The Influence of Body Mass Index on Growth Factor Composition in the Platelet-Rich Plasma in Patients with Knee Osteoarthritis
Abstract
1. Introduction
2. Methods
2.1. Patient Selection and Screening
2.2. ELISA
2.3. Statistical Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pereira, D.; Peleteiro, B.; Araújo, J.; Branco, J.; Santos, R.A.; Ramos, E. The effect of osteoarthritis definition on prevalence and incidence estimates: A systematic review. Osteoarthr. Cartil. 2011, 19, 1270–1285. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Rayman, M.P.; Gualillo, O.; Sellam, J.; van der Kraan, P.; Fearon, U. The role of metabolism in the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2017, 13, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Szwedowski, D.; Szczepanek, J.; Paczesny, Ł.; Pękała, P.; Zabrzyński, J.; Kruczyński, J. Genetics in Cartilage Lesions: Basic Science and Therapy Approaches. Int. J. Mol. Sci. 2020, 21, 5430. [Google Scholar] [CrossRef] [PubMed]
- Nowaczyk, A.; Szwedowski, D.; Dallo, I.; Nowaczyk, J. Overview of First-Line and Second-Line Pharmacotherapies for Osteoarthritis with Special Focus on Intra-Articular Treatment. Int. J. Mol. Sci. 2022, 23, 1566. [Google Scholar] [CrossRef]
- Huang, G.; Hua, S.; Yang, T.; Ma, J.; Yu, W.; Chen, X. Platelet-rich plasma shows beneficial effects for patients with knee osteoarthritis by suppressing inflammatory factors. Exp. Ther. Med. 2018, 15, 3096–3102. [Google Scholar] [CrossRef]
- van Buul, G.M.; Koevoet, W.L.; Kops, N.; Bos, P.K.; Verhaar, J.A.; Weinans, H.; Bernsen, M.R.; van Osch, G.J. Platelet-rich plasma releasate inhibits inflammatory processes in osteoarthritic chondrocytes. Am. J. Sports Med. 2011, 39, 2362–2370. [Google Scholar] [CrossRef] [PubMed]
- Everts, P.; Onishi, K.; Jayaram, P.; Lana, J.F.; Mautner, K. Platelet-Rich Plasma: New Performance Understandings and Therapeutic Considerations in 2020. Int. J. Mol. Sci. 2020, 21, 7794. [Google Scholar] [CrossRef]
- Szwedowski, D.; Szczepanek, J.; Paczesny, Ł.; Zabrzyński, J.; Gagat, M.; Mobasheri, A.; Jeka, S. The effect of platelet-rich plasma on the intra-articular microenvironment in knee osteoarthritis. Int. J. Mol. Sci. 2021, 22, 5492. [Google Scholar] [CrossRef]
