.; Moser, D.; Pflüger, M.; Gleiss, A.; Auff, E.; Stögmann, E.; Dal-Bianco, P.; Lehrner, J. The impact of depressive symptoms on health-related quality of life in patients with subjective cognitive decline, mild cognitive impairment, and Alzheimer’s disease. Int. Psychogeriatr. 2016, 28, 2045–2054. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, H.T.; Li, S.Y.; Yang, Y.P.; Lin, L.L.; Lin, S.I.; Wang, J.J. Cognitive function and quality of life in community-dwelling seniors with mild cognitive impairment in Taiwan. Community Ment. Health J. 2016, 52, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Landeiro, F.; Mughal, S.; Walsh, K.; Nye, E.; Morton, J.; Williams, H.; Ghinai, I.; Castro, Y.; Leal, J.; Roberts, N.; et al. Health-related quality of life in people with predementia Alzheimer’s disease, mild cognitive impairment or dementia measured with preference-based instruments: A systematic literature review. Alzheimer’s Res. Ther. 2020, 12, 154. [Google Scholar] [CrossRef]
- Power, M.; Quinn, K.; Schmidt, S. Development of the WHOQOL-old module. Qual. Life Res. 2005, 14, 2197–2214. [Google Scholar] [CrossRef]
- EQ. EQ-5D User Guides—EQ-5D. 2021. Available online: https://euroqol.org/publications/user-guides/ (accessed on 30 August 2021).
- Skevington, S.M.; Lotfy, M.; O’Connell, K.A.; WHOQOL Group. The World Health Organization’s WHOQOL-BREF quality of life assessment: Psychometric properties and results of the international field trial. A report from the WHOQOL group. Qual. Life Res. 2004, 13, 299–310. [Google Scholar] [CrossRef]
- Parker, S.G.; Bechinger-English, D.; Jagger, C.; Spiers, N.; Lindesay, J. Factors affecting completion of the SF-36 in older people. Age Ageing 2006, 35, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Perales, J.; Cosco, T.D.; Stephan, B.C.M.; Haro, J.M.; Brayne, C. Health-related quality-of-life instruments for Alzheimer’s disease and mixed dementia. Int. Psychogeriatr. 2013, 25, 691–706. [Google Scholar] [CrossRef] [PubMed]
- Logsdon, R.G.; Gibbons, L.E.; McCurry, S.M.; Teri, L. Assessing quality of life in older adults with cognitive impairment. Psychosom. Med. 2002, 64, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.C.; Lamping, D.L.; Banerjee, S.; Harwood, R.H.; Foley, B.; Smith, P.; Cook, J.C.; Murray, J.; Prince, M.; Levin, E.; et al. Development of a new measure of health-related quality of life for people with dementia: DEMQOL. Psychol. Med. 2007, 37, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Bárrios, H.; Narciso, S.; Guerreiro, M.; Maroco, J.; Logsdon, R.; de Mendonça, A. Quality of life in patients with mild cognitive impairment. Aging Ment. Health 2013, 17, 287–292. [Google Scholar] [CrossRef]
- Dean, K.; Walker, Z.; Jenkinson, C. Data quality, floor and ceiling effects, and test–retest reliability of the Mild Cognitive Impairment Questionnaire. Patient Relat. Outcome Meas. 2018, 9, 43–47. [Google Scholar] [CrossRef]
- Polit, D.; Beck, C. Developing and Testing Self-Report Scales Nursing Research: Generating and Assessing Evidence for Nursing Practice, 10th ed.; Wolters Kluwer: Alphen aan den Rijn, The Netherlands, 2017; pp. 351–378. [Google Scholar]
- Oxford University. Innovation. Translation and Linguistic Validation—Oxford University Innovation. Retreved 2021. 2021, Volumes 3–5. Available online: https://innovation.ox.ac.uk/clinical-outcomes/services/translation-linguistic-validation (accessed on 20 April 2021).