- Bennett, N.T.; Schultz, G.S. Growth factors and wound healing: Biochemical properties of growth factors and their receptors. Am. J. Surg. 1993, 165, 728–737. [Google Scholar] [CrossRef]
- Ornitz, D.M.; Itoh, N. The fibroblast growth factor signaling pathway. Wiley Interdiscip. Rev. Dev. Biol. 2015, 4, 215–266. [Google Scholar] [CrossRef]
- Wasterlain, A.S.; Braun, H.J.; Harris, A.H.; Kim, H.J.; Dragoo, J.L. The systemic effects of platelet-rich plasma injection. Am. J. Sports Med. 2013, 41, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Raud, B.; Gay, C.; Guiguet-Auclair, C.; Bonnin, A.; Gerbaud, L.; Pereira, B.; Duclos, M.; Boirie, Y.; Coudeyre, E. Level of obesity is directly associated with the clinical and functional consequences of knee osteoarthritis. Sci. Rep. 2020, 10, 3601. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, A.; Lad, D.; Karnatzikos, G. The effects of repeated intra-articular PRP injections on clinical outcomes of early osteoarthritis of the knee. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 2170–2177. [Google Scholar] [CrossRef] [PubMed]
- Szwedowski, D.; Mobasheri, A.; Moniuszko, A.; Zabrzyński, J.; Jeka, S. Intra-Articular Injection of Platelet-Rich Plasma Is More Effective than Hyaluronic Acid or Steroid Injection in the Treatment of Mild to Moderate Knee Osteoarthritis: A Prospective, Randomized, Triple-Parallel Clinical Trial. Biomedicines 2022, 10, 991. [Google Scholar] [CrossRef]
- Kanwat, H.; Mandeep Singh, D.; Devendra Kumar, C.; Alka, B.; Biman, S.; Aman, H. The effect of intra-articular allogenic platelet rich plasma in Dunkin-Hartley guinea pig model of knee osteoarthritis. Muscle Ligaments Tendons J. 2019, 7, 426. [Google Scholar] [CrossRef]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- Ha, C.W.; Park, Y.B.; Jang, J.W.; Kim, M.; Kim, J.A.; Park, Y.G. Variability of the Composition of Growth Factors and Cytokines in Platelet-Rich Plasma From the Knee With Osteoarthritis. Arthrosc.—J. Arthrosc. Relat. Surg. 2019, 35, 2878–2884.e1. [Google Scholar] [CrossRef]
- Taniguchi, Y.; Yoshioka, T.; Sugaya, H.; Gosho, M.; Aoto, K.; Kanamori, A.; Yamazaki, M. Growth factor levels in leukocyte-poor platelet-rich plasma and correlations with donor age, gender, and platelets in the Japanese population. J. Exp. Orthop. 2019, 6, 4–11. [Google Scholar] [CrossRef]
- Sundman, E.A.; Cole, B.J.; Fortier, L.A. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am. J. Sports Med. 2011, 39, 2135–2140. [Google Scholar] [CrossRef]
- Ziegler, C.G.; Van Sloun, R.; Gonzalez, S.; Whitney, K.E.; DePhillipo, N.N.; Kennedy, M.; Dornan, G.; Evans, T.A.; Huard, J.; LaPrade, R.F. Characterization of Growth Factors, Cytokines and Chemokines in Bone Marrow Concentrate and Platelet Rich Plasma: A Prospective Analysis. Orthop. J. Sport. Med. 2019, 7, 2325967119S0028. [Google Scholar] [CrossRef]
- Reyes, C.; Leyland, K.M.; Peat, G.; Cooper, C.; Arden, N.K.; Prieto-Alhambra, D. Association Between Overweight and Obesity and Risk of Clinically Diagnosed Knee, Hip, and Hand Osteoarthritis: A Population-Based Cohort Study. Arthritis Rheumatol. 2016, 68, 1869–1875. [Google Scholar] [CrossRef] [PubMed]
- Riddle, D.L.; Stratford, P.W. Body weight changes and corresponding changes in pain and function in persons with symptomatic knee osteoarthritis: A cohort study. Arthritis Care Res. 2013, 65, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Belk, J.W.; Kraeutler, M.J.; Houck, D.A.; Goodrich, J.A.; Dragoo, J.L.; McCarty, E.C. Platelet-Rich Plasma Versus Hyaluronic Acid for Knee Osteoarthritis: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Am. J. Sports Med. 2021, 49, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Caroleo, M.; Carbone, E.A.; Greco, M.; Corigliano, D.M.; Arcidiacono, B.; Fazia, G.; Rania, M.; Aloi, M.; Gallelli, L.; Segura-Garcia, C.; et al. Brain-Behavior-Immune Interaction: Serum Cytokines and Growth Factors in Patients with Eating Disorders at Extremes of the Body Mass Index (BMI) Spectrum. Nutrients 2019, 11, 1995. [Google Scholar] [CrossRef] [PubMed]
- Tucker, P.; Pfefferbaum, B.; Nitiéma, P.; Khan, Q.; Aggarwal, R.; Walling, E.E. Possible link of Interleukin-6 and Interleukin-2 with psychiatric diagnosis, ethnicity, disaster or BMI. Cytokine 2017, 96, 247–252. [Google Scholar] [CrossRef]
- Straczkowski, M.; Kowalska, I.; Nikolajuk, A.; Dzienis-Straczkowska, S.; Szelachowska, M.; Kinalska, I. Plasma interleukin 8 concentrations in obese subjects with impaired glucose tolerance. Cardiovasc. Diabetol. 2003, 2, 5. [Google Scholar] [CrossRef]