- Liu, R.; Wu, S.; Hao, Y.; Gu, J.; Fang, J.; Cai, N.; Zhang, J. The Chinese version of the world health organization quality of life instrument-older adults module (WHOQOL-OLD): Psychometric evaluation. Health Qual. Life Outcomes Chin. Version 2013, 11, 156. [Google Scholar] [CrossRef]
- Fang, J.; Power, M.; Lin, Y.; Zhang, J.; Hao, Y.; Chatterji, S. Development of short versions for the WHOQOL-OLD module. Gerontologist 2012, 52, 66–78. [Google Scholar] [CrossRef]
- Morley, J.E.; Malmstrom, T.K.; Miller, D.K. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J. Nutr. Health Aging 2012, 16, 601–608. [Google Scholar] [CrossRef]
- Yu, J.; Li, J.; Huang, X. The Beijing version of the Montreal Cognitive Assessment as a brief screening tool for mild cognitive impairment: A community-based study. BMC Psychiatry 2012, 12, 156. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Kääriäinen, M.; Kanste, O.; Elo, S.; Pölkki, T.; Miettunen, J.; Kyngäs, H. Testing and verifying nursing theory by confirmatory factor analysis. J. Adv. Nurs. 2011, 67, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Kline, R.B. Assessing statistical aspects of test fairness with structural equation modelling. Educ. Res. Eval. 2013, 19, 204–222. [Google Scholar] [CrossRef]
- Mhaoláin, A.M.N.; Gallagher, D.; Crosby, L.; Ryan, D.; Lacey, L.; Coen, R.F.; Coakley, D.; Walsh, J.B.; Cunningham, C.; Lawlor, B. Frailty and quality of life for people with Alzheimer’s dementia and mild cognitive impairment. Am. J. Alzheimer’s Dis. Other Dement. 2012, 27, 48–54. [Google Scholar] [CrossRef]
- Khamis, H. Measures of Association: How to Choose? J. Diagn. Med. Sonogr. 2008, 24, 155–162. [Google Scholar] [CrossRef]
- Cheng, Y.; Thorpe, L.; Kabir, R.; Lim, H.J. Latent class growth modeling of depression and anxiety in older adults: An 8-year follow-up of a population-based study. BMC Geriatr. 2021, 21, 550. [Google Scholar] [CrossRef]
- Hallit, S.; Daher, M.C.; Hallit, R.; Hachem, D.; Kheir, N.; Salameh, P. Correlates associated with mental health and nutritional status in Lebanese older adults: A cross-sectional study. Arch. Gerontol. Geriatr. 2020, 87, 103879. [Google Scholar] [CrossRef]
- McKay, M.A.; Copel, L. Factors associated with health-related quality of life in PACE participants. Geriatr. Nurs. 2021, 42, 145–150. [Google Scholar] [CrossRef]
- Salminen, K.S.; Suominen, M.H.; Kautiainen, H.; Pitkälä, K.H. Associations between Nutritional Status, Frailty and Health-Related Quality of Life among Older Long-Term Care Residents in Helsinki. J. Nutr. Health Aging 2020, 24, 319–324. [Google Scholar] [CrossRef]
- Arai, H.; Satake, S.; Kozaki, K. Cognitive frailty in geriatrics. Clin. Geriatr. Med. 2018, 34, 667–675. [Google Scholar] [CrossRef]
| Variables | N (%) | Mean (SD) /Median (Q1:Q3)) |
|---|---|---|
| Age | 79.49 (6.34) | |
| Number of children | 2.50 (2.00:3.00) | |
| Cognitive function (MoCA—Beijing Version) | 15.92 (6.36) | |
| Quality of life (WHOQOL-OLD) | 67.18 (14.84) | |
| Sex | ||
| Male | 98 (52.7) | |
| Female | 88 (47.3) | |
| Marital status | ||
| Married | 124 (66.7) | |
| Divorced/single/widow (er) | 62 (33.3) | |
| Living place | ||
| In town | 89 (47.8) | |
| In rural | 97 (52.2) | |
| Living situation | ||
| Living with family member | 145 (78.0) | |
| Living alone | 41 (22.0) | |
| Educational level | ||
| Primary school or lower | 139 (74.7) | |
| Middle school | 28 (15.1) | |
| High school | 16 (8.6) | |
| College or higher | 3 (1.6) | |
| Source of income | ||
| Retirement pension | 107 (57.5) | |
| Children or others | 79 (42.5) | |
| Level of income | ||
| Low | 89 (47.9) | |
| Middle | 86 (46.2) | |
| High | 11 (5.9) | |
| Main medical insurance type | ||
| Employment medical insurance or self payment | 28 (15.1) | |
| Basic residents medical insurance | 158 (84.9) | |
| Comorbidity | ||
| Yes | 79 (42.5) | |
| No | 107 (57.5) | |
| Frail status | ||
| Robust | 44 (23.7) | |
| Prefrail | 77 (41.4) | |
| Frail | 39 (21.0) |
| Chi-Square (df) p-Value | Chi-Square/df | CFI | GFI | SRMR | RMSEA (90%CI) | |
|---|---|---|---|---|---|---|
| <0.001 | 1.789 | 0.98 | 0.92 | 0.036 | 0.065 (0.045–0.085) | |