- Oh, J.H.; Kim, W.; Park, K.U.; Roh, Y.H. Comparison of the Cellular Composition and Cytokine-Release Kinetics of Various Platelet-Rich Plasma Preparations. Am. J. Sports Med. 2015, 43, 3062–3070. [Google Scholar] [CrossRef]
- Boswell, S.G.; Cole, B.J.; Sundman, E.A.; Karas, V.; Fortier, L.A. Platelet-rich plasma: A milieu of bioactive factors. Arthroscopy 2012, 28, 429–439. [Google Scholar] [CrossRef]
- Padilla, S.; Orive, G.; Sanchez, M.; Anitua, E. Platelet-rich plasma in orthopaedic applications: Evidence-based recommendations for treatment. J. Am. Acad. Orthop. Surg. 2014, 22, 469–470. [Google Scholar] [CrossRef]
- Nazli, S.A.; Loeser, R.F.; Chubinskaya, S.; Willey, J.S.; Yammani, R.R. High fat-diet and saturated fatty acid palmitate inhibits IGF-1 function in chondrocytes. Osteoarthr. Cartil. 2017, 25, 1516–1521. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Chubinskaya, S.; Schoeberl, B.; Florine, E.; Kopesky, P.; Grodzinsky, A.J. Effects of insulin-like growth factor-1 and dexamethasone on cytokine-challenged cartilage: Relevance to post-traumatic osteoarthritis. Osteoarthr. Cartil. 2015, 23, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Wallis, J.A.; Webster, K.E.; Levinger, P.; Taylor, N.F. What proportion of people with hip and knee osteoarthritis meet physical activity guidelines? A systematic review and meta-analysis. Osteoarthr. Cartil. 2013, 21, 1648–1659. [Google Scholar] [CrossRef] [PubMed]
- Marks, R. Obesity profiles with knee osteoarthritis: Correlation with pain, disability, disease progression. Obesity 2007, 15, 1867–1874. [Google Scholar] [CrossRef] [PubMed]
- Andia, I.; Maffulli, N. A contemporary view of platelet-rich plasma therapies: Moving toward refined clinical protocols and precise indications. Regen. Med. 2018, 13, 717–728. [Google Scholar] [CrossRef]
- Evanson, R.; Kelly Guyton, M.; Oliver, A.L.; Hire, J.M.; Topolski, R.L.; Zumbrun, S.D.; McPherson, J.C.; Bojescul, J.A. Gender and age differences in growth factor concentrations from platelet-rich plasma in adults. Mil. Med. 2014, 179, 799–805. [Google Scholar] [CrossRef]
- Cho, H.S.; Song, I.H.; Park, S.Y.; Sung, M.C.; Ahn, M.W.; Song, K.E. Individual variation in growth factor concentrations in platelet-rich plasma and its influence on human mesenchymal stem cells. Korean J. Lab. Med. 2011, 31, 212–218. [Google Scholar] [CrossRef]
- Xiong, G.; Lingampalli, N.; Koltsov, J.C.B.; Leung, L.L.; Bhutani, N.; Robinson, W.H.; Chu, C.R. Men and Women Differ. in the Biochemical Composition of Platelet-Rich Plasma. Am. J. Sports Med. 2018, 46, 409–419. [Google Scholar] [CrossRef]
- Castillo, T.N.; Pouliot, M.A.; Kim, H.J.; Dragoo, J.L. Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am. J. Sports Med. 2011, 39, 266–271. [Google Scholar] [CrossRef]
- Dragoo, J.L.; Braun, H.J.; Durham, J.L.; Ridley, B.A.; Odegaard, J.I.; Luong, R.; Arnoczky, S.P. Comparison of the acute inflammatory response of two commercial platelet-rich plasma systems in healthy rabbit tendons. Am. J. Sports Med. 2012, 40, 1274–1281. [Google Scholar] [CrossRef]
| Biomarker | Kit Name | Intra Assay CV | Inter Assay CV |
|---|---|---|---|
| Insulin-like growth factor 1 | Quantitative immunoenzymatic determination of human inuslin-like growth factor 1 (IGF-1) in human serum | ≤8.9% | ≤12.9% |
| Transforming growth factor beta | Human transforming growth factor β (TGF-β) ELISA kit | <10% | <12% |
| Fibroblast growth factor 2 | Human fibroblast growth factor 2 (FGF2) ELISA kit | <10% | <12% |
| Epidermal growth factor | Human epidermal growth factor 2 (EGF) ELISA kit | limited data | limited data |
| Vascular endothelial growth factor | Human vascular endothelial cell growth factor (VEGF) ELISA kit | <10% | <12% |
| Platelet-derived growth factor | Human platelet-derived growth factor (PDGF) ELISA Kit | <10% | <12% |
| Control Group | Study Group | ||
|---|---|---|---|
| Male | Number | 6 | 4 |
| Age [mean] | 62.17 | 52.50 | |
| BMI [mean][kg/m^2] | 23.60 | 33.35 | |
| Female | Number | 19 | 20 |
| Age [mean] | 56.58 | 54.10 | |
| BMI [mean][kg/m^2] | 21.31 | 32.08 | |
| Both genders | Number | 25 | 24 |
| Age [mean] | 57.92 | 53.83 | |
| BMI [mean][kg/m^2] | 21.86 | 32.29 | |
| Mean ± SEM (1) | Mean ± SEM (2) | Median (1) | Median (2) | U | p-Value | |
|---|---|---|---|---|---|---|
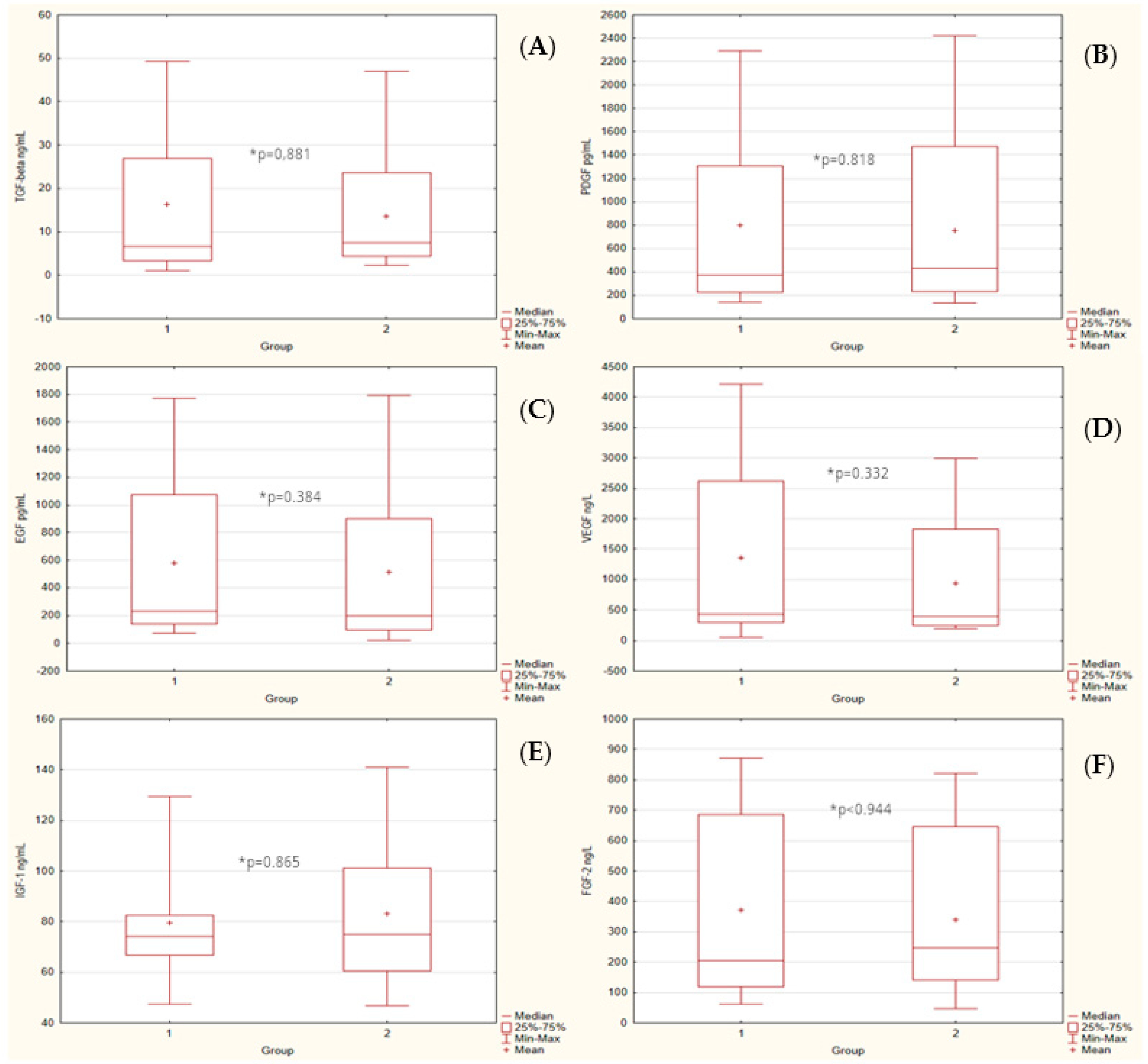
| TGF-β [ng/mL] | 16.41 ± 3.27 | 13.60 ± 2.75 | 6.58 | 7.55 | 292.00 | 0.881 |
| PDGF [pg/mL] | 801.05 ± 148.68 | 756.27 ± 146.31 | 372.06 | 433.28 | 288.00 | 0.818 |
| EGF [pg/mL] | 578.31 ± 110.25 | 514.43 ± 121.1 | 231.66 | 196.50 | 256.00 | 0.384 |