| Factors/items (Cronbach’s Alpha) | Factors loading ML | Factors loading ML/ Standardized | Mean (SD) | Median (Q1:Q3) | ||
| Practical concerns (0.923) | 44.75 (16.77) | 42.86 (31.43:54.29) | ||||
| 1. Worry about forgotten things | 1 | 0.69 | 2.62 (1.03) | 3 (2:3) | ||
| 2. Worry about sentence construction | 1.08 | 0.74 | 1.99 (1.03) | 2 (1:3) | ||
| 3. Worry about forgetting plans | 1.06 | 0.74 | 2.42 (1.03) | 2 (2:3) | ||
| 4. Worry about forgetting appointments | 1.15 | 0.78 | 2.28 (1.04) | 2 (1:3) | ||
| 5. Worry about feeling slowed down | 1.17 | 0.82 | 2.26 (1.01) | 2 (1:3) | ||
| 6. Worry about upsetting others because of memory problems | 1.16 | 0.87 | 1.97 (0.94) | 2 (1:3) | ||
| 7. Feeling less independent | 1.17 | 0.83 | 2.12 (1.01) | 2 (1:3) | ||
| Emotional concerns (0.962) | 38.76 (17.14) | 40.00 (20.00:50.00) | ||||
| 8. Irritation or frustration about memory problems | 1 | 0.95 | 1.88 (0.91) | 2 (1:3) | ||
| 9. Worry about memory | 0.98 | 0.89 | 2.03 (0.96) | 2 (1:2) | ||
| 10. Feeling downhearted about memory | 0.97 | 0.96 | 1.85 (0.88) | 2 (1:2) | ||
| 11. Worry about others’ reactions to memory problems | 0.97 | 0.93 | 1.91 (0.90) | 2 (1:2) | ||
| 12. Worry about memory is worse than peers | 0.94 | 0.85 | 1.92 (0.96) | 2 (1:3) | ||
| 13. Worry about memory worsening in the future | 0.92 | 0.81 | 2.03 (0.99) | 2 (1:3) | ||
| Variables | Practical Concern | Emotional Concern | MCQ | WHOQOL-OLD Version 1 | WHOQOL-OLD Version 2 | WHOQOL-OLD Version 3 | WHOQOL-OLD |
|---|---|---|---|---|---|---|---|
| Practical concern | -- | ||||||
| Emotional concern | 0.80 ** | -- | |||||
| MCQ | 0.96 ** | 0.94 ** | -- | ||||
| WHOQOL-OLD Version 1 | −0.55 ** | −0.48 ** | −0.54 ** | -- | |||
| WHOQOL-OLD Version12 | −0.55 ** | −0.51 ** | −0.56 ** | 0.92 ** | -- | ||
| WHOQOL-OLD Version 3 | −0.56 ** | −0.49 ** | −0.55 ** | 0.88 ** | 0.90 ** | -- | |
| WHOQOL-OLD | −0.58 ** | −0.51 ** | −0.57 ** | 0.9 3 ** | 0.94 ** | 0.94 ** | -- |
| Variables | Practical Concern Mean (SD) | Emotional Concern Mean (SD) | MCQ Total Mean (SD) |
|---|---|---|---|
| Comorbidity | |||
| Yes | 52.08 (17.14) | 45.36 (17.79) | 48.98 (16.66) |
| No | 39.33 (14.32) | 33.89 (14.94) | 36.82 (13.65) |
| Mann-Whitney U | 24.66 | 20.12 | 25.44 |
| p-value | <0.001 | <0.001 | <0.001 |
| Frail status | |||
| Robust ① | 34.41 (13.40) | 29.09 (12.25) | 31.96 (11.81) |
| Prefrail ② | 47.61 (15.45) | 41.47 (16.20) | 44.78 (15.07) |
| Frail ③ | 55.16 (18.04) | 49.23 (17.84) | 52.43 (17.08) |
| Kruskal-Wallis | 32.08 | 30.20 | 34.30 |
| p-value | <0.001 | <0.001 | <0.001 |
| Pairwise comparisons (p-value adjusted by Bonferroni correction) | ② > ①; ③ > ① | ② > ①; ③ > ① | ② > ①; ③ > ① |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, Q.; Su, H.; Zhou, Z.; Li, C.; Zou, J.; Zhou, Y.; Song, R.; Liu, Y.; Xu, L.; Zhou, Y. Psychometric Evaluation of the Chinese Version of Mild Cognitive Impairment Questionnaire among Older Adults with Mild Cognitive Impairment. Int. J. Environ. Res. Public Health 2023, 20, 498. https://doi.org/10.3390/ijerph20010498
Dai Q, Su H, Zhou Z, Li C, Zou J, Zhou Y, Song R, Liu Y, Xu L, Zhou Y. Psychometric Evaluation of the Chinese Version of Mild Cognitive Impairment Questionnaire among Older Adults with Mild Cognitive Impairment. International Journal of Environmental Research and Public Health. 2023; 20(1):498. https://doi.org/10.3390/ijerph20010498
Chicago/Turabian StyleDai, Qingmin, Hong Su, Zanhua Zhou, Caifu Li, Jihua Zou, Ying Zhou, Rhayun Song, Yang Liu, Lijuan Xu, and Yuqiu Zhou. 2023. "Psychometric Evaluation of the Chinese Version of Mild Cognitive Impairment Questionnaire among Older Adults with Mild Cognitive Impairment" International Journal of Environmental Research and Public Health 20, no. 1: 498. https://doi.org/10.3390/ijerph20010498
APA StyleDai, Q., Su, H., Zhou, Z., Li, C., Zou, J., Zhou, Y., Song, R., Liu, Y., Xu, L., & Zhou, Y. (2023). Psychometric Evaluation of the Chinese Version of Mild Cognitive Impairment Questionnaire among Older Adults with Mild Cognitive Impairment. International Journal of Environmental Research and Public Health, 20(1), 498. https://doi.org/10.3390/ijerph20010498