| VEGF [ng/L] | 1360.81 ± 288.44 | 939.03 ± 206.46 | 425.46 | 395.40 | 251.00 | 0.332 |
| IGF-1 [ng/mL] | 79.38 ± 4.27 | 82.97 ± 5.67 | 74.06 | 75.09 | 291.00 | 0.865 |
| FGF-2 [ng/L] | 372.58 ± 61.49 | 338.84 ± 53.13 | 204.50 | 246.93 | 296.00 | 0.944 |
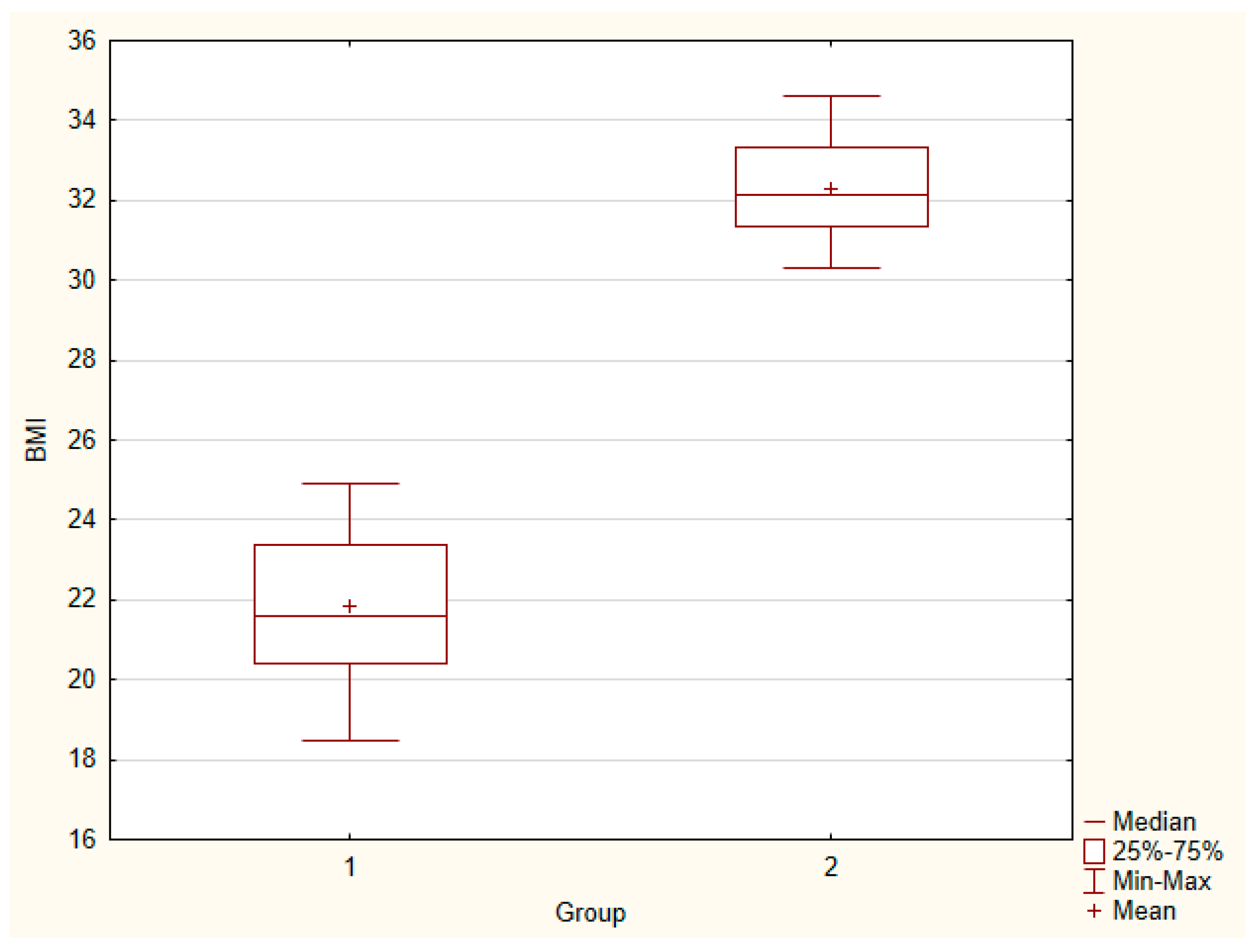
| BMI | 21.86 ± 0.42 | 32.29 ± 0.28 | 21.60 | 32.15 | 0.00 | 0.000 |
| All | Control | Study | ||
|---|---|---|---|---|
| BMI | TGF-β | r = −0.1715 p = 0.239 | r = −0.4165 p = 0.038 | r = 0.0484 p = 0.822 |
| PDGF | r = −0.0889 p = 0.543 | r = −0.3068 p = 0.136 | r = 0.0025 p = 0.991 | |
| EGF | r = −0.1173 p = 0.422 | r = −0.3747 p = 0.065 | r = 0.0446 p = 0.836 | |
| VEGF | r = −0.2326 p = 0.108 | r = −0.3484 p = 0.088 | r = 0.0424 p = 0.844 | |
| IGF-1 | r = 0.1136 p = 0.437 | r = 0.2121 p = 0.309 | r = 0.0659 p = 0.760 | |
| FGF-2 | r = −0.1018 p = 0.487 | r = −0.1932 p = 0.355 | r = −0.0463 p = 0.830 | |
| Age | TGF-β | r = −0.1088 p = 0.457 | r = −0.0794 p = 0.706 | r = −0.2095 p=0.326 |
| PDGF | r = −0.1917 p = 0.187 | r = −0.1592 p = 0.447 | r = −0.2561 p = 0.227 | |
| EGF | r = −0.1673 p = 0.251 | r = −0.0796 p = 0.705 | r = −0.2949 p = 0.162 | |
| VEGF | r = −0.0892 p = 0.542 | r = −0.0890 p = 0.672 | r = −0.2065 p = 0.333 | |
| IGF-1 | r = −0.2937 p = 0.041 | r = −0.1149 p = 0.585 | r = −0.4364 p = 0.033 | |
| FGF-2 | r = −0.2589 p = 0.072 | r = −0.1028 p = 0.625 | r = −0.5133 p = 0.010 | |
| Gender | TGF-β | r = −0.2096 p = 0.148 | r = −0.1814 p = 0.386 | r = −0.2766 p = 0.191 |
| PDGF | r = −0.2145 p = 0.139 | r = −0.1309 p = 0.533 | r = −0.3273 p = 0.118 | |
| EGF | r = −0.2171 p = 0.134 | r = −0.1568 p = 0.454 | r = −0.3003 p = 0.154 | |
| VEGF | r = −0.1842 p = 0.205 | r = −0.1529 p = 0.466 | r = −0.2924 p = 0.166 | |
| IGF-1 | r = 0.3924 p = 0.005 | r = 0.2688 p = 0.194 | r = 0.5395 p = 0.007 | |
| FGF-2 | r = −0.2466 p = 0.088 | r = −0.1864 p = 0.372 | r = −0.3485 p = 0.095 |
| All | Control | Study | ||
|---|---|---|---|---|
| TGF-β | PDGF | r = 0.8848 p = 0.000 | r = 0.8276 p = 0.000 | r = 0.9698 p = 0.000 |
| EGF | r = 0.9533 p = 0.00 | r = 0.9591 p = 0.000 | r = 0.9661 p = 0.000 | |
| VEGF | r = 0.9749 p = 0.00 | r = 0.9820 p = 0.000 | r = 0.9769 p = 0.000 | |
| IGF-1 | r = −0.0002 p = 0.999 | r = 0.1642 p = 0.433 | r = −0.1461 p = 0.496 | |
| FGF-2 | r = 0.7754 p = 0.000 | r = 0.9059 p = 0.000 | r = 0.5779 p = 0.003 | |
| PDGF | EGF | r = 0.9098 p = 0.00 | r = 0.8493 p = 0.000 | r = 0.9736 p = 0.000 |
| VEGF | r = 0.8646 p = 0.000 | r = 0.8235 p = 0.000 | r = 0.9748 p = 0.000 | |
| IGF-1 | r = 0.0161 p = 0.912 | r = 0.2007 p = 0.336 | r = −0.1327 p = 0.537 | |
| FGF-2 | r = 0.7051 p = 0.000 | r = 0.7872 p = 0.000 | r = 0.6029 p = 0.002 | |
| EGF | VEGF | r = 0.9461 p = 0.00 | r = 0.9736 p = 0.000 | r = 0.9725 p = 0.000 |
| IGF-1 | r = 0.0282 p = 0.847 | r = 0.1516 p = 0.469 | r = −0.0558 p = 0.796 | |
| FGF-2 | r = 0.7662 p = 0.000 | r = 0.8866 p = 0.000 | r = 0.8866 p = 0.000 | |
| VEGF | IGF-1 | r = 0.0218 p = 0.882 | r = 0.1916 p = 0.359 | r = −0.1406 p = 0.512 |
| FGF-2 | r = 0.7855 p = 0.000 | r = 0.9135 p = 0.000 | r = 0.5767 p = 0.003 | |
| IGF-1 | FGF-2 | r = 0.1375 p = 0.346 | r = 0.0988 p = 0.639 | r = 0.1905 p = 0.373 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiciński, M.; Szwedowski, D.; Wróbel, Ł.; Jeka, S.; Zabrzyński, J. The Influence of Body Mass Index on Growth Factor Composition in the Platelet-Rich Plasma in Patients with Knee Osteoarthritis. Int. J. Environ. Res. Public Health 2023, 20, 40. https://doi.org/10.3390/ijerph20010040
Wiciński M, Szwedowski D, Wróbel Ł, Jeka S, Zabrzyński J. The Influence of Body Mass Index on Growth Factor Composition in the Platelet-Rich Plasma in Patients with Knee Osteoarthritis. International Journal of Environmental Research and Public Health. 2023; 20(1):40. https://doi.org/10.3390/ijerph20010040
Chicago/Turabian StyleWiciński, Michał, Dawid Szwedowski, Łukasz Wróbel, Sławomir Jeka, and Jan Zabrzyński. 2023. "The Influence of Body Mass Index on Growth Factor Composition in the Platelet-Rich Plasma in Patients with Knee Osteoarthritis" International Journal of Environmental Research and Public Health 20, no. 1: 40. https://doi.org/10.3390/ijerph20010040
APA StyleWiciński, M., Szwedowski, D., Wróbel, Ł., Jeka, S., & Zabrzyński, J. (2023). The Influence of Body Mass Index on Growth Factor Composition in the Platelet-Rich Plasma in Patients with Knee Osteoarthritis. International Journal of Environmental Research and Public Health, 20(1), 40. https://doi.org/10.3390/ijerph20010040







